
Association between Childhood Asthma Control Test scores and lung pathophysiologic indicators in longitudinal measurements
Highlight box
Key findings
• Among 37 children with mild or moderate asthma, longitudinal changes in C-ACT score were significantly associated with large airway resistance and lung function.
What is known and what is new?
• The C-ACT is among most used questionnaires to monitor pediatric asthma status.
• We found the potential usefulness of C-ACT to monitor lung pathophysiologic changes in children with mild or moderate asthma.
What is the implication, and what should change now?
• In children with mild or moderate asthma, longitudinal C-ACT score changes could reflect changes in large airway resistance and lung function.
• Measures of small airway physiology would provide valuable complementary information for asthma control.
Introduction
With the hallmark of chronic lung inflammation and impaired lung function, pediatric asthma is a life-long risk factor for mortality and morbidity. Past decades have witnessed a rapid increase in the prevalence of pediatric asthma, particularly in low- and middle-income countries. The global prevalence of pediatric asthma was 4.2% in 2019 (1). Pediatric asthma has complex etiology and its phenotypes can be characterized by chronic airway inflammation, history of respiratory symptoms (wheeze, shortness of breath, chest tightness, and cough), and airflow limitation (2,3). The intensity of these symptoms may vary over time, due to changes in numerous exogenous factors such as exposure to allergens, viral infection, medication use, and endogenous factors such as airway remodeling (2,4-6). Therefore, asthma control should be highly personalized and the optimal asthma management plan should be based on a continuous cycle of “assess-adjust-review-response” (6,7), in which the precise assessment of asthma control is crucial. Several tools have been developed for asthma control assessment, including validated questionnaires and measurements of lung pathophysiology using spirometry, impulse oscillometry, and biomarkers of respiratory inflammation (7,8). The questionnaire-based assessment provides an easily accessible evaluation tool for patients and physicians, while the measurement of lung pathophysiology typically requires sophisticated instrumentation and trained personnel.
The Childhood Asthma Control Test (C-ACT) is among most commonly used questionnaires for the evaluation of asthma status in children of 4 to 11 years old (6,8), with inputs from both the child with asthma (for present symptoms) and caregivers (for recall of symptoms during the previous 4 weeks) (9). Clinically, C-ACT score ≤19 has been identified as the cut point for inadequately controlled asthma (9) and C-ACT score ≤12 as the cut point for poorly controlled asthma (10). A two-point change is considered as the minimal change of clinical importance (11).
Although C-ACT has been validated for numeric cut points to discern asthma control among children to guide initial diagnosis and treatment, research efforts have also been made to understand the relationship between C-ACT score and lung pathophysiology. Previous studies used a cross-sectional design to compare C-ACT scores among children with different degrees of asthma control (9,11-13). However, longitudinal studies have yet to be conducted to examine whether changes in C-ACT score can reflect changes in lung pathophysiologic indicators over time in the same individuals. Such studies can further validate the usefulness of the C-ACT tool in reflecting temporal changes in objectively measured asthma indicators.
In the present study, we conducted a secondary analysis of data collected from a clinical trial in which 43 children with mild or moderate asthma were followed bi-weekly for 6 weeks for the assessment of C-ACT as well as hospital-based measurements of lung function, airway mechanics, and respiratory inflammation. However, only 37 children had complete data on C-ACT score for the current analysis. We examined the associations of C-ACT score with concurrently (the same-day) measured and 4-week averages of lung pathophysiologic indicators, respectively. To our knowledge, this is the first study to investigate whether within-child variations in C-ACT score would reflect pathophysiologic changes in airway mechanics, lung function, and respiratory inflammation. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1383/rc).
Methods
Study design and participants
For the present study analysis, we used the data collected in a residential air purification intervention in 43 children with asthma (14). All participants were recruited from the outpatient clinic of the Shanghai General Hospital, had mild or moderate asthma according to the Global Initiative for Asthma (GINA) (15), and had at least one episode of asthma exacerbation during the past 12 months. Subjects were measured for lung pathophysiologic indicators at the clinic every 2 weeks over the period of 6 weeks. Among the 43 children, 37 participants were aged 4 to 11 years old (the age range applicable for C-ACT) and were asked to complete C-ACT questionnaires during clinic visits. These patients served as study subjects for the present analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of Shanghai General Hospital (Protocol #38) and Duke University Campus institutional review board (Protocol #D0742). All participants provided oral assent, and their parents gave written consent.
Outcome measurements
Upon enrollment, the children provided blood samples via venipuncture for immunoglobulin E (IgE)-mediated allergy testing and eosinophil counts. At each clinical visit, assessments of C-ACT, as well as measurements of lung function, airway mechanics, and respiratory inflammation were performed. During the study, stepping-up or down of asthma medication was allowed if deemed necessary based on physician’s evaluation at each clinical visit.
A validated Chinese version of the C-ACT was used in this study (16) and filled out by both the child and a caregiver. The C-ACT consists of four questions for the child to report the overall perception of asthma control, limitation of physical activities, coughing, and waking up at night, as well as three questions for the caregiver to recall symptoms over the previous 4 weeks including daytime symptoms, daytime wheezing, and waking up at night. A total score is calculated by summing the scores of all seven questions where the full score is 27 and indicates better asthma control (9). Airway mechanics were measured using a Jaeger MasterScreenTM impulse osillometer (IOS) (Becton, Dickinson and Company, Germany) (17). Main parameters of airway mechanics included airway resistance at 5 Hz (R5; total airway resistance), airway resistance at 20 Hz (R20; large airway resistance), difference between R5 and R20 (R5–R20; small airway resistance), airway reactance at 5 Hz (X5), and resonant frequency (Fres). Standard spirometry was performed following the American Thoracic Society (ATS) guideline and using Jaeger MasterScreenTM PFT System (Becton, Dickinson and Company) (18). Spirometry parameters included forced vital capacity (FVC), forced expiratory volume during the 1st second (FEV1), FEV1/FVC ratio, and forced expiratory flow during 25% to 75% of FVC (FEF25–75). Fractional exhaled nitric oxide (FeNO), a well-established biomarker of respiratory inflammation, was measured using a NIOX VERO machine (Circassia Pharmaceuticals Inc., USA) (19).
Statistical analysis
The association between changes in C-ACT scores and lung pathophysiology indicators in longitudinal measurements were analyzed using linear mixed-effects models in which a random intercept was specified to account for multiple measurements from the same participant and no other fixed-effect covariates were included. The raw values of lung function measurements by spirometry and impulse oscillometry, instead of percent predicted values, were used in this analysis. Due to the highly right-skewed distribution of FeNO data, transformed FeNO data {ln[FeNO]} was used in the analyses. As C-ACT evaluated children’s perception of near-term (present) asthma symptoms and caregivers’ recall of symptoms over the past 4 weeks, both same-day measurements and 4-week averages of respiratory physiologic indicators were assessed for their relationship with C-ACT score. Given that a two-point change in the C-ACT score is typically considered as the smallest change of clinical significance (10), we reported the change in lung pathophysiology indicators associated with a two-point decrease (deterioration) in C-ACT score. Percentage change in lung function and airway mechanics were calculated by dividing the absolute change with the average of the same indicator in all participants and multiplied by 100%. Statistical analyses were performed using the lme4 and lmeTest packages of the R software (version 3.6.3, R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria) (20-22).
Results
Participant characteristics
As shown in Table 1, 16 girls (43%) and 21 boys (57%) were included in the present analysis. Most of the participants (84%) had a mother with college or above education. All participants had physician-ascertained mild or moderate asthma. At baseline, most children had their asthma condition well controlled. Only three children (8%) had C-ACT <19, indicating inadequately controlled asthma (9) and none had C-ACT score <12 (the cut point for poorly controlled asthma) (11). Only one child (3%) had baseline FEV1% predicted <80% and no child had FEV1/FVC ratio % predicted <70%. A small portion of children (n=14, 38%) had eosinophilic airway inflammation at baseline, defined as having FeNO >35 ppb (n=5) (23) or blood eosinophil count >450/µL (n=9) (24). At baseline, 30% of children did not take any long-term asthma control medication. Dust mite was the most prevalent (59%) allergen among the allergens tested.
Table 1
| Baseline characteristics | Participants |
|---|---|
| Sample size | 37† |
| Female | 16 [43] |
| Age (years) | 7 [5, 10] |
| Height (cm) | 129±11 |
| Weight (kg) | 29±9 |
| C-ACT score | |
| Median [range] | 24 [17, 27] |
| ≤19 | 3 [8] |
| ≤12 | 0 [0] |
| FEV1 (% predicted)‡ | |
| Mean ± SD | 104±16 |
| <80% | 1 [3] |
| FEV1/FVC ratio (% predicted)‡ | |
| Mean ± SD | 99±8 |
| <70% | 0 [0] |
| FeNO | |
| Median [range] (ppb) | 13 [5, 56] |
| >35 ppb | 5 [14] |
| Blood eosinophil count | |
| Median [range] (/µL) | 330 [80, 850] |
| >450/µL | 9 [24] |
| Long-term asthma control medication | |
| None | 11 [30] |
| ICS alone | 5 [14] |
| LABA alone | 0 [0] |
| ICS + LABA | 17 [46] |
| H1 receptor antagonist | 7 [19] |
| Leukotriene receptor antagonists | 2 [5] |
| Positive for blood IgE test§ | 29 [78] |
| Dust mite allergy | 22 [59] |
| Mold allergy | 6 [16] |
| Mother with a college degree or above | 31 [84] |
Data are presented as n, n [%], median [range], or mean ± SD. †, the 37 participants reported in Table 1 is a subset (only those aged 4 to 11 years old) of the 44 participants reported previously by Cui et al. 2020 (14); ‡, percent predicted values for the spirometry values were calculated using the built-in proprietary formula of the spirometry machine (MasterScreenTM PFT System, Jaeger, Germany) developed by Zapletal for children aged 2 to 18 years old; §, upon enrollment, blood samples from each participant were analyzed using an allergen-specific IgE test (AllergyScreen®, Mediwiss Analytic GmbH, Germany). Positive indicated testing positive for any of the 19 allergens evaluated by this test. These 19 allergens included dust mite, mold, cat dander, dog dander, roach, tree pollens and foods (egg, milk, beef, shrimp), etc. Blood IgE levels >0.35 kU/L were considered positive. C-ACT, Childhood Asthma Control Test; FEV1, forced expiratory volume during the 1st second; SD, standard deviation; FVC, forced vital capacity; FeNO, fractional exhaled nitric oxide; ICS, inhaled corticosteroids; LABA, long-acting beta 2-agonists; IgE, immunoglobulin E.
Variability in C-ACT score
The summary statistics of the C-ACT scores are presented in Table 2. The median score was 24, with a range of 16 to 27. The intra-person variability of C-ACT score was 4.50 and inter-person variability was 1.48. The relatively large intra-person variability in C-ACT score afforded us to examine the associations between longitudinal changes in C-ACT score and changes in respiratory pathophysiologic indicators. Of the total C-ACT score (i.e., 27 points), 12 points were from the four questions answered by the children while 15 points were from the three questions answered by the caregivers. The intra-person variability for the children-derived C-ACT score was 2.20 and the intra-person variability for the caregiver-derived C-ACT score was 1.46.
Table 2
| Category | Questions | Score options | Median [range] | Variability | |
|---|---|---|---|---|---|
| Intra-child | Inter-child | ||||
| Answered by children | Q1: How is your asthma today? | 0 to 3 | 3 [1, 3] | 0.22 | 0.08 |
| Q2: How much of a problem is your asthma when you run, exercise or play sports? | 0 to 3 | 3 [0, 3] | 0.38 | 0.15 | |
| Q3: Do you cough because of your asthma? | 0 to 3 | 2 [0, 3] | 0.45 | 0.15 | |
| Q4: Do you wake up during the night because of your asthma? | 0 to 3 | 3 [2, 3] | 0.11 | 0.03 | |
| Subtotal: Q1–4 | 0 to 12 | 10 [4, 12] | 2.20 | 0.92 | |
| Answered by caregivers | Q5: During the last 4 weeks, how many days did your child have any daytime asthma symptoms? | 0 to 5 | 5 [0, 5] | 0.46 | 0.02 |
| Q6: During the last 4 weeks, how many days did your child sneeze during the day because of asthma? | 0 to 5 | 5 [2, 5] | 0.21 | 0.05 | |
| Q7: During the last 4 weeks, how many days did your child wake up during the night because of asthma? | 0 to 5 | 5 [3, 5] | 0.14 | 0.00 | |
| Subtotal: Q5–7 | 0 to 15 | 15 [8, 15] | 1.46 | 0.14 | |
| Total score | Q1–7: The sum of scores for all seven questions | 0 to 27 | 24 [16, 27] | 4.50 | 1.48 |
†, a Mandarin Chinese version of the C-ACT (16) was used in the study. C-ACT, Childhood Asthma Control Test.
Association between C-ACT score and respiratory health indicators
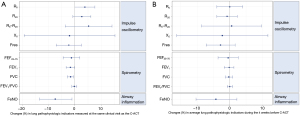
As shown in Figure 1A, for respiratory physiologic indicators measured on the same-day of the C-ACT assessment, a two-point decrease (deterioration) in C-ACT score was associated with a 1.7% decrease in FEV1 (P=0.04), a 1.6% decrease in FVC (P=0.01), and a 3.8% increase in R5 (P=0.052). No associations were found for other indicators of airway mechanics including R5–R20, X5, and Fres. Counter-intuitively, a two-point deterioration is associated with a 7.4% improvement in FeNO (P=0.02). However, by excluding the 14 children with eosinophilic inflammation at baseline, we found that a two-point worsening in C-ACT score was significantly associated with a 4.0% increase (worsening) in same-day measurements of FeNO (P<0.05). Although C-ACT incorporates three questions answered by caregivers for recall of symptoms over the previous 4 weeks, none of the respiratory pathophysiologic indicators averaged over the previous 4 weeks were associated with C-ACT score (Figure 1B).
C-ACT questions answered by children
As C-ACT includes four questions for the children about the present asthma conditions, the sum of all questions answered by children were evaluated with same-day measurements of respiratory health indicators (Figure S1). We found that worsening of C-ACT score by two points was significantly associated with (I) deteriorations of lung function including a 3.1% decrease in FEV1 (P=0.007) and a 2.5% decrease in FVC (P=0.003); (II) deterioration of airway mechanics including a 6.5% increase in R5 (P=0.02) and a 5.5% increase in R20 (P=0.03); and (III) a counter-intuitively improvement of respiratory inflammation, as reflected in a 10.4% decrease in FeNO (P=0.03). Among the four questions answered by the children, at least one question demonstrated significant associations with R5, R20, FEV1, FVC and FEF25–75 (Figure S2). Specifically, for the answer to the first question “How is your asthma today?”, a two-point decrease was significantly associated with significant deteriorations in FEF25–75 by 15.8% (P=0.03), FEV1 by 11.3% (P=0.003), FVC by 10.2% (P<0.001). In contrast, for the average of lung pathophysiologic measurements during the 4 weeks before C-ACT, none of these indicators were associated with the questions answered by the child (Figure S3).
C-ACT questions answered by caregivers
As shown in Figure S1, the sum of scores on the three questions answered by the caregivers was not associated with any respiratory pathophysiologic indicators measured on the same-day. In contrast, a two-point decrease in the sum score was marginally associated with a 2.0% decrease in FEV1 (P=0.055) averaged over the previous 4 weeks (Figure S3). Specifically, for caregivers’ answers to the fifth question (Q5) on daytime asthma symptoms during the previous 4 weeks, a two-point decrease was associated with significant deteriorations in lung function averaged over the previous 4 weeks, including FEV1 by 4.7% (P=0.02) and FVC by 3.0% (P=0.04), as well as a marginal deterioration in FEF25–75 by 7.9% (P=0.06) (Figure S2).
Discussion
C-ACT is among the most commonly used questionnaires for the asthma evaluation of children of 4 to 11 years old (9). Among 37 children with mild or moderate asthma, we found that longitudinal changes in C-ACT score were significantly associated with large airway resistance and lung function. Deterioration of C-ACT scores was significantly associated with deteriorations in same-day FeNO in participants without eosinophilic airway inflammation. However, the association reversed when evaluated in all participants, suggested a likely role of asthma phenotype in defining the relationship between C-ACT score and respiratory inflammation.
Asthma control assessment could guide the medication use and is essential for the personalized asthma management (6). In our study, the intra-person variability of C-ACT score (4.50) was substantially larger than inter-person variability of C-ACT score (1.48) (Table 2), highlighting the importance of understanding the within-child fluctuations in C-ACT score. In previous cross-sectional studies, weak correlations were observed between C-ACT score and lung function (9,12,13). In contrast, we found that within-person deteriorations in the C-ACT score were associated with small but significant deteriorations in same-day measurements of both FEV1 and FVC but not FEV1/FVC, a sign of air trapping in alveoli during exhalation due to airway obstruction (25,26). Consistently, increased total airway resistance (R5) was also associated with worsen C-ACT score. Nevertheless, C-ACT score changes can not reflect small airway condition changes (i.e., FEF50, FEF25–75, R5–R20, X5, and Fres), suggesting that small airway measurement can provide complementary information to the C-ACT.
FeNO is a validated indicator of airway inflammation (27). The relationship between FeNO and asthma control measures has been widely examined but the results are mixed. A systemic review of 58 studies revealed a weak association between FeNO levels and asthma control in adults and children (28). Nevertheless, Nguyen et al. reported associations between FeNO and Asthma Control Test (ACT) score in 410 adults (29). Stern et al. observed associations of FeNO with symptom scores in 151 atopic asthmatic children during 192 days when the use of inhaled corticosteroids (ICS) was adjusted every 3 weeks according to FeNO value (30). In our study, deteriorations in C-ACT score were significantly associated with deteriorations in FeNO only among a subgroup of children without eosinophilic airway inflammation, suggesting that asthma phenotype may modify the relationship between FeNO and C-ACT, which merit further evaluation with sample size large enough to represent distinct asthma phenotypes and medication adjustments.
C-ACT asks for children’s experience of asthma symptoms at present and caregivers’ recall of symptoms during the previous 4 weeks. Correspondingly, we assessed both same-day and 4-week averaged measurements of lung pathophysiological indicators. Worsening scores on the questions answered by children were significantly associated with deteriorations in FEV1, FVC, R5, and R20 on the same-day. Children’s input showed similar association as the total C-ACT score although the magnitude was much larger. In contrast, the questions filled out by the caregivers were only marginally associated with average FEV1 during the previous 4 weeks. These results suggests that children’s perception provides more accurate input for asthma control assessment than their caregivers, which is logically reasonable. For example, it is challenging for the caregiver to obtain accurate information of daytime and nighttime symptoms (16), if the child spends most of the daytime in school and sleeps in a bedroom separately from the caregiver.
Our findings provide initial evidence supporting the potential usefulness of C-ACT score in monitoring fluctuations of asthma control among children with mild or moderate asthma. As the web-based version of C-ACT has been validated to correlate well with paper-based version (10), physicians can track asthma control with the collaboration of children and their caregivers who can take the C-ACT at home. The qualitative association with these objective measurements reported can provide assurance on the utility of C-ACT. The quantitative relationship can provide a reference to physicians and caregivers where they can extrapolate from the changes seen in C-ACT to changes in lung function and airway mechanics if the hospital-based measurements are not readily available.
Our study has several limitations. First, we conducted repeated measurements within a relatively short period of 6 weeks during which no asthma exacerbation events were reported. A longer follow up period may be more desirable to examine the relationship between C-ACT score and lung pathophysiology, considering that clinical events such as asthma exacerbation and an asthma phenotype change may affect the relationship (31-33). Second, this study only included children with mild or moderate asthma due to the inclusion criteria of the parent study that provided data for the current analysis. Therefore, we cannot evaluate the relationship between C-ACT and lung pathophysiology in children with severe asthma. Third, this study followed up each subject for up to 6 weeks and only capture short-term variations in C-ACT score and lung pathophysiologic indictors. More importantly, although we assessed C-ACT score and measured lung pathophysiology every 2 weeks, the caregiver questions were designed for 4 weeks, which may partially explain the poor correlation between caregiver’s score and pathophysiologic indicators. Finally, results of FeNO suggested that the relationship between C-ACT and respiratory inflammation may vary across different phenotypes of asthma, which may limit the generalizability of using C-ACT score to reflect lung pathophysiology. A larger study is warranted to explicitly examine the potential difference by asthma phenotype in the relationship between changes in C-ACT score and changes in respiratory pathophysiology over time.
Conclusions
In this longitudinal study of 37 children with mild or moderate asthma, within-person changes in C-ACT scores were significantly associated with changes in same-day measurements of airway resistance and lung function. Children’s answers showed stronger associations with multiple indicators of airway resistance and lung function compared to caregiver’s answers, indicating the importance of children’s input despite their young age. In contrast, the caregiver-derived C-ACT score was associated with none of the same-day lung pathophysiologic measurements and was only marginally associated with average FEV1 during the previous 4 weeks. The deterioration in C-ACT score was significantly associated with increased pulmonary inflammation (FeNO) in children without eosinophilic airway inflammation, suggesting that asthma phenotype may modify the relationship between C-ACT score and lung inflammation. The lack of an association between C-ACT score and small airway physiology suggests the need for assessing small airways to complement the C-ACT in asthma control.
Acknowledgments
We are grateful to all study participants and their families for their contribution to the study. We would like to thank Dr. Yuh-Chin Tony Huang of Duke University for his kind advice on clinical interpretation.
Funding: This study was supported by funding from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1383/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1383/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1383/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1383/coif). JZ serves as an unpaid editorial board member of Journal of Thoracic Disease. XC reports a receival of a graduate fellowship from Duke University. MSB is an employee of Chemical Safety Research, Underwriters Laboratories Inc., Marietta, GA, USA. MSB and MHB report funding from Underwriters Laboratories Inc., Marietta, GA, USA. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of Shanghai General Hospital (Protocol#38) and Duke University Campus institutional review board (Protocol#D0742). All participants provided oral assent, and their parents gave written consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Safiri S, Carson-Chahhoud K, Karamzad N, et al. Prevalence, Deaths, and Disability-Adjusted Life-Years Due to Asthma and Its Attributable Risk Factors in 204 Countries and Territories, 1990-2019. Chest 2022;161:318-29. [Crossref] [PubMed]
- Olin JT, Wechsler ME. Asthma: pathogenesis and novel drugs for treatment. BMJ 2014;349:g5517. [Crossref] [PubMed]
- McCracken JL, Veeranki SP, Ameredes BT, et al. Diagnosis and Management of Asthma in Adults: A Review. JAMA 2017;318:279-90. [Crossref] [PubMed]
- Lewis TC, Robins TG, Dvonch JT, et al. Air pollution-associated changes in lung function among asthmatic children in Detroit. Environ Health Perspect 2005;113:1068-75. [Crossref] [PubMed]
- Busse WW, Lemanske RF Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010;376:826-34. [Crossref] [PubMed]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2020. Available online: https://ginasthma.org/
- Alzahrani YA, Becker EA. Asthma Control Assessment Tools. Respir Care 2016;61:106-16. [Crossref] [PubMed]
- Dinakar C, Chipps BE. Clinical Tools to Assess Asthma Control in Children. Pediatrics 2017;139:e20163438. [Crossref] [PubMed]
- Liu AH, Zeiger R, Sorkness C, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol 2007;119:817-25. [Crossref] [PubMed]
- Voorend-van Bergen S, Vaessen-Verberne AA, Landstra AM, et al. Monitoring childhood asthma: web-based diaries and the asthma control test. J Allergy Clin Immunol 2014;133:1599-605.e2. [Crossref] [PubMed]
- Liu AH, Zeiger RS, Sorkness CA, et al. The Childhood Asthma Control Test: retrospective determination and clinical validation of a cut point to identify children with very poorly controlled asthma. J Allergy Clin Immunol 2010;126:267-73, 273.e1.
- Chen HH, Wang JY, Jan RL, et al. Reliability and validity of childhood asthma control test in a population of Chinese asthmatic children. Qual Life Res 2008;17:585-93. [Crossref] [PubMed]
- Piacentini GL, Peroni DG, Bodini A, et al. Childhood Asthma Control Test and airway inflammation evaluation in asthmatic children. Allergy 2009;64:1753-7. [Crossref] [PubMed]
- Cui X, Li Z, Teng Y, et al. Association Between Bedroom Particulate Matter Filtration and Changes in Airway Pathophysiology in Children With Asthma. JAMA Pediatr 2020;174:533-42. [Crossref] [PubMed]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2017. Available online: https://ginasthma.org/
- GlaxoSmithKline Research & Development Limited. Childhood Asthma Control Test (C-ACT) (Mandarin Version for China). 2007. Available online: https://eprovide.mapi-trust.org/instruments/childhood-asthma-control-test
- Shi Y, Aledia AS, Tatavoosian AV, et al. Relating small airways to asthma control by using impulse oscillometry in children. J Allergy Clin Immunol 2012;129:671-8. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- American Thoracic Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. [Crossref] [PubMed]
- Bates D, Mächler M, Bolker B, et al. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 2015;67:1-48.
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests in Linear Mixed Effects Models. 2016. Available online: https://www.jstatsoft.org/article/view/v082i13
- R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2020. Available online: https://www.r-project.org/
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. [Crossref] [PubMed]
- Fowler SJ, Tavernier G, Niven R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J Allergy Clin Immunol 2015;135:822-4.e2. [Crossref] [PubMed]
- Park SW, Park JS, Jeong SH, et al. Air trapping is a major determinant of persistent airway obstruction in asthmatics. Respir Med 2012;106:786-93. [Crossref] [PubMed]
- Miller A, Palecki A. Restrictive impairment in patients with asthma. Respir Med 2007;101:272-6. [Crossref] [PubMed]
- Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell 1994;78:915-8. [Crossref] [PubMed]
- Wang Z, Pianosi P, Keogh K, et al. The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management. Rockville: Agency for Healthcare Research and Quality; 2017. Report No. 17(18)-EHC030-EF.
- Nguyen VN, Chavannes NH. Correlation between fractional exhaled nitric oxide and Asthma Control Test score and spirometry parameters in on-treatment-asthmatics in Ho Chi Minh City. J Thorac Dis 2020;12:2197-209. [Crossref] [PubMed]
- Stern G, de Jongste J, van der Valk R, et al. Fluctuation phenotyping based on daily fraction of exhaled nitric oxide values in asthmatic children. J Allergy Clin Immunol 2011;128:293-300. [Crossref] [PubMed]
- Brooks CR, Van Dalen CJ, Harding E, et al. Effects of treatment changes on asthma phenotype prevalence and airway neutrophil function. BMC Pulm Med 2017;17:169. [Crossref] [PubMed]
- Sonnenschein-van der Voort AM, Howe LD, Granell R, et al. Influence of childhood growth on asthma and lung function in adolescence. J Allergy Clin Immunol 2015;135:1435-43.e7. [Crossref] [PubMed]
- Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc 2009;6:301-5. [Crossref] [PubMed]


