
Clinical outcomes of the cuff wrapping technique in the modified Bentall procedure: a propensity score-matched study
Highlight box
Key findings
• The cuff wrapping technique effectively prevents and reduces bleeding at the proximal anastomotic sites, while also lowering the occurrence of contrast extravasation and persistent aortic-right atrial shunts.
What is known and what is new?
• The classic Bentall procedure carries the risk of persistent aortic root to right atrial shunt, thrombosis, and dangerous pulmonary embolism, while the modified Bentall procedure still presents a significant complication of hemorrhage at the proximal anastomosis.
• The safety and efficacy of the modified cuff wrapping Bentall procedure are evaluated.
What is the implication, and what should change now?
• The cuff wrapping technique could serve as an alternative option to aid in proximal anastomosis in patients undergoing the modified Bentall procedure.
Introduction
The Bentall procedure, first proposed by De Bono and Bentall in 1968, completely replaces the aortic valve and ascending aorta (1). Since then, using a composite valve graft for the replacement of the aortic root and ascending aorta with coronary reimplantation has become the gold standard for the treatment of aortic valve diseases combined with ascending aortic dilatation or aneurysm. However, since the inception of this procedure, coping with bleeding from the aortic root has always been the main issue that needs to be resolved.
To address the problem of difficult hemostasis at the aortic root, the Bentall procedure has undergone several refinements, gradually evolving over time. One notable refinement involves a technique that effectively controls proximal bleeding by using a right atrial shunt. This technique utilizes the aortic wall to wrap the aortic root, creating a fistula between the closed peri-graft space and the right atrium, commonly referred to as the Cabrol shunt. The Bentall procedure incorporating this technique is known as the classic Bentall (C-Bentall) procedure (2,3). However, the C-Bentall procedure creates new problems, such as, a possibly persistent aortic root to right atrial shunt, thrombosis, and dangerous pulmonary embolism. Later, Kouchoukos described the modified Bentall procedure using the Carrel patch technique, while abandoning the wrap-inclusion technique (4). This modification abandons directly anastomose the coronary ostia to the composite graft and the Cabrol shunt. Instead, the aortic root is mobilized and freed from the adjacent tissue, and then the two coronary ostia with button-like aortic tissue are excised from the aorta, which is then sutured to the corresponding site opposite to the graft. With improvements in surgical protocol and technique, this modified Bentall procedure avoids kinking of the coronary arteries and reduces tension at the anastomotic site of the coronary “button”. However, hemostasis at the proximal suture line reappears as a major problem.
Currently, oozing and hemorrhage at the proximal anastomosis remains a major complication of the modified Bentall procedure. To address this hemostatic problem, we proposed a modified cuff wrapping Bentall (M-Bentall) procedure, which involves the application of residual aortic tissue from the aortic root to wrap the proximal suture line by suturing the aortic tissue to the root of the artificial vessel graft. We compared the surgical outcomes of the M-Bentall procedure with those of the C-Bentall procedure at our institution during the same period. Propensity score matching (PSM) was performed to adjust for differences in baseline data between the C-Bentall and M-Bentall groups. The main objective of this retrospective study was to clarify the surgery-related parameters and postoperative complications and to assess the effect of our M-Bentall procedure on patients’ clinical outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-201/rc).
Methods
Patients
Between July 2017 and December 2021, a total of 159 consecutive patients who underwent the Bentall procedure in our institution were enrolled. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for this study protocol was obtained from the Ethics Committee of Fuwai Hospital (approval No. 2021-1490) and individual consent for this retrospective analysis was waived. Patients who received the classic Bentall procedure were enrolled in the C-Bentall group, while patients who received our modified cuff wrapping Bentall procedure were included in the M-Bentall group. The data were collected from electronic medical records and the picture archiving and communication system of all patients who had undergone the Bentall procedure. Participants met the following inclusion criteria: (I) patients aged 15–75 years and (II) patients with aortic root aneurysms treated with the Bentall procedure. Exclusion criteria were as follows: (I) contraindications to ceasing preoperative antithrombotic therapy; (II) severe cardiac dysfunction (ejection fraction <35%); (III) type A aortic dissection; and (IV) Behcet’s disease.
Surgical technique
In the M-Bentall group, we performed the Bentall procedure with a modification in the proximal suture line to improve hemostasis after weaning from cardiopulmonary bypass (CPB) (4).
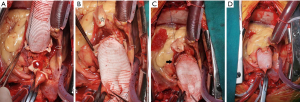
All patients were placed in the supine position with general anesthesia. The axillary artery or femoral artery was used for arterial perfusion, and a return cannula was placed in the right atrium for venous drainage. After initiating CPB and arresting the heart with cold cardioplegia, the aortic valve was resected, and the enlarged ascending aorta was excised ranging from 2 cm below the aortic cross-clamp to approximately 8 mm above the aortic annulus, and followed by dissecting and isolating the coronary artery ostia in a button-like shape (Figure 1A,1B). Then, implantation of the composite conduit was performed. If the patient chose to receive mechanical valve implantation, the Medtronic Open Pivot Valved Conduit (Medtronic, Minneapolis, MN, USA) was used. If the patient opted for bioprosthetic valve implantation, the Medtronic Hancock II valve (Medtronic, Minneapolis, MN, USA) was manually sutured to the collagen-impregnated, Hemashield Platinum straight graft (MAQUET Cardiovascular LLC, Wayne, NJ, USA) and implanted as a composite graft. Once the composite graft implantation was completed, a continuous suture line was used to anastomose the remnant aortic wall with the artificial vascular graft to wrap the proximal suture line between the composite graft and the aortic annulus (Figure 1C). Then, the left coronary artery ostium was attached to the composite graft with 5-0 prolene continuous suture. After the left coronary was well reimplanted, cardioplegia was used to fulfil and pressurize the composite graft, and the right ventricle was dilated by clamping of the venous cannula shortly followed by marking the tensionless position for the right coronary button reimplanted to the graft (Figure 1D). Any significant anastomotic bleeding at the left coronary button could be identified during this period. Finally, the distal graft was anastomosed to the transected ascending aorta. After careful deairing and hemostasis, CPB was weaned, followed by closing the sternum pectoral fascia, subcutaneous tissues, and skin.
Previously, the Bentall procedure with the inclusion technique has been well described, and we strictly adhered to the standard protocol of that procedure in the C-Bentall group (5). The cannulation site for the venous cannula differed from the M-Bentall procedure. The superior vena cava was cannulated, and the right atrial appendage was left intact for further use. CPB was initiated with moderate hypothermia. The ascending aorta was then cross-clamped, and the heart was arrested using cold cardioplegia. Next, an incision was made into the aortic root, and the aortic valve was excised. Then, the composite valve conduit, including a mechanical or bioprosthetic valve, was sewn into the aortic annulus. The conduit was carefully excised using cautery, creating two “round-hole” shaped buttons that corresponded to the locations of the coronary ostia, and then direct coronary artery reimplantation was performed by sewing the coronary ostium directly to these holes.
Then, rewarming was begun after the distal anastomosis was completed by sewing the conduit with the distal aorta together. After removing the cross-clamp and deairing, a fistula Cabrol shunt was created from the perigraft space to the right atrium as described by Cabrol (2,3). The remaining aorta tissue was used to wrap around the graft. Then, the patient was weaned from CPB, followed by removal of the cannulas and closure of the chest.
Postoperative follow-up
All patients who received the Bentall procedure were required to undergo a cardiac echocardiogram before discharge. Additionally, patients were instructed to return for follow-up visits at 3 months, 6 months, and 1 year after the surgery. They are specifically informed about the need to receive computed tomography (CT) angiography within the first 3 months after the procedure. Based on the CT results, the presence of contrast extravasation, thoracic aortic or coronary pseudoaneurysm, or persistent shunt after the surgery was assessed and documented.
Statistical analysis
Statistical analysis was performed using SPSS 24 (IBM SPSS, SPSS Inc., Chicago, IL, USA) and R-studio with R, and statistically significant differences were defined as two-tailed P values <0.05. Raw data with more than 10% missing values were not used, and those less than 10% were replaced by the mean value. To minimize baseline differences between groups, PSM matching was performed with the following covariates: age, sex, hypertension, coronary artery disease (CAD), New York Heart Association (NYHA) classification, preoperative left ventricular ejection fraction (LVEF), preoperative left ventricular end-diastolic diameter (LVED), preoperative aortic sinus diameter, preoperative serum creatinine (SCr), preoperative alanine transaminase (ALT), and preoperative D-dimer. Nearest-neighbor 1:1 PSM was conducted without replacement, with a caliper width of 0.1. Ninety patients were successfully matched in this way (45 pairs).
Normally distributed quantitative data were expressed as the mean ± standard deviation (SD), and the median and interquartile range (IQR) were used to represent quantitative data with a nonnormal distribution. The independent t-test and Mann-Whitney U test were used for comparisons of continuous variables. The chi-squared/χ2 test or Fisher’s exact test was applied for categorical data. Paired T-, Wilcoxon signed rank, and McNemar tests were applied after PSM.
Results
One hundred fifty-nine patients who met the inclusion criteria were enrolled in our research. There was only one in-hospital death, and the other patients all fully recovered and were discharged after postoperative cardiac-ultrasound examination. Among these 159 patients, 106 underwent the C-Bentall procedure, with 90 had successful follow-up radiographic data (C-Bentall group, n=90), and 53 underwent the M-Bentall procedure, of whom 46 had successful follow-up and postoperative imaging data (M-Bentall group, n=46), which are specified in Table S1.
Comparison of demographic data and preoperative data between the two groups
Before PSM, the two groups differed significantly in terms of baseline data. As shown in Table 1, baseline data, including age, sex, NYHA classification, hypertension, Marfan syndrome, bicuspid aortic valve, and CAD, showed no differences. Meanwhile, parameters such as SCr, ALT, D-dimer, and LVEF also had no statistical significance. However, the LVED and the severity of aortic insufficiency showed significant differences between the two groups (P=0.007 and P=0.042, respectively). The aortic sinus diameter also showed a trend of difference between the two groups (P=0.061). After PSM, the baseline data between the two groups were perfectly matched with no statistically significant differences. The demographic data of the study population after PSM are also shown in Table 1.
Table 1
| Item | Overall study population | Propensity-matched | |||||
|---|---|---|---|---|---|---|---|
| C-Bentall (n=90) | M-Bentall (n=46) | P | C-Bentall (n=45) | M-Bentall (n=45) | P | ||
| Sex (female) | 13 (14.44) | 4 (8.70) | 0.338 | 6 (13.33) | 4 (8.89) | 0.739 | |
| Age (years) | 53.62±12.86 | 52.96±12.63 | 0.774 | 53.38±13.52 | 52.53±12.17 | 0.954 | |
| Marfan | 5 (5.56) | 3 (6.52) | 1.000 | 3 (6.67) | 3 (6.67) | 1 | |
| BAV | 20 (22.22) | 8 (17.39) | 0.510 | 10 (22.22) | 8 (17.78) | 0.598 | |
| Hypertension | 41 (45.56) | 21 (45.65) | 0.992 | 20 (44.44) | 20 (44.44) | 1 | |
| CAD | 18 (20.00) | 8 (17.39) | 0.714 | 7 (15.56) | 8 (17.78) | 0.777 | |
| NYHA classification | 0.591 | 0.886 | |||||
| I | 14 (15.56) | 9 (19.57) | 7 (15.56) | 8 (17.78) | |||
| II | 48 (53.33) | 24 (52.17) | 25 (55.56) | 24 (53.33) | |||
| III | 26 (28.89) | 12 (26.09) | 12 (26.67) | 12 (26.67) | |||
| IV | 2 (2.22) | 1 (2.17) | 1 (2.22) | 1 (2.22) | |||
| SCr (μmol/L) | 86.34±23.12 | 84.63±13.42 | 0.587 | 85.93±22.16 | 85.06±13.23 | 0.823 | |
| ALT (IU/L) | 20.83±15.28 | 26.17±23.09 | 0.161 | 25.96±19.63 | 24.96±21.81 | 0.820 | |
| D-dimer (mg/L) | 0.81±2.21 | 0.40±0.39 | 0.215 | 0.37±0.38 | 0.39±0.39 | 0.819 | |
| Aortic sinus diameter (mm) | 56.00±10.34 | 52.64±8.70 | 0.061 | 56.00±10.34 | 52.64±8.70 | 0.735 | |
| LVED (mm) | 65.14±9.32 | 60.24±11.04 | 0.007 | 61.84±8.13 | 60.47±11.05 | 0.502 | |
| LVEF (%) | 58.22±6.88 | 58.93±5.89 | 0.550 | 58.44±7.46 | 58.96±5.95 | 0.720 | |
| Aortic insufficiency | 0.042 | 0.489 | |||||
| 0 | 1 (1.11) | 4 (8.70) | 1 (2.22) | 4 (8.89) | |||
| 1 | 6 (6.67) | 7 (15.22) | 5 (11.11) | 6 (13.33) | |||
| 2 | 12 (13.33) | 5 (10.87) | 9 (20.00) | 5 (11.11) | |||
| 3 | 28 (31.11) | 14 (30.43) | 11 (24.44) | 14 (31.11) | |||
| 4 | 43 (47.78) | 16 (34.78) | 19 (42.22) | 16 (35.56) | |||
Data are presented as n (%) or mean ± SD. BAV, bicuspid aortic valve; CAD, coronary artery disease; NYHA, New York Heart Association; SCr, serum creatinine; ALT, alanine transaminase; LVED, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; SD, standard deviation.
Analysis of surgical notes between the two groups after PSM
As shown in Table 2, no significant differences existed between the two groups in the type of concomitant procedures. There was no difference between the two groups in the amount of intraoperative blood products used, as well as no difference in the amount of intraoperative blood loss. However, the CPB time (126 vs. 154 min, P=0.018) and aortic cross-clamp time (92 vs. 113 min, P=0.009) of the M-Bentall group were significantly longer than those of the C-Bentall group. The prolonged operation time indicates that the cuff wrapping technique may increase the complexity and difficulty of the operation.
Table 2
| Item | C-Bentall (n=45) | M-Bentall (n=45) | P |
|---|---|---|---|
| Concomitant CABG | 5 (11.11) | 6 (13.33) | 0.748 |
| Concomitant MVS | 6 (13.33) | 2 (4.44) | 0.266 |
| Plasma transfusion (mL) | 0 (0–400) | 0 (0–400) | 0.741 |
| RBC transfusion (μ) | 0 (0–0) | 0 (0–0) | 0.452 |
| Platelet transfusion (μ) | 0 (0–0) | 0 (0–1) | 0.517 |
| Blood loss (mL) | 630 (600–663) | 630 (600–735) | 0.701 |
| CPB time (min) | 126.18±41.91 | 153.96±64.85 | 0.018 |
| Aorta cross-clamp time (min) | 92.09±34.51 | 113.18±40.18 | 0.009 |
Data are presented as n (%), median (IQR), or mean ± SD. PSM, propensity score matching; CABG, coronary artery bypass grafting; MVS, mitral valve surgery; RBC, red blood cell; CPB, cardiopulmonary bypass; IQR, interquartile range; SD, standard deviation.
Postoperative clinical outcomes
The in-hospital death rate was 0.63% (1/159) among all the patients in this study. Only one patient died after receiving the C-Bentall procedure during hospitalization. This patient was not matched to patients in the M-Bentall group and was excluded from the statistical analysis.
After PSM, the postoperative data are described in Table 3. There were no significant differences observed in postoperative adverse events [re-exploration, stroke, renal failure requiring continuous renal replacement therapy (CRRT), pneumonia, pulmonary thromboembolism] between the two groups. In terms of laboratory tests, the C-Bentall group showed a trend toward higher D-dimer values on the first postoperative day (P=0.091), and the peak D-dimer value showed a significant difference (P=0.019), with higher values in the C-Bentall group. There were no differences in postoperative length of stay, duration of ventilation time, or intensive care unit stay. The average chest tube drainage on the first postoperative day was low in both groups but did not show a significant difference. In terms of cardiac structure and function, postoperative echocardiography showed no significant differences in LVEF and LVED. However, the postoperative contrast extravasation (P=0.030) was different between the two groups. Although there was no significant difference between the two groups in the incidence of persistent shunts, in the M-Bentall group, its incidence was nil, while 4 cases (8.89%) existed in the C-Bentall group. In terms of pulmonary embolism, 2 cases with pulmonary thromboembolism were identified in the C-Bentall group after PSM. These two patients were treated with anticoagulant therapy only during hospitalization and eventually recovered and were discharged from the hospital. Meanwhile, 2 cases in the M-Bentall group were nonfatal pulmonary embolisms detected on examination occasionally.
Table 3
| Item | C-Bentall (n=45) | M-Bentall (n=45) | P |
|---|---|---|---|
| Early mortality | 0 (0.00) | 0 (0.00) | 1.000 |
| Re-exploration | 2 (4.44) | 0 (0.00) | 0.494 |
| Onset stroke | 1 (2.22) | 0 (0.00) | 1.000 |
| Renal failure required CRRT | 0 (0.00) | 0 (0.00) | 1.000 |
| Pneumonia | 1 (2.22) | 0 (0.00) | 1.000 |
| Pulmonary thromboembolism | 2 (4.44) | 2 (4.44) | 1.000 |
| Postoperative Day 1 SCr (μmol/L) | 96.10±29.94 | 93.45±20.22 | 0.624 |
| Postoperative peak SCr (μmol/L) | 115.00±47.55 | 103.90±22.56 | 0.160 |
| Postoperative Day 1 ALT (IU/L) | 19.27±12.05 | 22.42±13.64 | 0.248 |
| Postoperative peak ALT (IU/L) | 89.51±203.73 | 56.49±95.04 | 0.327 |
| Postoperative Day 1 D-dimer (mg/L) | 2.62±3.80 | 1.60±1.28 | 0.091 |
| Postoperative peak D-dimer (mg/L) | 4.73±4.77 | 2.89±1.95 | 0.019 |
| LVED (mm) | 51.93±7.82 | 51.00±6.89 | 0.549 |
| LVEF (%) | 56.29±8.74 | 56.33±8.47 | 0.981 |
| Aortic insufficiency | 0.317 | ||
| 0 | 44 (97.78) | 45 (100.00) | |
| 1 | 1 (2.22) | 0 (0.00) | |
| Chest drainage in postoperative Day 1 (mL) | 248.44±105.81 | 279.56±90.08 | 0.137 |
| Ventilation time (hours) | 19.51±19.28 | 14.78±10.04 | 0.148 |
| Intensive care unit stay (hours) | 76.03±54.10 | 77.57±54.04 | 0.893 |
| Postoperative hospital stay (days) | 8.31±2.71 | 8.11±2.48 | 0.716 |
| Contrast extravasation or pseudoaneurysm | 8 (17.78) | 1 (2.22) | 0.030 |
| Persistent shunt | 4 (8.89) | 0 (0.00) | 0.117 |
Data are presented as n (%) or mean ± SD. PSM, propensity score matching; CRRT, continuous renal replacement therapy; SCr, serum creatinine; ALT, alanine transaminase; LVED, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; SD, standard deviation.
There were no persistent aortic-right atrial shunts, and only one had active contrast extravasation in the M-Bentall group. None of the patients in this group underwent re-exploration for bleeding, suffered new onset of stroke or renal failure and required CRRT, and the clinical results were excellent. Meanwhile, there were 8 cases of extravasation of contrast material or pseudoaneurysms, along with 4 cases of persisting aortic-right atrial shunt in the C-Bentall group. Two patients in the C-Bentall group underwent re-exploration surgery to stop the bleeding, and one developed stroke.
Discussion
Our study demonstrated that the cuff wrapping technique is a feasible and simple method to facilitate hemostasis in the modified Bentall procedure.
The Bentall procedure is a landmark in the treatment field of aortic root lesions (1). However, bleeding from the aortic root and the coronary anastomosis have always been a bothersome problem for surgeons. Cabrol modified the Bentall procedure for coronary anastomosis while improving hemostasis at the aortic root by wrapping the aortic root with the residual aneurysm wall and performing a shunt to the right atrium, which is called the Cabrol shunt (2,3). Since then, the Bentall procedure has evolved into a direct implantation of the coronary artery to the graft (without mobilizing the coronary artery ostium) and then use the residual aortic aneurysm wall to wrap the aortic root and perform a shunt to the right atrium, which is called the C-Bentall procedure. Although the C-Bentall procedure successfully solves the bleeding problem, the root of the aorta is artificially anomalously connected to the right atrium. Bleeding at the anastomotic site has the possibility of producing small bleeding problems, which can lead to lateral compression of the coronary artery ostium or pseudoaneurysm, increasing the likelihood of reoperation and even the risk of thrombosis and pulmonary embolism due to the persistence of a left-to-right shunt.
In view of the drawbacks of the C-Bentall procedure, Kouchoukos et al. modified the C-Bentall procedure by abandoning the Cabrol shunt, mobilizing the coronary artery ostium in a “button” shape, and anastomosing the button-like coronary ostia directly to the prosthetic graft, which is called the modified Bentall procedure (4,6). This procedure has become mainstream in this field because it is similar to normal anatomy and physiology and has better clinical outcomes.
Bleeding and oozing from the proximal anastomotic line of the aortic root in the modified Bentall procedure is a major complication. This often makes intraoperative hemostasis difficult and increases the time for surgical hemostasis and the amount of blood loss and blood transfusion. There have been several subsequent modifications of this procedure for the hemostasis of the proximal suture line (7). One of the refinements is the flanged Bentall technique (8,9). However, oozing from the sewing cuff of the composite valve graft could not be wrapped. With this flanged Bentall procedure, after placement of the everting pledgeted 2-0 polyester sutures, only the pledgeted sutures could be wrapped between the residual aortic wall and the flange, leaving pinholes of the knots of these sutures on the sewing cuff between the flange and composite graft, which is “naked” as a potential bleeding point. In contrast, Cebi modified the C-Bentall procedure by directly implanting the coronary ostia to the composite valve graft, followed by proximal remnant ascending aorta tissue sutured to the graft above the anastomotic site of the coronary ostia to wrap the entire proximal ascending aorta, including the anastomotic site of the coronary ostia (10). However, it has the risk of producing compression in the coronary ostium if the tension in the wrapped space is high. Chen started to modify the composite valve graft by adding a short Dacron skirt to the graft root. The short skirt and the residual native aortic tissue were sewn together to tandem the proximal suture line (11). However, this creates an additional anastomotic line between the composite and the skirt and prolongs the operative time. The last method is similar to our method, but it directly sutures the residual aortic wall to the sewing cuff, which may bring a new problem in that the knots of sutures cannot be entirely wrapped (7). In addition to the appealed methods, there are many improvements for the proximal anastomosis of the modified Bentall procedure, but most of them are difficult to promote because of the complexity of the procedure or the difficulty of obtaining the surgical instruments and suture materials (12-15).
To solve the above problem, we introduced the M-Bentall procedure, which applied the residual tissue of the aortic root to wrap the proximal suture line between the composite valve graft and the aortic annulus, completely wrapped the proximal anastomotic line by suturing the residual aortic wall to the root of the graft conduit, promoted thrombus formation at the encased site, and effectively stopped oozing from suture lines. The advantage of this method is that it can completely wrap the area of possible bleeding without affecting the anastomosis of the coronary artery. We usually preserve at least 8 mm of the aortic wall tissue at the aortic root and then wrap it with the proximal end of the composite valve graft; thus, it has the advantage of wrapping all the oozing in the groove between the anastomosis of the composite valve graft and the aortic annulus. Our results showed that the incidence of contrast extravasation and pseudoaneurysm was very low in the M-Bentall group compared with the C-Bentall group. Moreover, since there is no aortic-right atrial Cabrol shunt, it is impossible for the thrombus around the aortic root to enter the right atrium through the fistula when applying our cuff wrapping technique. The absence of fatal pulmonary embolism in the M-Bentall group and the lower postoperative D-dimer index also support this opinion.
This modification resulted in a longer operative time. The CPB time and cross-clamp time in the M-Bentall group were longer than those in the C-Bentall group, but the clinical outcomes of our novel technique were satisfactory, without any increase in the incidence of adverse events. The application of this M-Bentall procedure did not result in any difficulty in hemostasis due to proximal anastomotic line bleeding or oozing, restarting CPB or re-exploration to stop bleeding. In addition, the patient’s postoperative recovery was ideal, with an average chest tube drainage of less than 300 mL on the first postoperative day and a decreasing trend in ventilation time.
Although the clinical results demonstrate a more satisfactory efficacy of the procedure, there are still some limitations to our study. Our cohort was small, and the follow-up period was short. We have not obtained long-term follow-up data to demonstrate the long-term efficacy of this technique. Further follow-up is needed to collect data for long-term outcomes. However, despite these limitations, our findings still provide an alternative technique for the help of anastomosis in the Bentall procedure, which is easier to perform.
Conclusions
The cuff wrapping technique provides good clinical results in preventing and reducing proximal anastomotic bleeding, while facilitating hemostasis during open-heart surgery. Furthermore, it reduces the incidence of contrast extravasation and persistent aortic-right atrial shunts, which significantly benefits the patient’s long-term survival.
Acknowledgments
We highly acknowledge the contribution of the following surgeons at Fuwai Hospital for their clinical support and linguistic assistance. Department of Vascular Surgery: Xiang-Yan Qian, Cun-Tao Yu, Xiao-Gan Sun, Bo Wei. Department of Cardiac Surgery: Long Deng, and Fang Fang.
Funding: The study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-201/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-201/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-201/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-201/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for this study protocol was obtained from the Ethics Committee of Fuwai Hospital (approval No. 2021-1490) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Cabrol C, Pavie A, Gandjbakhch I, et al. Complete replacement of the ascending aorta with reimplantation of the coronary arteries: new surgical approach. J Thorac Cardiovasc Surg 1981;81:309-15.
- Cabrol C, Pavie A, Mesnildrey P, et al. Long-term results with total replacement of the ascending aorta and reimplantation of the coronary arteries. J Thorac Cardiovasc Surg 1986;91:17-25.
- Kouchoukos NT, Wareing TH, Murphy SF, et al. Sixteen-year experience with aortic root replacement. Results of 172 operations. Ann Surg 1991;214:308-18; discussion 318-20. [Crossref] [PubMed]
- Lewis CT, Cooley DA, Murphy MC, et al. Surgical repair of aortic root aneurysms in 280 patients. Ann Thorac Surg 1992;53:38-45; discussion 45-6. [Crossref] [PubMed]
- Kouchoukos NT, Marshall WG Jr, Wedige-Stecher TA. Eleven-year experience with composite graft replacement of the ascending aorta and aortic valve. J Thorac Cardiovasc Surg 1986;92:691-705.
- Copeland JG 3rd, Rosado LJ, Snyder SL. New technique for improving hemostasis in aortic root replacement with composite graft. Ann Thorac Surg 1993;55:1027-9. [Crossref] [PubMed]
- Koshiyama H, Nakajima M, Amenomori S, et al. A refined flanged Bentall technique using Valsalva tube graft for proximal reinforcement. Eur J Cardiothorac Surg 2011;40:1537-9. [Crossref] [PubMed]
- Yakut C. A new modified Bentall procedure: the flanged technique. Ann Thorac Surg 2001;71:2050-2. [Crossref] [PubMed]
- Cebi N, Frömke J, Walterbusch G. Safe hemostasis by application of a new strict graft inclusion technique for replacement of the aortic root. Ann Thorac Surg 2003;76:631-2. [Crossref] [PubMed]
- Chen LW, Dai XF, Wu XJ. A modified composite valve Dacron graft for prevention of postoperative bleeding from the proximal anastomosis after Bentall procedure. Ann Thorac Surg 2009;88:1705-7. [Crossref] [PubMed]
- Della Corte A, Baldascino F, La Marca F, et al. Hemostatic modifications of the Bentall procedure: imbricated proximal suture and fibrin sealant reduce postoperative morbidity and mortality rates. Tex Heart Inst J 2012;39:206-10.
- Lee SI, Choi CH, Park KY, et al. "Wings of a Butterfly" Technique in Modified Bentall's Procedure. Thorac Cardiovasc Surg 2022;70:339-40. [Crossref] [PubMed]
- Mutsuga M, Usui A. "Double Cuff" Technique for Modified Bentall Procedure. Heart Lung Circ 2021;30:e109-11. [Crossref] [PubMed]
- Yan TD. Mini-Bentall Procedure: The "French Cuff" Technique. Ann Thorac Surg 2016;101:780-2. [Crossref] [PubMed]


