
Long-term survival in esophagectomy for early-stage esophageal cancer versus endoscopic resection plus additional chemoradiotherapy: a systematic review and meta-analysis
Highlight box
Key findings
• The research showed that CRT may be suitable for EC patients with high-risk relapse or unable to tolerate surgery, but cannot totally replace surgical treatment.
What is known and what is new?
• This study intended to comprehensively compare the long-term effects of esophagectomy with CRT after ER for the first time.
• Previous research has mostly comprised retrospective, single center, and small sample clinical studies. Therefore, it is fundamental to make more comparisons on the pros and cons of the 2 treatment methods for further investigation.
What is the implication, and what should change now?
• This study found that esophagectomy remained a higher priority for patients with early-stage EC, as evidenced by a longer disease-free survival, while CRT after ER was indicated for patients who were known to be at high risk of recurrence and could not tolerate surgery.
Introduction
As one of the most severe malignant digestive neoplasms, esophageal cancer (EC) is highly aggressive, has a poor prognosis, and the 5-year overall survival (OS) is less than 25% (1,2). Recently, endoscopic resection (ER) has been recognized for its ability to diagnose and even cure early-stage superficial EC, which can increase the 5-year survival rate to about 85% (3,4). Mönig et al. (5) proposed that the lymph node metastasis rate at T1a stage is 0–13% in esophageal squamous cell carcinoma (ESCC), the lymph node metastasis rate at T1b stage is 8–26.5% in the superficial submucosa (SM1), and the risk of lymph node metastasis can increase to 22–61% when infiltrated into the submucosal layer (SM2) and below. Shen et al. (6) declared that the risk factors of lymph node metastasis were tumor differentiation, vascular invasion, and depth of tumor invasion. Therefore, it is essential to conduct esophagectomy or adjuvant chemoradiotherapy (CRT) after ER.
Traditionally, esophagectomy combined with lymph node dissection has been accepted as the standard treatment for cT1a/bN0M0 early EC. Although it has a reliable long-term effect, patients usually must accept the risk of postoperative complications and downtrend of living quality. According to a retrospective analysis by Saeki et al. (7), during the 87-month follow-up period, a total of 34 patients with early-stage EC were included without any recurrence and metastasis; the 3- and 5-year OS rates were 97.4% and 89.9%, respectively, whereas the postoperative complication rates were 18% and 10%, respectively. Additionally, the whole effect of treatment was found to largely depend on the experience of the surgical team and the patient’s physical condition.
Compared to esophagectomy, CRT after ER involves less trauma and a lower risk of postoperative complications and is gradually becoming another acceptable treatment choice. A multi-center, single-arm prospective study by Minashi et al. (8) reported that among 176 enrolled patients, the metastasis recurrence rate was 8.5% when the pathological stage was T1b (SM1-2) N0M0; the 3-year progression-free survival rate was 89.7%, and the 3-year OS rate was 90.7%. This long-term treatment effect was considered equivalent to that of radical surgery, but not limited to surgical patients only on account of the unilateral results.
A recent phase II trial revealed that combined ER and CRT is equally effective for clinical stage ESCC as esophagectomy, however, the long-term survival rate and the risk of recurrence and metastasis between the 2 methods have not been reported in detail (9). Furthermore, the current literature is mostly composed of retrospective, single center, and small sample clinical studies. Therefore, it is fundamental to conduct further comparisons of the pros and cons of the 2 treatment methods. Meta-analysis provides a summary of clinical research data by implementing statistical methods and a quantitative and comprehensive analysis of the results, which is widely used to solve clinical problems and provide corresponding decision-making and guidance. Therefore, we aimed to use meta-analysis methods to comprehensively analyze the long-term effect of esophagectomy compared with CRT after ER, and to contribute to the body of evidence-based medicine for the scientific management of early EC. We present this article in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-376/rc).
Methods
Search strategy
A comprehensive systematic literature search was conducted for all suitable controlled trials and observational studies in electronic databases including PubMed, Cochrane library, Embase, and Web of Science from inception to September 2020. The search terms and relative variants presented in the title and abstract were as follows: “esophageal neoplasms”, “esophageal cancer”, “esophagectomy”, “chemoradiotherapy”, “chemoradiation”, “radiochemotherapy”, “esophagoscopy”, “endoscopic mucosal resection”, and logical combinations of these terms using the Boolean operators “AND” and “OR”. Based on the identified study reference list, a manual search was conducted to identify any studies that may have been missed in the initial search.
Inclusion criteria
The following inclusion criteria were established before collecting articles: (Ⅰ) studies comparing survival data of esophagectomy with CRT after ER. (Ⅱ) participants: patients had EC with pT1a-pT1b; (Ⅲ) intervention: esophagectomy versus additional CRT; (Ⅳ) study design: randomized controlled trials (RCTs) or observational studies including cohort and case-control studies; (V) outcomes: complete data.
Exclusion criteria
(I) case reports or studies that did not perform a comparison; (II) letters and expert opinions; (III) meta-analyses or animal studies; (IV) reviews, news, comments, and other literature without original data; (V) incomplete literature and overlapped studies.
Data extraction and quality assessment
The formal full-length publications of studies which met the inclusion criteria previously described were independently skimmed through by 2 reviewers independently. Data were extracted from article texts, tables, and figures. The primary endpoints included any patterns of recurrence: locoregional recurrence rate (LRR), distant metastases rate (DMR), and LRR plus DMR. The secondary endpoints were OS and disease-free survival (DFS). OS was defined as the time from the date of ER to that of death from any cause or interruption of follow-up. DFS was defined as the duration of patient survival following ER without signs or symptoms of cancer recurrence. Discrepancies between the 2 reviewers were mutually resolved through discussion with a third reviewer.
Since many trials did not report this information directly, the time-to-event data were extracted from the survival curves. Kaplan–Meier curves were read by Engauge Digitizer version 4.1 (free software downloaded from http://sourceforge.net). Data combining was performed by RevMan 5.3 software (Cochrane Collaboration, Oxford, UK).
The quality evaluation of included studies was performed using the modified Newcastle-Ottawa Scale (NOS) (10,11), and the evaluation criteria included 3 main parts: the selection of research objects, comparability between the groups, and outcome measurement (total NOS score: 9 points). Any disagreements between the 2 extracting authors were settled by consensus. If consensus could not be reached between the 2 reviewers after discussion, a third author was invited for final arbitration.
Sensitivity and publication of bias assessment
The sensitivity analysis was evaluated using the odds ratio (OR) value with the 95% confidence interval (CI) of the random effects model, and the stability of the OR and 95% CI were observed by sequentially eliminating the literature. If little effect was observed after elimination, the combined result was considered relatively stable. Publication bias was tested using Funnel plots tests, Egger’s and Begg’s tests, and a P-value >0.05 was taken to indicate no significant publication bias (12,13). Data analyses were conducted by Stata 13.0 (Stata Corp., College Station, TX, USA), with P≤0.05 considered statistically significant.
Statistical analysis
All statistical analyses were assessed by RevMan 5.3 software. We calculated the pooled hazard ratio (HR) and 95% CI for OS and DFS and the pooled OR difference with 95% CI for recurrence or metastasis rate. A random effects model was used when substantial heterogeneity (I2>50%) was detected, and a fixed effects model was used in the absence of significant heterogeneity (I2<50%) (14,15). Statistical significance was considered when P<0.05. Furthermore, when a study included medians and interquartile ranges, we calculated the mean ± standard deviation via a method proposed by Hozo et al. (12).
Results
Study selection and characteristics
Our search strategy yielded a total of 39 publications suitable for full-text screening. After screening according to the inclusion criteria, a total of 9 suitable articles remained (16-24). Figure 1 illustrates the literature retrieval process. A total of 6 of the 9 included articles had been conducted in Japan. The total number of participants was 790. ESCC was the predominant condition, whose primary treatments were endoscopic submucosal dissection; the chemotherapy regimen included 5-fluorouracil and cisplatin. We conducted NOS on the non-RCTs with a score of 5–8, based on the scoring system. A study with a NOS score of 5 or higher was regarded as of high quality. The baseline characteristics of the selected studies are summarized in Table 1.
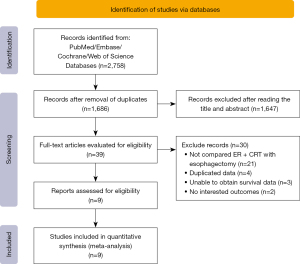
Table 1
| Author | Publication year | Country | Study type | Sample size | T stage | Pathology | Treatment regimen | Dose of RT | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Tanaka (16) | 2019 | Korea | Re | 52 | T1b | SCC | ER + CRT/ER + surgery | >50 | 7 |
| Lorenzo (17) | 2019 | France | Re | 10 | T1 | SCC | ESD + CRT/ESD + surgery | NA | 5 |
| Lu (18) | 2019 | America | Re | 501 | T1b | AC | ER + RT ± CT/surgery | NA | 5 |
| Suzuki (19) | 2018 | Japan | Re | 32 | T1b | SCC + AC | ESD + CRT/ESD + surgery | 40/50 | 6 |
| Takeuchi (21) | 2018 | Japan | Re | 32 | M3–T1b | SCC + AC | ER + CRT/ER + surgery | 50.4 | 7 |
| Koterazawa (20) | 2018 | Japan | Re | 59 | T1 | SCC | ESD + CRT/ESD + surgery | 41.4/50.4 | 8 |
| Ohki (22) | 2018 | Japan | Re | 23 | T1 | NA | ESD + CRT/ESD + surgery | NA | 6 |
| Ikeda (23) | 2015 | Japan | Re | 26 | T1 | NA | ESD + CRT/ESD + surgery | 41.4/50.4 | 5 |
| Shimizu (24) | 2004 | Japan | Re | 55 | T1 | SCC | EMR + CRT/surgery | 40–46 | 6 |
RT, radiotherapy; NOS, Newcastle-Ottawa Scale; Re, retrospective; SCC, squamous cell carcinoma; AC, adenocarcinoma; NA, not available; ER, endoscopic resection; CRT, chemoradiotherapy; ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection.
Recurrence and metastasis
Recurrence and metastasis analysis included a total of 234 patients from 7 studies (16,17,19-24). A statistically significant improvement in the recurrence and metastasis rate was observed for esophagectomy when compared to CRT after ER (OR =6.08, 95% CI: 1.96 to 18.84, P=0.002). No heterogeneity was revealed in the result (I2=0%, P=0.79) (Figure 2).

OS
A total of 6 studies contributed a total of 225 patients, including 113 who underwent esophagectomy and 112 controls (CRT after ER group) (16-17,20,22-24). No statistically significant OS difference could be established between these groups (HR =1.02, 95% CI: 0.57 to 1.82, P=0.94), with a low between-study heterogeneity (I2=0%, P=0.42) (Figure 3). A fixed effects model was chosen for remaining groups because of the minimal heterogeneity.

DFS
There were 2 studies that reported data on DFS rates, consisting of a total of 91 patients, 44 in the esophagectomy and 47 in the CRT after ER (19,20). Despite the small sample size, the results demonstrated the significant superiority of the esophagectomy over the control group (HR =0.24, 95% CI: 0.07 to 0.85, P=0.03) with insignificant heterogeneity (I2=0%, P=0.39) (Figure 4).

Sensitivity analysis and evaluation of publication bias
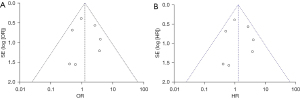
The included literature had good stability. Funnel plots were created for the primary outcome measures of recurrence and metastasis and OS (Figure 5A,5B), both of which revealed no publication bias. The results of recurrence and metastasis were from Begg’s test (P=0.072) and Egger’s test (P=0.649); the result of OS was from Begg’s test (P=1.000) and Egger’s test (P=0.847). No statistically significant difference was detected, suggesting a small bias in publication.
Discussion
The treatment of early EC is largely based on radical surgery and endoscopic treatment. The treatment plan for EC is mainly determined according to its stage. The clinical effect of ER is equivalent to that of radical surgery, but the former can be used as the first choice of treatment due to it being associated with less trauma, fewer postoperative complications, and shorter hospital stay. Moreover, for some patients with T1b stage who are at risk of lymph node metastasis, ER cannot achieve lymph node sampling or dissection, and the tumor may be incompletely removed (25). Adjuvant therapy is accepted to improve long-term survival and reduce recurrence rates. At present, the main adjuvant treatment methods are esophagectomy and CRT after ER (26). However, some controversies still exist. For one thing, we must consider the safety and effectiveness of CRT after ER before deciding whether it can completely replace radical surgery. For another, for patients with pathological high-risk factors such as lymph node metastasis or vascular nerve invasion, it must be clarified whether radical surgery can provide better long-term benefits. In this meta-analysis, we focused on analyzing the details of both sides of intervention.
Traditionally, radical esophagectomy for early EC has been a standard treatment method but with obvious treatment risks, such as lung infection or stenosis, anastomotic leakage, and even death. Yang et al. (27) conducted a retrospective study including a total of 179 patients. Lung infection and esophageal perforation occurred in the radical surgery group, yet no serious complications occurred in the CRT after ER group. In the radical surgery group, the local recurrence rate was 1%, the LRR was 5%, and DMR was 4%; in addition, both local and regional recurrences in the CRT after ER group were 8%, and no distant metastasis was found. No significant differences were identified in the 5-year OS rate between the 2 groups (P=0.405). A was shown in Figure 2, the 9 articles included in our study involved a total of 790 patients with early EC, and the recurrence and metastasis rate of the CRT after ER group was 6.08 times that of the radical surgery group. Further, in the radical surgery group, DFS was also better than CRT after ER (HR =0.24, 95% CI: 0.07 to 0.85, P=0.03). There were 2 studies that reported radical surgery can achieve better disease control for tumors ≥ SM2 with lymphatic vascular invasion (19,20). We identified 2 reasons to explain why radical surgery can achieve better performance. Firstly, compared to ER, esophagectomy can completely remove the lesion and perform lymph node dissection, thereby obtaining a more accurate pathological staging and long-term prognosis judgment. Secondly, the lack of accurate pathological staging after ER contributed to insufficient radiation field exposure. The study by Lee et al. also reported similar views (28). Additionally, the included literature showed that no significant differences were found in OS. The results were basically consistent with the retrospective study conducted by Yang et al. (27). A single arm confirmatory Japanese study (JCOG0508) (29) reported on 176 patients with stage I ESCC treated with ER (combined with CRT or not) in 2016. A total of 96 patients were treated with CRT; the 3-year OS was 92.6% among all patients and 90.7% in the CRT group. Therefore, the study concluded that the efficacy of ER combined with adjuvant CRT for T1b esophageal SCC is equivalent to that of radical surgery. In 2020, Tsou et al. (30) conducted a review study, summarizing and reviewing 3 previous related studies, and concluded that patients with a high risk of recurrence should be recommended to use radical surgery. Although the thoughts and conclusions of the current analysis are similar to previously reported results, a total of 9 documents were included herein, making the conclusions more credible and practical.
No differences in survival benefits were found between the 2 treatment strategies according to our study. Compared to adjuvant CRT, radical esophagectomy, as a highly invasive operation with a high incidence rate of postoperative complications (40–50%) and mortality (2.0–9.5%), is not suitable for elderly patients or patients with more complications, yet it can prolong the hospital stay and reduce the quality of life of patients after operation, and these patients should consider adjuvant CRT (31). This study analyzed the application value of CRT after ER of early EC, and argued that CRT can be the preferred choice for the elderly, and patients who are in poor physical condition or unable to tolerate surgery. However, there are no large-scale RCTs to confirm these observations and an ongoing multicenter RCT from China is expected to provide new insights into treatment options for patients with early-stage esophageal cancer (Chest 201908, ClinicalTrials.gov number: NCT04135664) (32). Meanwhile, early EC should be evaluated by a multidisciplinary team, including the participation of gastroenterology, oncology, and thoracic surgeons, all striving to select a reasonable treatment plan.
This study has certain limitations. First, the included studies are basically non-RCTs, most studies are limited to single-institution case series, small sample size, and retrospective study design. Large amounts of retrospective data in studies can create uncertainties and questions about final conclusions that should be addressed with more RCTs. Selection bias in retrospective studies may be unavoidable unless the propensity score matching method is employed. Besides, due to trust in the efficacy of traditional surgery, patients with a greater risk of tumor recurrence are more likely to undergo esophagectomy, whereas those with a relatively low probability of recurrence may undergo CRT after ER. This difference in subjective choice may be increasing the observable curative effect of CRT after ER, thereby weakening the observable curative effect of radical surgery. Nevertheless, our research results show that surgical resection has better DFS and less probability of metastasis and recurrence, which highlights the reliability of our results. Similarly, in the included literature, the total number of participants from Lu et al. (18) is 501, but the number of patients in the radical surgery group is as high as 477 (78%). Considering the overall stability of the research results, the relevant research results were not included for overall comparison. This reduced the overall sample size of the study. In addition, of the 9 articles included in this study, 6 articles are from Japan, and relatively few are from Europe and America. Furthermore, the included articles mainly focus on long-term effects and do not provide detailed short-term effects, such as postoperative complications, radiotherapy and chemotherapy toxicity. Therefore, it is impossible to detect the difference in the recent treatment effects of the 2 methods in a more comprehensive manner. Last, due to the limitations of the included literature data, this study did not conduct further subgroup analysis on the tumor staging of patients, and failed to clarify the difference in the efficacy of radical surgery and CRT after ER for specific substages.
Conclusions
For the adjuvant treatment after ER of early EC, CRT cannot completely replace esophagectomy, and both are equally essential treatments in survival benefits. For patients with tumor invasion ≥ SM2 or high risk of lymph node metastasis, radical surgery is recommended. Moreover, for patients with high risk factors for surgery or intolerance, CRT is the preferred option, but this conclusion still needs to be supported by more large-scale and sample RCTs.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-376/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-376/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-376/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018;154:360-73. [Crossref] [PubMed]
- Nelson DB, Dhupar R, Katkhuda R, et al. Outcomes after endoscopic mucosal resection or esophagectomy for submucosal esophageal adenocarcinoma. J Thorac Cardiovasc Surg 2018;156:406-413.e3. [Crossref] [PubMed]
- Marino KA, Sullivan JL, Weksler B. Esophagectomy versus endoscopic resection for patients with early-stage esophageal adenocarcinoma: A National Cancer Database propensity-matched study. J Thorac Cardiovasc Surg 2018;155:2211-2218.e1. [Crossref] [PubMed]
- Mönig S, Chevallay M, Niclauss N, et al. Early esophageal cancer: the significance of surgery, endoscopy, and chemoradiation. Ann N Y Acad Sci 2018;1434:115-23. [Crossref] [PubMed]
- Shen W, Shen Y, Tan L, et al. A nomogram for predicting lymph node metastasis in surgically resected T1 esophageal squamous cell carcinoma. J Thorac Dis 2018;10:4178-85. [Crossref] [PubMed]
- Saeki H, Watanabe M, Mine S, et al. Esophagectomy for superficial esophageal cancer after non-curative endoscopic resection. J Gastroenterol 2015;50:406-13. [Crossref] [PubMed]
- Minashi K, Nihei K, Mizusawa J, et al. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2019;157:382-390.e3. [Crossref] [PubMed]
- Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today 2020;50:12-20. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:ED000142. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139-45. [Crossref] [PubMed]
- Ng KH, Peh WC. Presenting the statistical results. Singapore Med J 2009;50:11-4.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Tanaka T, Ueno M, Iizuka T, et al. Comparison of long-term outcomes between esophagectomy and chemoradiotherapy after endoscopic resection of submucosal esophageal squamous cell carcinoma. Dis Esophagus 2019;32:doz023. [Crossref] [PubMed]
- Lorenzo D, Barret M, Leblanc S, et al. Outcomes of endoscopic submucosal dissection for early oesophageal squamous cell neoplasia at a Western centre. United European Gastroenterol J 2019;7:1084-92. [Crossref] [PubMed]
- Lu DJ, David J, Anderson E, et al. Alternative Strategies to Esophagectomy in the Management of T1b Esophageal Adenocarcinoma. Int J Radiat Oncol 2019;105:E191.
- Suzuki G, Yamazaki H, Aibe N, et al. Endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer: choice of new approach. Radiat Oncol 2018;13:246. [Crossref] [PubMed]
- Koterazawa Y, Nakamura T, Oshikiri T, et al. A comparison of the clinical outcomes of esophagectomy and chemoradiotherapy after noncurative endoscopic submucosal dissection for esophageal squamous cell carcinoma. Surg Today 2018;48:783-9. [Crossref] [PubMed]
- Takeuchi M, Suda K, Hamamoto Y, et al. Technical feasibility and oncologic safety of diagnostic endoscopic resection for superficial esophageal cancer. Gastrointest Endosc 2018;88:456-65. [Crossref] [PubMed]
- Ohki S, Hikichi T, Yamada L, et al. Evaluation of additional treatment after non-curative endoscopic submucosal resection for esophageal cancer. Dis Esophagus 2018;31:147-8.
- Ikeda A, Hoshi N, Yoshizaki T, et al. Endoscopic Submucosal Dissection (ESD) with Additional Therapy for Superficial Esophageal Cancer with Submucosal Invasion. Intern Med 2015;54:2803-13. [Crossref] [PubMed]
- Shimizu Y, Kato M, Yamamoto J, et al. EMR combined with chemoradiotherapy: a novel treatment for superficial esophageal squamous-cell carcinoma. Gastrointest Endosc 2004;59:199-204. [Crossref] [PubMed]
- Nagami Y, Ominami M, Shiba M, et al. The five-year survival rate after endoscopic submucosal dissection for superficial esophageal squamous cell neoplasia. Dig Liver Dis 2017;49:427-33. [Crossref] [PubMed]
- Kitagawa Y, Ishihara R, Ishikawa H, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus 2023;20:343-72.
- Yang AJ, Choi SH, Byun HK, et al. Management of Clinical T1N0M0 Esophageal Cancer. Gut Liver 2019;13:315-24. [Crossref] [PubMed]
- Lee HJ, Lee H, Park JC, et al. Treatment Strategy after Endoscopic Resection of Superficial Esophageal Squamous Cell Carcinoma: A Single Institution Experience. Gut Liver 2015;9:714-9. [Crossref] [PubMed]
- Muto M, Minashi K, Nihei K, et al. Efficacy of combined endoscopic resection and chemoradiotherapy for clinical stage I esophageal squamous cell carcinoma (ESCC): A single-arm confirmatory study (JCOG0508). J Clin Oncol 2016;34.
- Tsou YK, Lee CH, Le PH, et al. Adjuvant therapy for pT1a-m3/pT1b esophageal squamous cell carcinoma after endoscopic resection: Esophagectomy or chemoradiotherapy? A critical review. Crit Rev Oncol Hematol 2020;147:102883. [Crossref] [PubMed]
- Ahmed O, Ajani JA, Lee JH. Endoscopic management of esophageal cancer. World J Gastrointest Oncol 2019;11:830-41. [Crossref] [PubMed]
- Yang Y, Su Y, Zhang X, et al. Esophagectomy versus definitive chemoradiotherapy for patients with clinical stage N0 and pathological stage T1b esophageal squamous cell carcinoma after endoscopic submucosal dissection: study protocol for a multicenter randomized controlled trial (Ad-ESD Trial). Trials 2020;21:603. [Crossref] [PubMed]
(English Language Editor: J. Jones)


