
Impact of intraoperative use of venovenous extracorporeal membrane oxygenation on the status of von Willebrand factor large multimers during single lung transplantation
Highlight box
Key findings
• The von Willebrand factor (vWF) large multimer index at the end of single lung transplantation was significantly lowered by venovenous extracorporeal membrane oxygenation (VV ECMO) use.
What is known and what is new?
• It is known that a reduction in vWF large multimers develops during extracorporeal life support (ECLS), which can cause excessive-high shear stress in the blood flow and result in hemostatic disorders. We demonstrated that the vWF large multimer index was significantly lower in the patients who underwent VV ECMO support during single lung transplantation compared to those without ECMO at the end of the surgery.
What is the implication, and what should change now?
• The short duration of time of VV ECMO use in our study did not significantly affect the intra- and postoperative outcomes including blood loss, blood transfusion, and re-exploration thoracotomy for bleeding. To comprehensively evaluate the actual influence of this decrease in the vWF large multimer index, a multicenter larger-scale study is warranted.
Introduction
One of the most severe complications in lung transplantation is perioperative blood loss. Large-volume transfusions of red blood cells (RBCs) and platelets have been reported to be associated with various complications in lung transplant recipients in multiple studies (1-3). Diamond et al. have identified large-volume RBC transfusion (>1 L) as a risk factor for grade 3 primary graft dysfunction (PGD) in a prospective, multicenter cohort study (2). Platelet transfusions were shown to be substantial risk factors for the production of anti-human leukocyte antigen (HLA) antibodies (4), which can potentially cause chronic lung allograft dysfunction (CLAD) (5).
Intraoperative use of extracorporeal life support (ECLS) is one of the risk factors for the increased amount of bleeding and transfusion in lung transplantation. Among the different types of ECLS, cardiopulmonary bypass (CPB) had been reported to increase blood transfusion due to coagulopathy and inflammatory response (6). Currently, venoarterial extracorporeal membrane oxygenation (VA ECMO), which is associated with lower blood loss during lung transplant surgeries compared with CPB (7), is preferably used for lung transplant cases unless intracardiac repairs are required (8). Moreover, venovenous (VV) ECMO has been used as an intraoperative extracorporeal mechanical support for lung transplantation at some lung transplant centers in recent years (9). We started utilizing VV ECMO in 2015 and reported that VV ECMO could be chosen for intraoperative extracorporeal mechanical support during single lung transplantation (SLT) (10).
Although the increase in the amount of bleeding during ECLS is primarily due to the use of heparin, recent studies have suggested the involvement of acquired von Willebrand syndrome (AvWS), which is one of the hemostatic disorders. von Willebrand factor (vWF) is a hemostatic factor that is produced and secreted as large multimers by endothelial cells and megakaryocytes. vWF initially forms a huge multimer and is then shear stress-dependently cleaved in the bloodstream by its specific cleaving enzyme ADAMTS13, and then is present as multimers consisting of 2–80 vWF monomers (11,12). The conformation change of the A2 domain of vWF by shear stress in the blood flow triggers its cleavage by ADAMTS13. Among the vWF multimers, higher molecular weight multimers (large multimers) are known to play a critical role in hemostasis (11,12). The lack of ADAMTS13 results in excessive platelet thrombus formation by the function of ultra-large vWF multimers, as seen in patients with hereditary thrombotic thrombocytopenic purpura (13). On the other hand, some genetic mutations in the vWF A2 domain cause a reduction in large multimers and induce a hemostatic disorder by increasing the susceptibility of vWF to cleavage by ADAMTS13 (12). A reduction in vWF large multimers develops in various other conditions including cardiovascular diseases such as aortic stenosis and the use of ECLS, which can cause excessive-high shear stress in the blood flow and result in hemostatic disorders (12). This hemostatic disorder caused by excessive cleavage of vWF is defined as AvWS (14).
Kalbhenn et al. reported that patients treated with VV ECMO support developed AvWS (15). They have shown that using VV ECMO for pneumonia, ARDS, and so forth can cause dangerously high shear stress in the blood stream, leading to AvWS within 24 hours after ECMO exposure (15). However, it remains unclear whether VV ECMO use during a short period of time during lung transplant surgery can cause a decrease of vWF large multimers. The objective of this prospective study was to investigate the impact of VV ECMO use on the status of vWF large multimers and hemostatic disorders during SLT. We present this article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-275/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Tohoku University Graduate School of Medicine approved this prospective study (approval No. 2016-1-629; protocol No. UMIN000018135), and written informed consent was obtained from all participants.
Patients, blood sample and data collection, and study groups
We prospectively enrolled 12 patients who would undergo SLT under VV ECMO or without ECMO at Tohoku University Hospital from April 2017 to December 2019 (participant recruitment start date, January 10, 2017; last follow-up date, December 31, 2020) (Figure 1).

For the analysis of ADAMTS13 activity and vWF large multimers, blood samples from the patients were collected in tubes containing 3.2% sodium citrate immediately before lung transplantation, before reperfusion (immediately before reperfusion of the lung graft), and at the end of surgery. The plasma of the collected blood was stored at −80 ℃ until analysis. The collected data were as follows: pretransplant demographics of the recipients, operative characteristics, postoperative characteristics, and mortality. The levels of pleural adhesions were assessed intraoperatively and graded as follows according to the area of adhesion: severe, 50% to whole thoracic cavity; moderate, partial (only band adhesions) to <50%; mild, no to partial adhesion; none, no adhesion.
ECMO criteria, induction procedure, and intraoperative management
We utilized VV ECMO in cases that did not show a dominant perfusion ratio on the SLT side (>80%) by pretransplant lung perfusion scintigraphy or severe pulmonary hypertension [systolic pulmonary arterial pressure (PAP) of >70 mmHg]. The selection of the ECMO modality (VA or VV ECMO) or the decision not to employ ECMO was made prior to the surgical procedure. Furthermore, immediately after the induction of general anesthesia, percutaneous insertion of a Swan-Ganz catheter through the right or left internal jugular vein was performed to enable monitoring of the PAP. During the surgery, we remained prepared to switch VA to VV ECMO support in response to an emergence of circulatory support requirements.
We initiated VV ECMO support immediately after the induction of general anesthesia. We established VV ECMO using right and left femoral veins for the cannulation sites. For the inflow, a 15–22 Fr cannula (CAPIOX EBS, Terumo Corp., Tokyo, Japan or HLS Cannulae, Getinge Group Japan K.K., Tokyo, Japan) was inserted, and the tip was adjusted within a caudal part of the inferior vena cava to minimize the recirculation. For the outflow, a 15–21 Fr cannula (CAPIOX EBS, Terumo Corp., Tokyo, Japan or HLS Cannulae, Bio-Medicus, Medtronic Japan Co., Ltd., Tokyo, Japan) was inserted and the tip was placed at the right atrium using transesophageal echocardiography guidance. The blood flow rate of VV ECMO was started at 50% of the estimated cardiac output of the recipient and adjusted according to the intraoperative results of the arterial blood gas analysis (1.5–2.5 L/min). Heparin administration was controlled to target the activated clotting time (ACT) from 160–180 s. Weaning from VV ECMO support was attempted upon completion of the surgery if the patient demonstrated tolerance. If not, we continued VV ECMO support and transfer the patient to the intensive care unit (ICU).
Measurement of ADAMTS13 activity and quantitative method for calculation of the vWF large multimer index
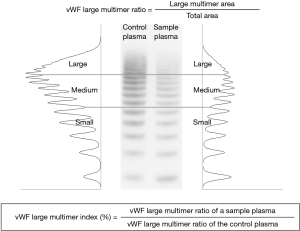
ADAMTS13 activity was measured based on the FRETS-VWF73 assay by SRL Co. (Tokyo, Japan) (16). We first determined the concentration of vWF antigen (vWF:Ag) in standard human plasma (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) by quantitative immunoelectrophoresis using an automatic analyzer. Then, the same amount of vWF:Ag in the plasma of each sample and that in the standard human plasma were analyzed side-by-side by the so-called vWF multimer analysis, which is a western blot analysis under non-reduced conditions. The bands of the 11th and those over the 11th were defined as large multimers. The vWF large multimer proportion was calculated as the densitometric area of those bands using Image J (National Institutes of Health, Bethesda, MD, USA). Then, we determined the parameter, “vWF large multimer index”, as described previously (17). Briefly, the vWF large multimer index (%) was defined as the ratio of the large multimer proportion in the total vWF (vWF large multimer ratio) derived from a sample to that from the standard human plasma in the adjacent lane (17,18) (Figure 2). Thus, a patient’s vWF large multimer index was expressed as a ratio (percentage) of the standard.
Statistical analysis
Continuous data are expressed as group mean ± standard error with ranges. Categorical variables are presented as numbers and percentages. For comparison between the groups, Student’s or Welch’s t-test was used for the parametric data. Mann-Whitney U test was used for the nonparametric data. Fisher’s exact test or the chi-square test was used for the categorical values. The ADAMTS13 activity, vWF:Ag, and vWF large multimer index at each time point were analyzed by repeated measures two-way ANOVA. Differences were considered significant at the P<0.05 level. Statistical analyses were performed using Prism 5 (GraphPad Software Inc, La Jolla, Calif) and SPSS Statistics 21.0 for Windows (IBM Corporation, Chicago, IL, USA).
Results
Study groups and pre-transplant demographics of the recipients
All enrolled patients were followed up until one year after lung transplantation at Tohoku University Hospital, except for one 90-day mortality case. In the present study, we analyzed 5 SLT cases without ECMO use and 7 SLT cases with VV ECMO use. Table 1 shows the pretransplant demographics of the recipients. Recipient age, gender, height, weight, and body mass index (BMI) were similar between the 2 groups. Forced vital capacity (FVC) was comparable between the groups. Because the control group included 3 cases of chronic obstructive pulmonary disease (COPD), forced expiratory volume in one second (FEV1) in the control group tended to be lower than that in the ECMO group; however, the difference was not statistically significant. There was no significant difference between the 2 groups in prothrombin time–international normalized ratio (PT-INR), activated partial thromboplastin time (APTT), or platelet count.
Table 1
| Demographics | Control group (n=5) | ECMO group (n=7) | P value |
|---|---|---|---|
| Age (years) | 48.4±3.8 (34.0–57.0) | 47.1±4.1 (23.0–59.0) | 0.85 |
| Gender (male/female) | 3/2 | 4/3 | 0.92 |
| Height (cm) | 164.4±3.2 (153.5–175.0) | 162.4±2.6 (150.4–173.0) | 0.66 |
| Weight (kg) | 50.5±6.4 (35.0–75.0) | 55.6±6.9 (30.0–91.8) | 0.64 |
| BMI (kg/m2) | 18.5±2.0 (13.0–26.4) | 20.7±2.0 (13.3–30.7) | 0.50 |
| Indication | – | ||
| IPF | 0 (0.0) | 4 (57.1) | |
| COPD | 3 (60.0) | 0 (0.0) | |
| LAM | 1 (20.0) | 2 (28.6) | |
| CTD-ILD | 1 (20.0) | 1 (14.3) | |
| Pulmonary function test | |||
| FVC (% predicted) | 55.1±5.0 (46.4–76.0) | 50.9±8.0 (23.6–85.0) | 0.71 |
| FEV1 (% predicted) | 35.1±7.5 (17.8–57.6) | 43.7±6.5 (26.3–78.4) | 0.44 |
| PT-INR | 0.99±0.02 (0.93–1.04) | 1.00±0.02 (0.99–1.00) | 0.79 |
| APTT (sec) | 32.2±2.7 (27.4–33.9) | 30.3±1.0 (27.4–33.9) | 0.53 |
| Platelet count (×103/µL) | 258±12 (221.0–301.0) | 240±13 (189.0–282.0) | 0.39 |
Values are expressed as mean ± standard error (range) or number (%). ECMO, extracorporeal membrane oxygenation; BMI, body mass index; IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease; LAM, lymphangioleiomyomatosis; CTD-ILD, connective tissue disease-associated interstitial lung disease; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; PT-INR, prothrombin time-international normalized ratio; APTT, activated partial thromboplastin time.
Operative characteristics
Table 2 shows the operative characteristics. There was no significant difference in the operative and cold ischemic time (CIT) between the 2 groups. The mean intraoperative ECMO time in the ECMO group was 487±14 minutes (range, 443–557). The intraoperative blood loss in the ECMO group (1,728±734) tended to be greater than that in the control group (774±308); however, the difference was not statistically significant (P=0.36). In the control group, 1 patient (20.0%) intraoperatively required a transfusion of more than 1,000 mL of RBC. On the other hand, 3 patients (42.9%) were given more over than 1,000 mL of intraoperative RBC in the ECMO group. The mean amount of transfusion of RBC in the ECMO group (920±319) tended to be greater than that in the control group (336±210); however, the difference was not statistically significant (P=0.28). In the control group, 1 patient (20.0%) needed more than 1,000 mL of fresh frozen plasma (FFP). In the ECMO group, the requirement of FFP was over 1,000 mL in 2 patients (28.6%). No patient in the control group and 1 patient (14.3%) in the ECMO group required an intraoperative transfusion of platelets. Table 2 shows the inflow and outflow cannula sizes in each case in the ECMO group. The cases with a relatively smaller outflow cannula (case #9 and 12) tended to require more transfusion products.
Table 2
| Case # | Group | OP time (min) (P=0.29) | CIT (min) (P=0.34) | Intra-OP ECMO time (min) | Cannula size (Fr) | Intra-OP blood loss (mL) (P=0.36) |
Pleural adhesion grade* | Intra-OP blood transfusion | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflow | Outflow | RBC (mL) (P=0.28) | FFP (mL) (P=0.44) | Platelet (mL) |
|||||||
| 1 | Control | 365 | 361 | – | – | – | 249 | None | 0 | 0 | 0 |
| 2 | Control | 383 | 456 | – | – | – | 644 | Severe | 280 | 480 | 0 |
| 3 | Control | 366 | 457 | – | – | – | 307 | None | 0 | 0 | 0 |
| 4 | Control | 452 | 480 | – | – | – | 2,122 | Severe | 1,400 | 1,200 | 0 |
| 5 | Control | 430 | 564 | – | – | – | 550 | Moderate | 280 | 0 | 0 |
| 6 | ECMO | 504 | 457 | 459 | 22 | 22 | 2,332 | None | 1,680 | 2,880 | 0 |
| 7 | ECMO | 423 | 453 | 517 | 19.5 | 18 | 547 | Moderate | 280 | 0 | 0 |
| 8 | ECMO | 356 | 447 | 455 | 21 | 21 | 869 | None | 560 | 480 | 0 |
| 9 | ECMO | 482 | 656 | 557 | 21 | 15 | 6,228 | Severe | 1,960 | 1,440 | 200 |
| 10 | ECMO | 380 | 457 | 443 | 21 | 16.5 | 748 | Mild | 0 | 0 | 0 |
| 11 | ECMO | 409 | 550 | 485 | 21 | 16.5 | 177 | None | 0 | 0 | 0 |
| 12 | ECMO | 468 | 539 | 492 | 16.5 | 15 | 1,198 | None | 1,960 | 480 | 0 |
*, pleural adhesion grade: severe, 50% to the whole thoracic cavity; moderate, partial (only band adhesions) to < 50%; mild, no to partial adhesion; none, no adhesion. OP, operative; CIT, cold ischemia time; ECMO, extracorporeal membrane oxygenation; RBC, red blood cell; FFP, fresh frozen plasma.
Intraoperative ADAMTS13 activity and vWF:Ag
Figure 3A shows the comparisons of ADAMTS13 activity between the 2 groups in the intraoperative period (before lung transplantation, before reperfusion, and at the end of surgery). There was no significant difference in the ADAMTS13 activity between the ECMO and the control group during the intraoperative period. Figure 3B shows the comparisons of vWF:Ag between the 2 groups in the intraoperative period. The level of vWF:Ag, representing the total amount of vWF multimers, was expressed as its ratio to that in the standard human plasma because the value of vWF:Ag varies significantly according to the age, ABO blood group, and so forth (19). There was no significant difference in vWF:Ag between the two groups during the intraoperative period.

Intraoperative vWF large multimer index
Figure 4A shows the vWF large multimer index comparisons between the 2 groups in the intraoperative period. The vWF large multimer index in the control group maintained a level around 100% throughout the perioperative period [mean vWF large multimer index; before lung transplantation (LT), 76.7%±10.0%; before reperfusion, 100.1%±10.9%; end of LT, 112.6%±10.6%]. On the other hand, the vWF large multimer index in the ECMO group decreased before reperfusion and at the end of LT (mean vWF large multimer index; before LT, 93.8%±7.0%; before reperfusion, 74.7%±8.4%; end of LT, 75.8%±7.4%). The vWF large multimer index at the end of LT was significantly lower in the ECMO group than that in the control group (P<0.05). There were 3 patients who did not receive intraoperative transfusion of FFP in the control group and the ECMO group, respectively. In order to exclude the influence of FFP transfusion on the vWF large multimer indices, we compared the indices in these patients. Figure 4B shows each patient’s intraoperative vWF large multimer index without intraoperative FFP transfusion from the control and the ECMO group. The vWF large multimer index of each case in the control group was 66.8%/86.6%/59.5% at before LT, 89.0%/106.0%/59.5% before reperfusion, and, 89.0%/113.5%/147.7% at the end of LT. The vWF large multimer index of each case in the ECMO group was 84.6%/85.9%/74.2% before LT, 70.6%/65.5%/29.9% before reperfusion, and 92.3%/59.8%/41.8% at the end of LT. Namely, intraoperative vWF large multimer indices decreased to as low as 30–40% in the ECMO group without FFP transfusion, whereas that in the control group was maintained at >60% during SLT surgery.

Postoperative characteristics and mortality
Table 3 shows each group’s postoperative 30-day, 90-day, and 1-year mortality. No patient was on ECMO at the intensive care unit upon arrival in the control group, and 2 patients (28.6%) were not weaned from VV ECMO in the ECMO group. There was no re-exploration thoracotomy for bleeding in either group. There was no mortality case in the control group within 1 year after transplantation. There was one 90-day mortality case in the ECMO group. The cause of the mortality was PGD.
Table 3
| Characteristics | Control group (n=5) | ECMO group (n=7) |
|---|---|---|
| ECMO on ICU arrival | 0 (0.0) | 2 (28.6) |
| Re-exploration thoracotomy for bleeding | 0 (0.0) | 0 (0.0) |
| Mortality | ||
| 30-day | 0 (0.0) | 0 (0.0) |
| 90-day | 0 (0.0) | 1 (14.3)* |
| 1-year | 0 (0.0) | 0 (0.0) |
Values are expressed as numbers (%). *, the cause of death was primary graft dysfunction. ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Discussion
This is the first study that quantitatively analyzed the intraoperative changes in vWF large multimers in patients who underwent lung transplantation. We demonstrated that vWF large multimer index, which has been regarded as the most reliable predictor of AvWS in recent years, was significantly lower in the patients who underwent VV ECMO support during SLT compared to those without ECMO at the end of the surgery. However, due to the limited statistical power resulting from the small sample size, we were unable to establish a significant association between the decrease in the large multimer index and intra- and postoperative outcomes, including blood loss, blood transfusion, and re-exploration thoracotomy for bleeding.
In the present study, the ADAMTS13 activity and vWF:Ag showed no significant change by the intraoperative VV ECMO use. On the other hand, there was a significant decrease in the vWF large multimer index at the end of the surgery in SLT. However, the mean vWF large multimer index at the end of surgery was relatively maintained at 75.8% in the ECMO group. The intraoperative blood loss and the required amounts of intraoperative transfusion products in the ECMO group tended to be greater than those in the control group, but the difference was not statistically significant. We previously reported a more severe decrease in the vWF large multimer index in 5 patients with acute cardiovascular diseases treated with VA ECMO (11). These patients exhibited vWF large multimer indices of 20.8% at 24 hours, 51.0% at 23 hours, 27.6% at 47 hours, 28.8% at 19 hours, and 31.0% at 82 hours after VA ECMO initiation (11). Furthermore, among the 5 patients, 2 experienced significant bleeding events, while all 5 patients exhibited bleeding at the skin sites where the cannulas were inserted, suggesting an evident disturbance in hemostasis (11).
The results regarding AvWS in our study were also quite different from those in a previous study reported by Kalbhenn et al. They demonstrated that patients receiving VV ECMO support for respiratory failure were all diagnosed with AvWS, as shown by the ratio of the von Willebrand factor collagen binding capacity (vWF:CB) to vWF:Ag decreasing to about 0.25 in average (vWF:CB/vWF:Ag, normal: >0.7). They also reported that 17 of the 18 patients developed bleeding complications from 3 to 30 days after ECMO (15). The difference in the results in our study compared to those in previous studies may be primarily explained by the short duration of time of ECMO use in our study. The relatively low threshold for the use of intraoperative FFP transfusion in our cohort, the thicker venous outflow cannulas in VV ECMO compared to those in VA ECMO in general (11), and the lower blood flow rate of 1.5–2.5 L/min in the present study compared to that in VA ECMO used for patients with severe respiratory failure may also be responsible for the difference.
We acknowledge that there are several limitations in the present study. First, it was a single-center analysis with a small number of patients, and thus we cannot accurately compare the outcome of each group. To accurately assess the comprehensive influence of the reduction in vWF large multimer index due to VV ECMO on the intra- and postoperative outcomes, such as blood loss, blood transfusion, and re-exploration thoracotomy for bleeding, a more extensive study will be essential. Given the total number of lung transplantations performed at our center, it is prudent to consider planning a nationwide study to ensure robust data and generalize the findings effectively. Second, there were few patients receiving an intraoperative transfusion of FFP in both groups. Therefore, an accurate comparison of the vWF large multimer indices was impossible. Whereas we compared the vWF large multimer indices in the patients without intraoperative FFP transfusion between the control and the ECMO group, the number of patients was not adequate for statistical analysis. Third, we were not able to enroll a large enough number of patients to undergo lung transplantation under CPB or VA ECMO in this study, which would be a future challenge.
Conclusions
To summarize, the intraoperative use of VV ECMO for SLT caused a decrease in the vWF large multimer index. The short duration of time of VV ECMO use in our study did not significantly affect the intra- and postoperative outcomes including blood loss, blood transfusion, and re-exploration thoracotomy for bleeding. Nevertheless, to comprehensively evaluate the actual influence of this decrease in the vWF large multimer index on intra- and postoperative outcomes, a multicenter larger-scale study is warranted.
Acknowledgments
A part of this study was conducted as collaborative research with Sysmex Corporation (Kobe, Japan).
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-275/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-275/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-275/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-275/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Tohoku University Graduate School of Medicine approved this prospective study (approval No. 2016-1-629; protocol No. UMIN000018135), and written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pena JJ, Bottiger BA, Miltiades AN. Perioperative Management of Bleeding and Transfusion for Lung Transplantation. Semin Cardiothorac Vasc Anesth 2020;24:74-83. [Crossref] [PubMed]
- Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34. [Crossref] [PubMed]
- Hayes D Jr, Tumin D, Yates AR, et al. Transfusion with packed red blood cells while awaiting lung transplantation is associated with reduced survival after lung transplantation. Clin Transplant 2016;30:1545-51. [Crossref] [PubMed]
- Snyder LD, Wang Z, Chen DF, et al. Implications for human leukocyte antigen antibodies after lung transplantation: a 10-year experience in 441 patients. Chest 2013;144:226-33. [Crossref] [PubMed]
- Lau CL, Palmer SM, Posther KE, et al. Influence of panel-reactive antibodies on posttransplant outcomes in lung transplant recipients. Ann Thorac Surg 2000;69:1520-4. [Crossref] [PubMed]
- Dalibon N, Geffroy A, Moutafis M, et al. Use of cardiopulmonary bypass for lung transplantation: a 10-year experience. J Cardiothorac Vasc Anesth 2006;20:668-72. [Crossref] [PubMed]
- Machuca TN, Collaud S, Mercier O, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2015;149:1152-7. [Crossref] [PubMed]
- Magouliotis DE, Tasiopoulou VS, Svokos AA, et al. Extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation: a meta-analysis. Gen Thorac Cardiovasc Surg 2018;66:38-47. [Crossref] [PubMed]
- Moreno Garijo J, Cypel M, McRae K, et al. The Evolving Role of Extracorporeal Membrane Oxygenation in Lung Transplantation: Implications for Anesthetic Management. J Cardiothorac Vasc Anesth 2019;33:1995-2006. [Crossref] [PubMed]
- Oishi H, Matsuda Y, Ejima Y, et al. Changes in haemodynamics during single lung transplantation under venovenous extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg 2022;35:ivac101. [Crossref] [PubMed]
- Tamura T, Horiuchi H, Obayashi Y, et al. Acquired von Willebrand syndrome in patients treated with veno-arterial extracorporeal membrane oxygenation. Cardiovasc Interv Ther 2019;34:358-63. [Crossref] [PubMed]
- Horiuchi H, Doman T, Kokame K, et al. Acquired von Willebrand Syndrome Associated with Cardiovascular Diseases. J Atheroscler Thromb 2019;26:303-14. [Crossref] [PubMed]
- Furlan M, Robles R, Solenthaler M, et al. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood 1997;89:3097-103.
- Alsidawi S, Couto M, López-Candales A. Acquired Von Willebrand Syndrome In Aortic Stenosis: Case Report And Review. Bol Asoc Med P R 2015;107:86-8.
- Kalbhenn J, Schmidt R, Nakamura L, et al. Early diagnosis of acquired von Willebrand Syndrome (AVWS) is elementary for clinical practice in patients treated with ECMO therapy. J Atheroscler Thromb 2015;22:265-71. [Crossref] [PubMed]
- Kokame K, Nobe Y, Kokubo Y, et al. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol 2005;129:93-100. [Crossref] [PubMed]
- Tamura T, Horiuchi H, Imai M, et al. Unexpectedly High Prevalence of Acquired von Willebrand Syndrome in Patients with Severe Aortic Stenosis as Evaluated with a Novel Large Multimer Index. J Atheroscler Thromb 2015;22:1115-23. [Crossref] [PubMed]
- Sakatsume K, Saito K, Akiyama M, et al. Association between the severity of acquired von Willebrand syndrome and gastrointestinal bleeding after continuous-flow left ventricular assist device implantation. Eur J Cardiothorac Surg 2018;54:841-6. [Crossref] [PubMed]
- Wang Z, Dou M, Du X, et al. Influences of ABO blood group, age and gender on plasma coagulation factor VIII, fibrinogen, von Willebrand factor and ADAMTS13 levels in a Chinese population. PeerJ 2017;5:e3156. [Crossref] [PubMed]


