
Risk factors for mitral valve dysfunction, reoperation and death following repair of the primary mitral valve disease in children
Highlight box
Key findings
• Mixed mitral valve (MV) pathology (P=0.014) predicted potential MV dysfunction. ≥ moderate MV regurgitation at postoperative 24 h (P=0.014) indicated potential MV reoperation. Double-orifice MV technique (P=0.002), MV reoperation (P=0.023), and severe MV regurgitation at 24 h after first surgery (P=0.028) were identified as the independent risk factors for death.
What is known and what is new?
• The main treatment of primary MV disease is valve repair rather than replacement. However, characterized by a complicated deformity, valve repair was difficult and result in high rate of valve reoperation.
• This study reveals that through meticulous repair and individual therapeutic regimens, the left ventricular function and the rate of MV reoperation can be controlled well.
• The study states the relationship among ≥ moderate/severe MV regurgitation at postoperative 24 h, double-orifice technique, mixed MV pathology, MV reoperation and MV prognosis.
What is the implication, and what should change now?
• Surgeons must have adequate patience to repair MV precisely on the first attempt to keep the degree of MV regurgitation below moderate at postoperative 24 h to avoid adverse prognosis.
Introduction
Primary mitral valve (MV) diseases in children are characterized by various anatomical abnormalities, the most common of which are a complex structural malformation of the valve annulus, valve leaflets, chordae tendineae, and papillary muscles, as well as the valve’s abnormal anatomical relationship with the left atrium, left ventricle, and aortic valve. MV repair is the preferred treatment for children, while mechanical MV replacement is employed as the final or “bailout” approach after MV repair fails (1). Primary MV disease is characterized by a complicated deformity, making valve repair difficult and resulting in a relatively high rate of MV dysfunction and MV reoperation. In this regard, a 5-year MV reoperation rate of 11–14% has been reported by del Nido et al. and Baird et al. (2,3). A 36-year MV dysfunction rate of 62.5% has been reported by Stellin et al. (4). Nevertheless, valve repair offers advantages over mechanical valve replacement, which include avoiding lifelong anticoagulation and facilitating normal valve development.
In the case of primary MV diseases, surgeons have already reached a consensus in considering MV repair as the best treatment option and have made every effort to treat it carefully (2,3,5). In recent years, owing to the advancements in surgical techniques and the knowledge about the MV nature, the proportion of MV reoperation rate has reduced to <10% in the middle to long-term follow-up studies (4,6,7). However, despite the repair of primary MV diseases, it is not unusual for more than moderate mitral valve regurgitation (MR) or mitral valve stenosis (MS) to reoccur. In this context, this study aimed to analyze the surgical results of primary MV illnesses and to identify the risk factors associated with MV dysfunction, reoperation and death following the repair of primary MV diseases. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-270/rc).
Methods
Patients
From January 2013 to December 2021, 98 patients diagnosed with primary MV diseases were selected from the database of the Cardiovascular Center of the Children’s Hospital of Fudan University. Patients with atrioventricular septal defect, single-ventricle, an abnormal origin of coronary arteries, complex congenital heart diseases (CHD), secondary MR, and those who underwent direct mechanical MV replacement at the initial surgery were excluded from the analyses. The patient flow chart is displayed in Figure 1. Demographic and surgical data were obtained from the center. Follow-up data were collected from clinical examinations and echocardiography at the outpatient clinics.

The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of the Children’s Hospital of Fudan University [No. (2022)131]. The need for patient consent was waived off owing to the retrospective nature of the study.
MR and MS types
Primary mitral pathology was divided into MR and MS based on Carpentier’s functional categorization (8). Type I MR was characterized by normal leaflet motion, while Type II MR was characterized by leaflet prolapse. Type III MR was characterized by restricted leaflet motion. Type A MS, characterized by normal papillary muscles, and Type B MS, characterized by abnormal papillary muscles, were identified as the distinct subtypes of the disease.
Preoperative evaluation
Patients underwent preoperative transthoracic echocardiography (TTE) for MV analysis. According to the American Society of Echocardiography guidelines (9,10), MR was graded as mild, moderate, and severe based on the color Doppler jet area and the mitral inflow. MS was graded based on the mean pressure gradient across the MV before and after surgery: mild <5 mmHg; moderate ≥5 mmHg, but ≤10 mmHg; severe >10 mmHg. Children with combined MR and MS were assigned to either MR or MS group based on the major lesions. An intraoperative transesophageal echocardiogram was performed to analyze the MV before and after the repair.
Follow-up
All patients underwent TTE 24 h after surgery, at 3 months, at 1 year, and annually after MV surgery. Early death was defined as death within 30 days of surgery, and late death as death after 30 days. Complications after surgery, such as pulmonary hypertensive crisis, delayed chest closure, infection, and pericardial effusion, were recorded during the follow-up. MV dysfunction was defined as moderate and more recurrent MV regurgitation/stenosis.
Study endpoints
The primary outcome was the occurrence of MV reoperation and death. The secondary outcome was the occurrence of MV dysfunction. Time to MV dysfunction and reoperation was measured from the date of the first MV repair. For patients who did not experience MV dysfunction and reoperation, the times were censored at the last follow-up date or the time of death. The indications of MV dysfunction and reoperation were based on residual or recurrent MR and MS that appeared during the follow-up.
Statistical analysis
Mean ± standard deviation (SD) was used to describe the continuous variables in normal distribution and median [interquartile range (IQR)] to describe those that were not. Categorical variables were presented by n (%). Differences between the no-MV reoperation and MV reoperation groups were analyzed by Chi-squared test or Fisher’s exact test for categorical variables, and independent t-test or Mann-Whitney U test for continuous variables. Freedom from dysfunction and reoperation was assessed based on the Kaplan-Meier survival analysis.
The univariable Cox regression analysis was performed to evaluate the risk factors for dysfunction and reoperation and death: age ≤6 months, weight ≤8 kg, gender, left ventricular ejection fractions (LVEF) <0.6 before surgery, annular diameter/body surface area (BSA) before surgery, left atrium diameter (LAD)/BSA, left ventricular end-diastolic diameter (LVEDD)/BSA, left ventricular end-systolic diameter (LVSDD)/BSA, left ventricular end-diastolic volume index (LVEDVI), left ventricular end-systolic volume index (LVESVI), double-orifice MV technique, concomitant procedure, without annuloplasty, mixed MV pathology, isolated MR diseases, MR combined with CHD diseases, MS diseases, MV reoperation, ≥ moderate MV regurgitation before surgery and within 24 h after the first surgery, severe MV regurgitation at first 24 h after first surgery, ≥ moderate MV stenosis before surgery and within 24 h after the first surgery, and surgical era (2013 to 2016). The results of the models were reported by hazard ratio (HR), 95% confidence interval (CI), and P value. Variables with P<0.1 were included in the multivariable Cox regression analysis. Statistical significance was set at P<0.05.
All statistical analyses were conducted using the IBM SPSS statistics 25.0 on Windows (SPSS, Inc., Chicago, IL, USA). Images were created with the GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA) and Microsoft Office Word (Microsoft Software, Inc., Redmond, WA, USA).
Results
Patient characteristics
Eight patients with direct mechanical MV replacement and one with Ross II replacement were excluded from the analysis during data collection.
Table 1 summarizes the baseline characteristics of the patients. In total, 98 patients were diagnosed with primary MV disease, and nine underwent additional MV surgery. The total cohort had a mean follow-up period of 23.5 months (IQR, 9–44.5). Group NR (had no MV reoperations) had a mean follow-up period of 18 months (IQR, 4–36.1), while Group R (had MV reoperations), had a mean follow-up period of 30 months (IQR, 4.5–51). One- and 3-year survival rates were 95.7%±2.1%, while 5- and 7-year survival rates were 89.3%±6.5% (Figure 2A). Perioperatively severe MR and MS occurred in 42.7% (n=38) and 15.7% (n=14) of patients in Group NR and 55.6% (n=5) and 0% of patients in Group R. Before surgery, 70 (78.7%) patients in the Group NR and 7 (77.8%) patients in Group R had ≥ moderate MR. In comparison, 22 (24.7%) patients in Group NR and 3 (33.3%) in Group R had ≥ moderate MS.
Table 1
| Variables | Total cohort (n=98) | Group NR (n=89) | Group R (n=9) | P value† |
|---|---|---|---|---|
| Demographics | ||||
| Age (months) | 24 (7.78–70.5) | 24 (7.35–72) | 36 (9.85–84) | 0.735 |
| Age ≤6 months | 21 (21.4) | 20 (22.5) | 1 (11.1) | 0.429 |
| Male | 47 (48.0) | 43 (48.3) | 4 (44.4) | 0.825 |
| Weight (kg) | 11.2 (6.93–17) | 11.2 (6.9–17) | 14 (8.5–16) | 0.815 |
| Weight ≤8 kg | 31 (31.6) | 29 (32.6) | 2 (22.2) | 0.524 |
| CTR | 0.60±0.07 | 0.60±0.07 | 0.59±0.08 | 0.707 |
| BSA (m2) | 0.49 (0.34–0.70) | 0.49 (0.34–0.70) | 0.59 (0.40–0.66) | 0.806 |
| Annular diameter/BSA (mm/m2) | 39.94 (29.51–56.72) | 39.93 (29.47–56.03) | 46.48 (30.16–63.64) | 0.567 |
| LAD/BSA (mm/m2) | 40.58 (29.90–61.99) | 40.11 (29.71–61.91) | 60.38 (37.88–66.13) | 0.448 |
| LVEDD/BSA (mm/m2) | 64.86 (49.27–95.69) | 63.94 (49.18–93.37) | 77.06 (56–98.11) | 0.645 |
| LVSDD/BSA (mm/m2) | 38.4 (30.63–56.74) | 38.01 (30.49–55.09) | 50.15 (35.59–57.86) | 0.441 |
| LVEDVI (mL/m2) | 95.74 (71.24–156.52) | 94.98 (70.57–147.71) | 109.68 (81.6–183.87) | 0.345 |
| LVESVI (mL/m2) | 26.90 (19.79–38.89) | 26.42 (19.03–37.98) | 33.60 (29.03–45.28) | 0.156 |
| LVEF | 0.70±0.07 | 0.70±0.07 | 0.67±0.09 | 0.100 |
| Previous CoA surgery | 3 (3.1) | 2 (2.2) | 1 (11.1) | 0.141 |
| MR grade before surgery | ||||
| Severe MR | 43 (43.9) | 38 (42.7) | 5 (55.6) | 0.698 |
| ≥ moderate MR | 77 (78.6) | 70 (78.7) | 7 (77.8) | 1.000 |
| MS grade before surgery | ||||
| Severe MS | 14 (14.3) | 14 (15.7) | 0 (0.0) | 0.199 |
| ≥ moderate MS | 25 (25.5) | 22 (24.7) | 3 (33.3) | 0.572 |
| Shone’s syndrome | 2 (2.0) | 2 (2.2) | 0 (0.0) | 0.650 |
| Mixed mitral valve pathology | 40 (40.8) | 35 (39.3) | 5 (55.6) | 0.345 |
| Combined with other cardiac malformations | 68 (69.4) | 63 (70.8) | 5 (55.6) | 0.345 |
| Preoperative mechanical ventilation | 6 (6.1) | 5 (5.6) | 1 (11.1) | 0.512 |
| Death | 5 (5.1) | 2 (2.2) | 3 (33.3) | 0.001 |
| Follow-up time (months) | 23.5 (9–44.5) | 18 (4–36.1) | 30 (4.5–51) | 0.156 |
Group NR: Group (had no MV reoperations); Group R: Group (had MV reoperations). Data are reported as mean ± SD, median (IQR) or n (%). †, the P value shows the difference between Group NR (n=89) and Group R (n=9). CTR, cardiothoracic ratio; BSA, body surface area; LAD, left atrium diameter; LVEDD, left ventricular end-diastolic diameter; LVSDD, left ventricular end-systolic diameter; LVEDVI (LVEDV/BSA), left ventricular end-diastolic volume index; LVESVI (LVESV/BSA), left ventricular end-systolic volume index; LVEF, left ventricular ejection fraction; CoA, coarctation; MR, mitral valve regurgitation; MS, mitral valve stenosis; MV, mitral valve; SD, standard deviations; IQR, interquartile range.

Types of MV and classification of mixed MV pathologies
The primary MV diseases were outlined in Table 2. Pathological types of MR patients (n=76) included Type I MR (n=65), Type II MR (n=32) and Type III MR (n=9). Pathological types of MS patients (n=22) included Type A MS (n=20) and Type B MS (n=2). MV annulus, MV leaflets, chordae tendineae, and the papillary muscle were the main lesion sites of the MV, and their specifics are displayed in Table 2.
Table 2
| Classification | No. of cases (%)# |
|---|---|
| Mechanism of MR (n=76) | |
| Type I (normal leaflet motion) | 65 (85.5) |
| Annular dilatation | 18 (23.7) |
| Cleft leaflet | 39 (51.3) |
| Leaflet defect | 31 (40.8) |
| Type II (leaflet prolapse) | 32 (42.1) |
| Ruptured chordae | 7 (9.2) |
| Elongated chordae | 19 (25.0) |
| Absent chordae | 6 (7.9) |
| Type III (restricted leaflet motion) | 9 (11.8) |
| Short chordae | 7 (9.2) |
| Papillary muscle fusion | 2 (2.6) |
| Mechanism of MS (n=22) | |
| Type A (normal papillary muscle) | 20 (90.9) |
| Supravalvular ring | 16 (72.7) |
| Junctional fusion of papillary muscles | 2 (9.1) |
| Leaflet fusion | 2 (9.1) |
| Type B (abnormal papillary muscle) | 2 (9.1) |
| Parachute valve | 2 (9.1) |
#, patients can have mixed mitral valve pathologies. MR, mitral valve regurgitation; MS, mitral valve stenosis.
Classification of mixed MV pathologies (n=40) were displayed in Table 3. Cleft leaflet combined with Leaflet defect (n=6), Cleft leaflet combined with Elongated chordae (n=5), Annular dilatation combined with Leaflet defect (n=5) and Annular dilatation combined with Elongated chordae (n=4) were the most common comorbidities. The details are displayed in Table 3.
Table 3
| Classification | No. of cases |
|---|---|
| Annular dilatation & elongated chordae | 4 |
| Annular dilatation & absent chordae | 1 |
| Annular dilatation & leaflet defect | 5 |
| Annular dilatation & cleft leaflet & short chordae | 1 |
| Annular dilatation & cleft leaflet & elongated chordae | 1 |
| Annular dilatation & cleft leaflet & ruptured chordae | 1 |
| Annular dilatation & ruptured chordae | 1 |
| Annular dilatation & leaflet defect & elongated chordae | 2 |
| Annular dilatation & cleft leaflet & leaflet defect | 1 |
| Annular dilatation & leaflet defect & papillary muscle fusion | 1 |
| Cleft leaflet & leaflet defect & ruptured chordae | 2 |
| Cleft leaflet & short chordae | 1 |
| Cleft leaflet & elongated chordae | 5 |
| Cleft leaflet & leaflet defect | 6 |
| Cleft leaflet & absent chordae & short chordae | 1 |
| Cleft leaflet & leaflet defect & elongated chordae | 2 |
| Cleft leaflet & leaflet defect & ruptured chordae & short chordae | 1 |
| Elongated chordae & short chordae | 2 |
| Leaflet defect & elongated chordae | 1 |
| Leaflet defect & short chordae | 1 |
Surgical techniques and concomitant procedures
The surgical techniques and concomitant procedures were implemented separately or in combination during the surgeries. The details are listed in Table 4. Based on each patient’s specific condition, techniques such as supravalvular ring resection (16/22, 72.7%), commissurotomy (n=5/22, 22.7%), and papillary muscle splitting (n=5/22, 22.7%) were applied mostly during the procedures of MS disease repair. Furthermore, annuloplasty (n=43/76, 56.6%), cleft closure (n=39/76, 51.3%), and leaflet plication (n=9/76, 11.8%) were employed mostly during the procedures of MR disease repair. As for the concomitant procedures such as ventricular septal defect closure (33/98, 33.7%), atrial septal defect closure (20/98, 20.4%), and patent ductus arteriosus ligation (20/98, 20.4%), they were most frequently conducted intraoperatively.
Table 4
| Variables | No. of cases (%)# |
|---|---|
| Mitral valve repair techniques | |
| Supravalvular ring resection | 16 (16.3) |
| Annuloplasty | 44 (44.9) |
| Cleft closure | 39 (39.8) |
| Leaflet extension or augmentation | 3 (3.1) |
| Leaflet resection | 7 (7.1) |
| Leaflet plication | 9 (9.2) |
| Artificial chordae | 8 (8.2) |
| Double-orifice MV technique | 3 (3.1) |
| Commissurotomy | 5 (5.1) |
| Chordal splitting/papillary muscle splitting | 9 (9.2) |
| Concomitant procedure | |
| Atrial septal defect closure | 20 (20.4) |
| Ventricular septal defect closure | 33 (33.7) |
| Patent ductus arteriosus ligation | 20 (20.4) |
| Tricuspid valvuloplasty | 8 (8.2) |
| Pulmonary valve valvuloplasty | 3 (3.1) |
| Aortic valve valvuloplasty | 5 (5.1) |
| Aortic repair | 6 (6.1) |
| Others | 4 (4.1) |
#, surgical techniques and concomitant procedures were implemented either separately or in combination. MV, mitral valve.
Perioperative data
Table 5 provides in-depth information about the perioperative period. The postoperative mechanical ventilation time in Group NR was 21 h (IQR, 8–46.75) which was significantly lesser compared to Group R, which was approximately 92 h (IQR, 18.25–813.5) (P=0.030). The ICU stay days after surgery in Group NR was 3 days (IQR, 2–4.5) which was significantly lesser compared to Group R, which was approximately 5 days (IQR, 2.25–43.75) (P=0.047). Twenty-four hours after surgery, 20.2% of patients (n=18) were detected with ≥ moderate MR in Group NR, which was not significantly different (P=0.083) from that in Group R (about 55.6%, n=5). The number of patients in Group NR who had ≥ moderate MS at 24 h following surgery was not significantly different (P=0.452) from that in Group R.
Table 5
| Variables | Total cohort (n=98) | Group NR (n=89) | Group R (n=9) | P value† |
|---|---|---|---|---|
| CPB time (min) | 78 (62.75–98.75) | 78 (62.25–98) | 87 (73–105) | 0.315 |
| Cross-clamp time (min) | 48 (34.75–62.25) | 46.5 (34.25–61.75) | 67 (48–73) | 0.072 |
| CPB temperature (℃) | 33.55 (32–34.3) | 33.5 (32–34.38) | 34 (33–34.2) | 0.365 |
| Postoperative mechanical ventilation time (h) | 21.75 (8–50.25) | 21 (8–46.75) | 92 (18.25–813.5) | 0.030 |
| ICU stay after surgery (days) | 3 (2.0–5.0) | 3 (2–4.5) | 5 (2.25–43.75) | 0.047 |
| Postoperative hospital stay (days) | 9 (8.0–15.0) | 9 (8.0–13.0) | 18 (10.0–32.0) | 0.050 |
| Complications | ||||
| Pulmonary hypertensive crisis | 4 (4.1) | 4 (4.5) | 0 (0.0) | 0.516 |
| Delayed chest closure | 1 (1.0) | 1 (1.1) | 0 (0.0) | 0.749 |
| Infection | 4 (4.1) | 3 (3.4) | 1 (11.1) | 0.263 |
| Pericardial effusion | 3 (3.1) | 3 (3.4) | 0 (0.0) | 0.576 |
| ≥ moderate MR at 24 h after surgery | 23 (23.5) | 18 (20.2) | 5 (55.6) | 0.083 |
| ≥ moderate MS at 24 h after surgery | 3 (3.1) | 2 (2.2) | 1 (11.1) | 0.452 |
Group NR: Group (had no MV reoperations); Group R: Group (had MV reoperations). Data are reported as the median (IQR) or n (%). †, the P value shows the difference between Group NR (n=89) and Group R (n=9). CPB, cardiopulmonary bypass; ICU, intensive care unit; MR, mitral valve regurgitation; MS, mitral valve stenosis; MV, mitral valve; IQR, interquartile range.
Mortality
In total, 5 patients died, 3 in Group R (33.3%) and 2 in Group NR (2.2%). Significant difference was found in death between the two groups (Table 1, P=0.001; Figure 2B, P<0.001). In Group R, one patient experienced pulmonary hemorrhage, acute renal insufficiency, and ultimately died of cardiopulmonary insufficiency and shock after undergoing fourth operation. One patient had difficulty weaning off the ventilator and died of cardiopulmonary failure. One patient with recurrent ventricular fibrillation after surgery died of heart failure, who we suspected associated with potential myocardial disease. In Group NR, one patient developed acute renal insufficiency and pulmonary hypertension crisis after surgery, and ultimately died of cardiopulmonary failure. Another patient was unable to maintain oxygen saturation and blood pressure after surgery, and the effectiveness of extracorporeal membrane oxygenation (ECMO) treatment was poor, ultimately resulting in death from cardiopulmonary failure and shock.
MV operations and subsequent reprocessing
In the primary MR disease group (34 moderate MR & 42 severe MR patients), 86 operations were performed on 76 patients, while in the primary MS disease group (7 moderate MS & 15 severe MS patients), 26 operations were performed on 22 patients. Figure 1 depicts the flowchart of the entire analysis.
Seven patients from the primary MR disease group underwent MV reoperations due to residual severe MR (n=6) and recurrent severe MS (n=1). Three patients had a third operation, while the fourth was continuously monitored and did not require the third operation. After the initial operation, 5 MV repairs and 5 MV replacements were implemented in the subsequent reprocessing.
Two patients in the primary MS disease group underwent MV reoperations due to recurrent severe MS. One patient did not require further surgery and is currently being monitored, while another patient underwent the third and fourth operations successively. After the initial operation, 4 MV repairs were implemented in the subsequent reprocessing stage.
The Kaplan-Meier plots revealed that the percentages of patients free from MV dysfunction at 1, 3, 5, and 7 years were 65.2%±5.1%, 58.2%±6.0%, 58.2%±6.0%, and 58.2%±6.0% respectively in the total cohort; 61.7%±5.9%, 55.3%±7.0%, 55.3%±7.0%, and 55.3%±7.0% respectively in the primary MR disease group; 77.5%±10.0%, 69.8%±11.6%, 69.8%±11.6%, and 69.8%±11.6% respectively in the primary MS disease group (Figure 3A). The percentage of patients free from MV reoperation at 1, 3, 5, and 7 years were 91.5%±3.1%, 91.5%±3.1%, 86.1%±6.0%, and 75.3%±11.4% respectively in the total cohort; 91.2%±3.5%, 91.2%±3.5%, 82.1%±9.2%, and 82.1%±9.2% respectively in the primary MR disease group; 92.3%±7.4%, 92.3%±7.4%, 92.3%±7.4%, 69.2%±20.7% respectively in the primary MS disease group (Figure 3B). No significant difference was recorded between the primary MR diseases group and the primary MS diseases group regarding freedom from MV dysfunction (Figure 3A, P=0.326) and reoperation (Figure 3B, P=0.954).

Treatment effects of MS and MR
Fifteen (68.2%) patients in the primary MS disease group (Figure 4A) had severe MS, whereas 7 (31.8%) patients had moderate MS preoperatively. At 24 h after the initial operation, moderate MS was detected only in 2 (9.1%) patients and mild MS in 20 (90.9%) patients. Two (9.1%) patients had severe MS regurgitation at the most recent follow-up, while it was moderate in 8 (36.4%) and mild in 12 (54.5%) patients. Three patient (13.6%) developed moderate MR and 1 patient (4.5%) developed severe MR in the last follow-up without undergoing any repair.
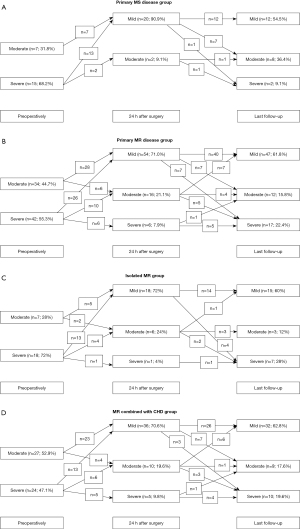
Figure 4B depicts that, among those with primary MR disease, 42 (55.3%) patients had severe MR, and 34 (44.7%) had moderate MR before surgery. At 24 h following the initial operation, severe MR was detected in only 6 (7.9%) patients, moderate MR in 16 (21.1%), and mild MR in 54 (71.0%). At the most recent follow-up, severe MR was detected in 17 (22.4%) patients, whereas moderate MR was detected in 12 (15.8%) and mild MR in 47 (61.8%) patients. Severe MS was detected in 1 patient (1.3%) whereas moderate MS was detected in 1 patient (1.3%). Both of them did not undergo MV repair in the entire follow-up.
Patients were subsequently divided into two groups—those with isolated MR disease (n=25) and those with MR combined with CHD disease (n=51). In the isolated MR group, 18 (72%) patients had severe MR, whereas only 7 (28%) patients had moderate MR preoperatively. At 24 h after the initial operation, 1 (4%) patient was detected with severe MR, 6 (24%) had moderate MR, and 18 (72%) had mild MR. Thirteen individuals with severe MR improved to have mild symptoms, four improved to have moderate symptoms, and one remained severely affected. A total of 5 patients with moderate MR improved to mild, while two were stable at the moderate level. In the last follow-up, 7 (28%) patients had severe MR, 3 (12%) had moderate MR, and 15 (60%) had mild MR. Only one patient with severe MR remained in a severe state. Two patients with moderate MR progressed to severe, one progressed to mild, and three maintained their moderate MR status. A total of 4 patients with mild MR progressed to severe, and 14 with mild MR stayed in the moderate category (Figure 4C). One patient (4%) developed moderate MS in the last follow-up without undergoing any repair.
Preoperatively, in MR combined with the CHD group, 24 (47.1%) patients were detected with severe MR and 27 (52.9%) with moderate MR. At 24 h after the initial operation, 5 (9.8%) patients had severe MR, 10 (19.6%) had moderate MR, and 36 (70.6%) had mild MR. A total of 13 patients with severe MR improved to the mild level, 6 improved to the moderate level, and 5 remained unchanged in the severe category. Twenty-three patients with moderate MR improved to the mild level, but four did not change. In the last follow-up, 10 (19.6%) patients were detected with severe MR, 9 (17.6%) with moderate MR, and 32 (62.8%) with mild MR. The MR severity for 4 patients stayed at the severe level, while 1 patient’s MR improved to the moderate level. The severity of MR increased in 3 patients, decreased in 6, and remained the same in 1. Three patients with mild MR changed into severe, 7 into moderate, and 26 remained as mild (Figure 4D). One patient (2.0%) developed severe MS in the last follow-up without undergoing any repair.
Left ventricular function
The changes in left ventricular (LV) function in children with MR and MS diseases were assessed from the follow-up data of LVEF and LVEDVI. The LVEF at pre-operation, 24 h, 3 months, 1 year, 3 years, and 5 years after operation was 0.694±0.068, 0.623±0.101, 0.698±0.046, 0.691±0.041, 0.709±0.054, and 0.690±0.037 in the MR disease group and 0.726±0.068, 0.663±0.100, 0.706±0.046, 0.693±0.042, 0.710±0.054, and 0.700±0.037 in the MS disease group, respectively, indicating a transient decrease in the LV systolic function, albeit a rapid recovery in both the groups (Figure 5A).

The LVEDVI at pre-operation, 24 h, 3 months, 1 year, 3 years, and 5 years after the operation was 129.16±70.72, 87.67±43.08, 87.46±27.68, 96.75±27.31, 149.61±70.14, and 171.08±45.97 mL/m2 in the MR diseases group and 82.34±71.79, 61.66±44.03, 73.40±28.42, 84.02±27.46, 97.62±69.78, and 118.00±45.90 mL/m2 in the MS diseases group respectively, indicating an ideal restoration of the LV shape and function in both the groups after approximately one year of the surgery (Figure 5B).
Risk factors for MV dysfunction, reoperation and death
Preoperative LVEDVI, preoperative LVESVI, and mixed MV pathology were all found to increase the likelihood of MV dysfunction in a univariable Cox regression analysis. This study found that mixed MV pathology [P=0.014; hazard ratio (HR) =2.492] was the only independent risk factor for MV dysfunction (Table 6).
Table 6
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | 1.001 | 0.992–1.009 | 0.895 | – | – | – | |
| Age ≤6 months | 0.972 | 0.422–2.238 | 0.946 | – | – | – | |
| Weight | 0.998 | 0.965–1.033 | 0.920 | – | – | – | |
| Weight ≤8 kg | 1.023 | 0.488–2.144 | 0.953 | – | – | – | |
| Gender (male =0) | 1.342 | 0.677–2.657 | 0.399 | – | – | – | |
| LVEF <0.6 before surgery | 1.917 | 0.459–8.004 | 0.372 | – | – | – | |
| Annular diameter/BSA before surgery | 1.009 | 0.991–1.028 | 0.316 | – | – | – | |
| LAD/BSA before surgery | 1.007 | 0.996–1.019 | 0.235 | – | – | – | |
| LVEDD/BSA before surgery | 1.004 | 0.993–1.016 | 0.487 | – | – | – | |
| LVSDD/BSA before surgery | 1.009 | 0.991–1.027 | 0.321 | – | – | – | |
| LVEDVI before surgery | 1.004 | 1.000–1.009 | 0.050 | 0.998 | 0.985–1.010 | 0.701 | |
| LVESVI before surgery | 1.012 | 1.003–1.022 | 0.012 | 1.014 | 0.986–1.042 | 0.341 | |
| Double-orifice MV technique | 2.580 | 0.615–10.824 | 0.195 | – | – | – | |
| Concomitant procedure | 0.811 | 0.406–1.620 | 0.552 | – | – | – | |
| Without annuloplasty | 0.672 | 0.342–1.321 | 0.249 | – | – | – | |
| Mixed mitral valve pathology | 2.852 | 1.418–5.737 | 0.003 | 2.492 | 1.203–5.161 | 0.014 | |
| Isolated MR diseases | 1.260 | 0.602–2.638 | 0.540 | – | – | – | |
| MR combined with CHD diseases | 1.286 | 0.653–2.533 | 0.467 | – | – | – | |
| MS diseases | 0.501 | 0.193–1.299 | 0.155 | – | – | – | |
| ≥ moderate MV regurgitation before surgery | 1.835 | 0.708–4.752 | 0.212 | – | – | – | |
| ≥ moderate MV stenosis before surgery | 0.708 | 0.308–1.631 | 0.418 | – | – | – | |
| Surgical era (2013 to 2016) | 0.688 | 0.342–1.383 | 0.294 | – | – | – | |
MV, mitral valve; HR, hazard ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; BSA, body surface area; LAD, left atrium diameter; LVEDD, left ventricular end-diastolic diameter; LVSDD, left ventricular end-systolic diameter; LVEDVI (LVEDV/BSA), left ventricular end-diastolic volume index; LVESVI (LVESV/BSA), left ventricular end-systolic volume index; MR, mitral valve regurgitation; CHD, congenital heart diseases; MS, mitral valve stenosis.
In the univariable Cox regression analysis, we found that LVESVI before surgery, the use of double-orifice MV technique, and ≥ moderate MV regurgitation within the first 24 h after initial surgery all acted as risk factors for MV reoperations. Independent risk factors for MV reoperation in the multivariate Cox regression analysis only included ≥ moderate MV regurgitation during the first 24 h following initial operation (P=0.014; HR =8.493) (Table 7).
Table 7
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | 1.005 | 0.990–1.021 | 0.498 | – | – | – | |
| Age ≤6 months | 0.389 | 0.048–3.140 | 0.375 | – | – | – | |
| Weight | 1.004 | 0.936–1.077 | 0.915 | – | – | – | |
| Weight ≤8 kg | 0.640 | 0.133–3.090 | 0.579 | – | – | – | |
| Gender (male =0) | 1.520 | 0.385–6.001 | 0.550 | – | – | – | |
| LVEF <0.6 before surgery | 1.084 | 0.286–4.110 | 0.906 | – | – | – | |
| Annular diameter/BSA before surgery | 1.012 | 0.978–1.048 | 0.482 | – | – | – | |
| LAD/BSA before surgery | 1.001 | 0.978–1.024 | 0.949 | – | – | – | |
| LVEDD/BSA before surgery | 1.004 | 0.982–1.026 | 0.721 | – | – | – | |
| LVSDD/BSA before surgery | 1.009 | 0.974–1.045 | 0.612 | – | – | – | |
| LVEDVI before surgery | 1.006 | 0.998–1.015 | 0.122 | – | – | – | |
| LVESVI before surgery | 1.020 | 1.002–1.038 | 0.027 | 1.016 | 0.994–1.038 | 0.167 | |
| Double-orifice MV technique | 8.728 | 1.046–72.856 | 0.045 | 8.906 | 0.946–83.822 | 0.056 | |
| Concomitant procedure | 0.558 | 0.147–2.122 | 0.392 | – | – | – | |
| Without annuloplasty | 0.533 | 0.139–2.038 | 0.358 | – | – | – | |
| Mixed mitral valve pathology | 2.179 | 0.579–8.194 | 0.249 | – | – | – | |
| Isolated MR diseases | 1.554 | 0.383–6.300 | 0.537 | – | – | – | |
| MR combined with CHD diseases | 0.836 | 0.223–3.134 | 0.790 | – | – | – | |
| MS diseases | 0.776 | 0.154–3.894 | 0.758 | – | – | – | |
| ≥ moderate MV regurgitation before surgery | 1.224 | 0.242–6.184 | 0.807 | – | – | – | |
| ≥ moderate MV regurgitation at first 24 h after first surgery | 11.874 | 2.266–62.208 | 0.003 | 8.493 | 1.532–47.079 | 0.014 | |
| ≥ moderate MV stenosis before surgery | 1.270 | 0.307–5.245 | 0.741 | – | – | – | |
| ≥ moderate MV stenosis at first 24 h after first surgery | 4.571 | 0.552–37.888 | 0.159 | – | – | – | |
| Surgical era (2013 to 2016) | 0.453 | 0.087–2.355 | 0.346 | – | – | – | |
MV, mitral valve; HR, hazard ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; BSA, body surface area; LAD, left atrium diameter; LVEDD, left ventricular end-diastolic diameter; LVSDD, left ventricular end-systolic diameter; LVEDVI (LVEDV/BSA), left ventricular end-diastolic volume index; LVESVI (LVESV/BSA), left ventricular end-systolic volume index; MR, mitral valve regurgitation; CHD, congenital heart diseases; MS, mitral valve stenosis.
Besides being risk factors in the univariable Cox regression analysis, the use of double-orifice MV technique (P=0.002; HR =39.319), the experience of MV reoperation (P=0.023; HR =8.764) and severe MV regurgitation at first 24 h after first surgery (P=0.028; HR =10.856) were also acted as independent risk factors for death following repair of primary MV disease (Table 8).
Table 8
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | 0.974 | 0.930–1.019 | 0.254 | – | – | – | |
| Age ≤6 months | 2.264 | 0.372–13.779 | 0.375 | – | – | – | |
| Weight | 0.906 | 0.548–1.496 | 0.699 | – | – | – | |
| Weight ≤8 kg | 1.495 | 0.250–8.963 | 0.660 | – | – | – | |
| Gender (male =0) | 0.705 | 0.116–4.296 | 0.704 | – | – | – | |
| LVEF <0.6 before surgery | 0.846 | 0.140–5.112 | 0.855 | – | – | – | |
| Annular diameter/BSA before surgery | 1.018 | 0.975–1.063 | 0.422 | – | – | – | |
| LAD/BSA before surgery | 1.017 | 0.993–1.041 | 0.168 | – | – | – | |
| LVEDD/BSA before surgery | 0.997 | 0.966–1.029 | 0.872 | – | – | – | |
| LVSDD/BSA before surgery | 0.992 | 0.941–1.045 | 0.765 | – | – | – | |
| LVEDVI before surgery | 0.999 | 0.986–1.013 | 0.895 | – | – | – | |
| LVESVI before surgery | 1.010 | 0.985–1.036 | 0.441 | – | – | – | |
| Double-orifice MV technique | 43.047 | 5.843–317.13 | 0.00002 | 39.319 | 3.653–423.213 | 0.002 | |
| Concomitant procedure | 1.826 | 0.202–16.530 | 0.592 | – | – | – | |
| Without annuloplasty | 54.722 | 0.031–96818.99 | 0.294 | – | – | – | |
| Mixed mitral valve pathology | 2.469 | 0.406–15.006 | 0.326 | – | – | – | |
| Isolated MR diseases | 0.032 | 0.000005–219.308 | 0.444 | – | – | – | |
| MR combined with CHD diseases | 1.397 | 0.233–8.386 | 0.714 | – | – | – | |
| MS diseases | 2.110 | 0.341–13.073 | 0.422 | – | – | – | |
| MV reoperation | 13.181 | 2.191–79.293 | 0.005 | 8.764 | 1.354–56.751 | 0.023 | |
| Severe MV regurgitation before surgery | 2.120 | 0.345–13.032 | 0.417 | – | – | – | |
| Severe MV regurgitation at first 24 h after first surgery | 14.422 | 2.026–102.653 | 0.008 | 10.856 | 1.285–91.682 | 0.028 | |
| Surgical era (2013 to 2016) | 1.284 | 0.181–9.119 | 0.803 | – | – | – | |
HR, hazard ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; BSA, body surface area; LAD, left atrium diameter; LVEDD, left ventricular end-diastolic diameter; LVSDD, left ventricular end-systolic diameter; LVEDVI (LVEDV/BSA), left ventricular end-diastolic volume index; LVESVI (LVESV/BSA), left ventricular end-systolic volume index; MV, mitral valve; MR, mitral valve regurgitation; CHD, congenital heart diseases; MS, mitral valve stenosis.
Discussion
MV repair in children has always been relatively challenging due to its complex anatomical structure. In our cohort, a total death rate of 5.1% (5/98) and MV reoperation rate of 9.2% (9/98) at 10 years were achieved, which is similar to the 7–12.2% death rate, 9.2% MV reoperation rate reported by Sivalingam et al. and Geoffrion et al. (11,12). The goal of this study was to better understand the outcomes of MV repair for patients with primary MR and MS, as well as to identify risk factors for MV dysfunction, reoperation and death.
Prognosis of MV repair in primary MR diseases and primary MS diseases
To date, no definitive research has been reported on the surgical outcome of MV repair in primary MR and MS diseases. The severity of MR and MS has been mainly assessed by the occurrence of MV reoperation and the LV function index in children.
Consistent with the findings published by Stellin (4) and Vida (13), we found no statistically significant difference in freedom from MV reoperation between the primary MR and MS disease groups (Figure 3B). Supravalvular ring in most of the etiologies of MS (n=16) could be one of the possible reasons for this phenomenon. Only 2 out of 16 patients in our cohort with a supravalvular ring required a second operation on their MV due to MV re-stenosis. Cho (14) observed that the release of the restricted valve annulus with a sufficient diameter following the removal of all ring components is frequently associated with successful outcomes. As Lamberti (15) indicated, we can perform a complete resection on the periphery of the supravalvular ring during its resection by sewing a traction suture on the ring to expose the field of view.
In addition, the repair of MR diseases is complex, especially isolated MR diseases with mixed MV pathologies. In our cohort, those with isolated MR showed a greater prevalence of shorter and smaller MV leaflets [32% (8/25) vs. 29.4% (15/51)] and leaflet prolapse [44% (11/25) vs. 41.2% (21/51)] compared to those in the MR combined with CHD group. Notably, the percentage of patients with cleft leaflets was much higher in the isolated MR group than in the MR combined with the CHD group [76% (19/25) vs. 39.2% (20/51)]. Greater MR in the isolated MR group, the impact of high-velocity reflux blood flow during LV contraction, the development of leaflet hyperplasia and fibrosis, the consequent worsening of leaflet mal-apposition, and the enlargement of the valve annulus all occurred in the presence of a leaflet cleft (16). These thickened, stiff, and stunted MV were prone to further development of MR, which could then require MV replacement. Figure 4C,4D illustrates the progression of MR after MV surgery despite a thorough repair, with more numbers of patients in the isolated MR group (28%; 7/25) experiencing severe MR compared to those in the MR combined with CHD group (19.6%; 10/51) during the last follow-up. Finally, 3 (12%) patients in the isolated MR group and 4 (7.8%) patients in MR combined with the CHD group received MV reoperations.
The LV function index is often employed to evaluate the efficacy of MV repair in addition to the rate of reoperation and the extent of postoperative MR and MS. Both the LVEF in the primary MR and MS groups depicted a transient decrease post-operation, but a rapid restoration during the early postoperative period indicated a good recovery of LV function (Figure 5A,5B). Five years after the MV operation, the LVEF in these two groups remained at a relatively normal level of >0.6, which Wunderlich et al. have put forward as a borderline. Per their assessment, LVEF below this threshold indicates LV dysfunction and poor prognosis (17). The TTE showed a variation of LVEDVI from 87.67±43.08 mL/m2 at 24 h after surgery to 171.08±45.97 mL/m2 at 5 years in the MR group and 61.66±44.03 mL/m2 at 24 h after surgery to 118.00±45.90 mL/m2 at 5 years in the MS group. According to our data, the LV function of most children indeed improved after MV repair and remained stable with the growth and development of children.
Risk factors for MV dysfunction, reoperation and death
The multivariable Cox regression analysis indicated that mixed MV pathology acted as an independent risk factor for MV dysfunction (Table 5; P=0.014, HR =2.492). Most patients with mixed MV pathology progressed rapidly to dysfunction following the operation (Figure 6; P=0.002) in contrast to those without mixed MV pathology. This finding can be attributed to the MV not supporting the perfect repair of mixed pathologies.

Our different MV repair techniques for children may interact with one another during the repair phase, making it challenging for the mitral annulus shape to return to the typical, nonplanar, and stable saddle structure after surgery (18). The instability of leaflets after repair may be hastened by the effect of blood flow from the systemic circulation, which may also accelerate the onset of early MV dysfunction.
Our results also corroborated those of Sughimoto, who reported that ≥ moderate MV regurgitation on the first postoperative day was an independent risk factor for MV reoperation (Table 7; P=0.014, HR =8.493) (19). Because MR results from 24 h after surgery usually indicate the prognosis (Figure 7, P<0.001), we concluded that surgeons must have adequate patience to repair MV precisely on the first attempt. In our cohort, 24 patients were diagnosed with moderate or severe MR at 24 h following surgery. In these patients, six who did not receive MV reoperations were diagnosed as having severe MR during the last follow-up, and five who received MV reoperations were already suffering from severe MR before reoperation.

As for the analysis of the death event, the use of double-orifice MV technique (Table 8, P=0.002, HR =39.319; Figure 8A, P<0.001), the occurrence of MV reoperation (Table 8, P=0.023, HR =8.764; Figure 2B, P<0.001) and the detection of severe MV regurgitation at first 24 h after first surgery (Table 8, P=0.028, HR =10.856; Figure 8B, P<0.001) all acted as the independent risk factor for death. Although the use of double-orifice MV technique helped a few patients, we did not recommend it as the first choice because it has some serious flaws. One reason might be that the MV of the younger patient may have been more susceptible to tears during surgery because of its fragility. Postoperative severe MR often results from suturing the anterior and posterior valve tissue with excessive tension, which can easily lead to tearing of the posterior valve leaflets. In addition, the development of MV leaflets and changes in the structure of the valve annulus in the late postoperative period could result in a high tension of the valve coaptation position, resulting in MR. Furthermore, extensive stitching of the valves, considering the tiny valve size in younger children, might alter the valve geometry, thereby exposing the surface of the valve leaflet to uneven blood flow impact. Based on the experience of our center, the double-orifice MV technique is unsuitable for patients with severe MV dysplasia and annular stenosis.

In the early postoperative stage, tearing of the valve due to high tension can cause severe MR regurgitation, while emergency MV repair surgery is not effective. Among the 5 patients who died in our cohort, 40% (2/5) adopted the double-orifice MV technique, 40% (2/5) were detected severe MV regurgitation at first 24 h after first surgery, and 60% (3/5) underwent MV reoperation. Nevertheless, high tension during repair can be avoided by following the recommendations made by Cao et al., which involves repeatedly injecting saline to monitor and adjust the coaptation point (which is not necessarily the midpoint of the anterior and posterior valves), keeping the leaflet width moderate, having the force points act on the chordae tendineae rather than the leaflets, and decreasing the amount of leaflet suture (20,21).
Limitations
Our study is conclusive, albeit it has some limitations. First and foremost, being a retrospective case study that included patients from a single center, the findings of this research should be interpreted with caution. Moreover, the proportion of patients with primary MV diseases and reoperations was relatively small, which could not be enough to evaluate the risk factors. Another limitation of our study was that patients were classified as having MR or MS diseases based on whether regurgitation or stenosis was predominated. As a result, some patients had coexisting MR and MS, which may have affected the analysis outcomes.
Conclusions
No statistically significant difference was found in freedom from MV dysfunction and reoperation between the primary MR and MS disease groups. LV function was satisfactorily restored through meticulous surgery. Future MV dysfunction was predicted in patients with mixed MV pathology. ≥ moderate MV regurgitation at 24 h after the first surgery were identified as the independent risk factor for MV reoperation. The use of double-orifice MV technique, MV reoperation and severe MV regurgitation at 24 h after first surgery were identified as the independent risk factors for death.
Acknowledgments
We want to thank Medjaden Inc. for their help in polishing our paper.
Funding: The work was supported by the Biomedical Science and Technology Support Project of Shanghai Science and Technology Innovation Action Plan (No. 21S3904600).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-270/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-270/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-270/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-270/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Board of the Children’s Hospital of Fudan University [No. (2022)131]. The need for patient consent was waived off because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raghuveer G, Caldarone CA, Hills CB, et al. Predictors of prosthesis survival, growth, and functional status following mechanical mitral valve replacement in children aged <5 years, a multi-institutional study. Circulation 2003;108:II174-9. [Crossref] [PubMed]
- del Nido PJ, Baird C. Congenital mitral valve stenosis: anatomic variants and surgical reconstruction. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2012;15:69-74. [Crossref] [PubMed]
- Baird CW, Myers PO, Marx G, et al. Mitral valve operations at a high-volume pediatric heart center: Evolving techniques and improved survival with mitral valve repair versus replacement. Ann Pediatr Cardiol 2012;5:13-20. [Crossref] [PubMed]
- Stellin G, Padalino MA, Vida VL, et al. Surgical repair of congenital mitral valve malformations in infancy and childhood: a single-center 36-year experience. J Thorac Cardiovasc Surg 2010;140:1238-44. [Crossref] [PubMed]
- Caldarone CA, Raghuveer G, Hills CB, et al. Long-term survival after mitral valve replacement in children aged <5 years: a multi-institutional study. Circulation 2001;104:I143-7. [Crossref] [PubMed]
- Yakub MA, Krishna Moorthy PS, Sivalingam S, et al. Contemporary long-term outcomes of an aggressive approach to mitral valve repair in children: is it effective and durable for both congenital and acquired mitral valve lesions? Eur J Cardiothorac Surg 2016;49:553-60; discussion 560. [Crossref] [PubMed]
- Yakub MA, Dillon J, Krishna Moorthy PS, et al. Is rheumatic aetiology a predictor of poor outcome in the current era of mitral valve repair? Contemporary long-term results of mitral valve repair in rheumatic heart disease. Eur J Cardiothorac Surg 2013;44:673-81. [Crossref] [PubMed]
- Carpentier A, Branchini B, Cour JC, et al. Congenital malformations of the mitral valve in children. Pathology and surgical treatment. J Thorac Cardiovasc Surg 1976;72:854-66.
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Wunderlich NC, Beigel R, Siegel RJ. Management of mitral stenosis using 2D and 3D echo-Doppler imaging. JACC Cardiovasc Imaging 2013;6:1191-205. [Crossref] [PubMed]
- Sivalingam S, Haranal M, Moorthy PSK, et al. Mid-Term Results Comparing the Use of Artificial Chords Versus Native Chords for Mitral Valve Repair in Children. World J Pediatr Congenit Heart Surg 2020;11:579-86. [Crossref] [PubMed]
- Geoffrion TR, Pirolli TJ, Pruszynski J, et al. Mitral Valve Surgery in the First Year of Life. Pediatr Cardiol 2020;41:334-40. [Crossref] [PubMed]
- Vida VL, Carrozzini M, Padalino M, et al. Surgical Treatment of Congenital Mitral Valve Dysplasia. J Card Surg 2016;31:352-6. [Crossref] [PubMed]
- Cho S, Kim WH, Kwak JG, et al. Surgical results of mitral valve repair for congenital mitral valve stenosis in paediatric patients. Interact Cardiovasc Thorac Surg 2017;25:877-82. [Crossref] [PubMed]
- Lamberti JJ, Mitruka SN. Congenital anomalies of the mitral valve. In: Mavroudis C, Backer CL. editors. Pediatric Cardiac Surgery. 3rd edition. Philadelphia, PA, USA: Mosby Inc.; 2003.
- Sénéchal M, MacHaalany J, Bertrand OF, et al. Predictors of left ventricular remodeling after surgical repair or replacement for pure severe mitral regurgitation caused by leaflet prolapse. Am J Cardiol 2013;112:567-73. [Crossref] [PubMed]
- Wunderlich NC, Beigel R, Rader F, et al. Degenerative Mitral Regurgitation: Assessment, Physical Examination, and Imaging. Curr Cardiol Rep 2019;21:85. [Crossref] [PubMed]
- Nam HH, Dinh PV, Lasso A, et al. Dynamic Annular Modeling of the Unrepaired Complete Atrioventricular Canal Annulus. Ann Thorac Surg 2022;113:654-62. [Crossref] [PubMed]
- Sughimoto K, Konstantinov IE, d'Udekem Y, et al. Mid-term outcomes of congenital mitral valve surgery: Shone's syndrome is a risk factor for death and reintervention. Interact Cardiovasc Thorac Surg 2017;25:734-9. [Crossref] [PubMed]
- Cao F, Mo XM, Chen J, et al. Follow-up study of edge-to-edge mitral repair in children. Chin J Thorac Cardiovasc Surg 2017;33:456-61.
- De Bonis M, Lapenna E, La Canna G, et al. Mitral valve repair for functional mitral regurgitation in end-stage dilated cardiomyopathy: role of the "edge-to-edge" technique. Circulation 2005;112:I402-8. [Crossref] [PubMed]


