Detection of folate receptor–positive circulating tumor cells by ligand-targeted polymerase chain reaction in non-small cell lung cancer patients
Currently circulating tumor cells (CTCs) represent attractive predictive and prognostic biomarkers. CTCs may be used to evaluate the treatment efficacy, to assess prognosis, to predict the risk of recurrence in several cancers, including lung cancer (1). CTCs are extremely rare in the bloodstream approximately with a frequency of 1 CTC per 106–107 leukocytes, therefore highly specific and sensitive methods are required to their detection (2).
In the last years, several methods to detect CTCs in peripheral blood have been proposed (3). To date, the US Food and Drug Administration approved only the CellSearch System, a method based on the immunomagnetic isolation of epithelial cell adhesion molecule (EpCAM) positive CTCs (4). However, this assay was approved for the prognostic detection of CTCs exclusively in patients with metastatic breast, colon and prostate cancer, whereas it is not currently recommended for lung cancer.
Several limits are reported for CellSearch System use in lung cancer, despite it represents the gold standard in other cancer. Previous studies showed that CTCs have low or no EpCAM expression in approximately 26% of non-small cell lung cancer (NSCLC), due to the epithelial-to-mesenchymal transition. Therefore, EpCAM-based technology use in NSCLC may miss a high percentage of EpCAM negative CTCs. In addition, the CellSearch platform could not identify the circulating tumor microemboli, frequently diffused in lung cancer. Unfortunately, for these reasons, the CellSearch System is able to identify CTCs only in about 23–39% of stage IV NSCLC patients (5,6). Therefore, several alternative methods have been tested in thoracic oncology, in order to improve the number of detectable CTCs (7).
For example, the isolation by size of tumor cells (ISET) an antigen/EpCAM independent assay showed a higher sensitivity for CTCs detection compared with CellSearch System in NSCLC. However ISET assay may miss cells less than eight in size, despite it captures EpCAM negative CTCs (6,8).
Recently, a new methods using as criterion of detection the folate receptors (FRs) expression on CTCs surface showed promising results in NSCLC patients (9-12). FRs are negligibly expressed in the blood, normal cells excluding FR-positive activated macrophages detectable barely in the blood of healthy donors (HD). On the contrary, FRs are over-expressed on tumoral cells surface in various cancers, particularly in about 72–83% of NSCLCs (9,13).
Several studies have reported that the FR-positive CTCs detection by a novel ligand-targeted polymerase chain reaction (LT-PCR) could be an effective and feasible method in NSCLC patients (9-12). This technique is based on the immunomagnetic depletion of leukocytes followed by CTCs detection labeling with folate-linked oligonucleotide and subsequent quantification through RT-PCR. The binding of folate-linked oligonucleotide and FR leads to an increase of 1 CTC in the peripheral blood by almost 100,000 order of magnitude, followed by a further amplification by RT-PCR (9).
Previous studies have established by receiver operating characteristic (ROC) analysis the optimal cut-off threshold between malignant lung disease and control group in a range of 8.5–8.94 CTC units (CU). FR-based CTCs detection by LT-PCR in NSCLC showed a sensitivity of 73.2–81.8% and a specificity of 84.1–93.2% (Table 1).
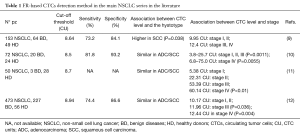
Full table
Moreover, Wan et al. have emphasized the utility of the FR-based CTC detection method in order to assess a possible relationship between CTC and the clinical features of NSCLC. They reported that the blood CTC level is significantly higher in patients with advanced lung cancer than in those with early-stage disease, in agreement with previous studies using this assay (Table 1).
In NSCLC, correlation between the CTC level and clinicopathological features has not been defined yet, however further studies on wider series using this sensitive methods could clarify a possible relationship. Therefore, LT-PCR represents a non-invasive ultrasensitive assay that could enable an advanced stratification of lung cancer identifying the subgroup of patients with poor prognosis in early stage. In conclusion, FR-based CTC detection by LT-PCR may be optimal method in NSCLC, however further studies are needed to validate the technique, to overcome the analysis bias and to define internationally recognized criteria.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Franco R, Cantile M, Marino FZ, et al. Circulating tumor cells as emerging tumor biomarkers in lung cancer. J Thorac Dis 2012;4:438-9. [PubMed]
- Tognela A, Spring KJ, Becker T, et al. Predictive and prognostic value of circulating tumor cell detection in lung cancer: a clinician's perspective. Crit Rev Oncol Hematol 2015;93:90-102. [Crossref] [PubMed]
- Toss A, Mu Z, Fernandez S, et al. CTC enumeration and characterization: moving toward personalized medicine. Ann Transl Med 2014;2:108. [PubMed]
- Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 2007;13:920-8. [Crossref] [PubMed]
- O'Shannessy DJ, Davis DW, Anderes K, et al. Isolation of Circulating Tumor Cells from Multiple Epithelial Cancers with ApoStream(®) for Detecting (or Monitoring) the Expression of Folate Receptor Alpha. Biomark Insights 2016;11:7-18. [Crossref] [PubMed]
- Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-15. [Crossref] [PubMed]
- Hofman V, Ilie M, Long E, et al. Detection of circulating tumor cells from lung cancer patients in the era of targeted therapy: promises, drawbacks and pitfalls. Curr Mol Med 2014;14:440-56. [Crossref] [PubMed]
- Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for nonsmall-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-60. [Crossref] [PubMed]
- Yu Y, Chen Z, Dong J, et al. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol 2013;6:697-702. [Crossref] [PubMed]
- Lou J, Ben S, Yang G, et al. Quantification of rare circulating tumor cells in non-small cell lung cancer by ligand-targeted PCR. PLoS One 2013;8:e80458. [Crossref] [PubMed]
- Wan JW, Gao MZ, Hu RJ, et al. A preliminary study on the relationship between circulating tumor cells count and clinical features in patients with non-small cell lung cancer. Ann Transl Med 2015;3:352. [PubMed]
- Chen X, Zhou F, Li X, et al. Folate Receptor-Positive Circulating Tumor Cell Detected by LT-PCR-Based Method as a Diagnostic Biomarker for Non-Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1163-71. [Crossref] [PubMed]
- Nunez MI, Behrens C, Woods DM, et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J Thorac Oncol 2012;7:833-40. [Crossref] [PubMed]


