
Comparison of uniport versus triport thoracoscopic single or combined basal segmentectomy for stage IA lung cancer
Highlight box
Key findings
• UTBS provided similar perioperative outcomes and oncology prognosis compared to TTBS for stage IA basal segmental lung cancer.
What is known and what is new?
• As our previous study reported, TTBS was reliable and feasible.
• Our study further filled the gap regarding the perioperative outcomes, and oncological prognosis of UTBS via a single-direction approach and further evaluate the perioperative and oncological outcomes of UTBS with that of TTBS.
What is the implication, and what should change now?
• UTBS has potential advantages, such as better pain control and less blood loss. UTBS is recommended for stage IA basal segmental lung cancer.
Introduction
With the popularity of low-dose spiral computed tomography (CT), more small pulmonary nodules, especially ground glass nodules, are detected, and thus reducing tumor-related deaths (1). In 2022, the results of JCOG0802/WJOG4607L (2) released by the Japan Clinical Oncology Group showed that for peripheral non-small cell lung cancer (NSCLC) of 2 cm or less in size, segmentectomy is not inferior to lobectomy in terms of overall survival (OS) and recurrence-free survival (RFS). Recently, Cancer and Leukemia Group B (CALGB) reported their multicenter randomized clinical trial [CALGB140503 (3)], which similarly showed that sublobar resection is not inferior to lobar resection in terms of disease-free survival (DFS) and OS in patients with peripheral NSCLC (T1aN0 <2 cm). Over the past decades, segmentectomy has primarily been carried out using a minimally invasive technique. Studies (4,5) have shown that uniport thoracoscopic surgery, which is increasingly used in segmentectomy, has comparable short- and long-term outcomes to triport thoracoscopic surgery. In addition, uniport thoracoscopic surgery has potential advantages, such as better pain control and cost-effectiveness (6).
Single or combined basal segmentectomy (CBS), excluding common basal segmentectomy, is the most difficult of all types of segmentectomies due to the deeper location of the hilar structures, more structural variation, and more complex intersegmental plane adjacencies. Currently, only a few studies (7,8) have reported on thoracoscopic anatomical basal segmentectomy, and few studies have reported on the uniport approach (9). We previously published the largest case series on basal segmentectomy (10) with single-direction lobectomy (11) and we gradually began exploring uniport thoracoscopic basal segmentectomy (UTBS) and had good results (12). There is no definite criterion for surgeons to determine whether a uniport or triport thoracoscopic surgery should be performed when a basal segmentectomy is needed. On the basis of these studies mentioned, we conduct this study to explore the differences in perioperative and oncological outcomes between UTBS and triport thoracoscopic basal segmentectomy (TTBS). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-477/rc).
Methods
Patient selection
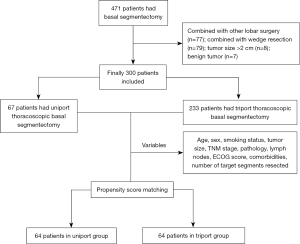
Between April 2015 and May 2022, 300 patients who met the inclusion and exclusion criteria were consecutively included and divided into the UTBS group (n=67) and the TTBS group (n=233) according to the surgical incisions used. The patients who underwent different port approaches were not selected, but from different periods. The surgeons were from the same team, and up until 2019, they mainly performed TTBS. After 2019, almost all surgeons switched from TTBS to UTBS (Figure S1). Clinical data and follow-up data of all patients were obtained from the Western Lung Cancer Database. This study was approved by the institutional review board of West China Hospital (No. 2023-0138) and conducted according to the Declaration of Helsinki (as revised in 2013). In this study, each patient provided written informed consent for surgery and the publication of the study data.
The inclusion criteria included: (I) pulmonary nodules with a diameter of <2 cm, ground-glass opacity (GGO) composition ≥50%; (II) underwent uniport or triport thoracoscopic intended basal segmentectomy; (III) pathological diagnosis of lung cancer. The exclusion criteria: (I) compromised segmentectomy due to poor cardiopulmonary function; (II) combined with other lobar operation (lobectomy, segmentectomy, or wedge resection); (III) Robot-assisted thoracoscopic basal segmentectomy; (IV) simultaneous bilateral segmentectomy; (V) Previous surgical history of lung cancer. (VI) common basal segmentectomy such as left S8+9+10 resection and right S7+8+9+10 resection (Figure 1).
The surgical margin was more than 2 cm or greater than the maximum diameter of the tumor. CBS was planned for patients with lesions close to the intersegmental boundaries. Thus, surgical methods were split into single segmentectomies and combined dual- or tri-segmentectomies based on where the target nodule was located. Specimens were sent for intraoperative frozen-section pathology to determine if the resection margins were adequate. All adenocarcinoma cases were staged and histologically classified using the 8th edition of the TNM staging system (13) and the new proposed histological classification system (14).
Operative procedure
Preoperative preparation
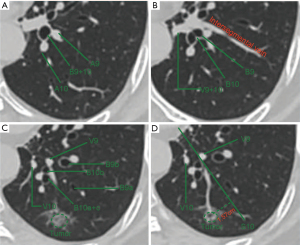
All patients had standard preoperative preparation, including hematological, biochemical, and cardiopulmonary function tests, as well as imaging tests of the brain, lung, upper abdomen, and bone. The location and adjacent structures of the target nodules, anatomical variation, and positional relationship of the bronchi and blood vessels in the basal segment were carefully assessed and identified on high-resolution computed tomography (HRCT) to design an appropriate surgical resection (Figure 2A-2D) (15).
Surgical procedures
Incision strategy
The observation, main operation, and auxiliary ports were in the 7th, 4th, and 9th intercostal spaces (ICS) at the midaxillary line, the anterior axillary line, and the posterior axillary line, respectively. The uniport incision (4 cm) was performed in the 4th or 5th ICS across the midaxillary line, according to the surgeon’s preference (port strategies are shown in Figure 3). The target nodule was localized by tactile sensation, and marking was made by sutures ligating on the surface of the visceral pleura (16). All surgical procedures were performed in a single-direction approach through the inferior pulmonary ligament (17) or the interlobar fissure approach. When performing segmentectomies of S9 and/or S10, the trans-inferior ligament approach was routinely selected. When performing segmentectomies of S7 and/or S8, the interlobar fissure approach may be adopted if the interlobar fissure is complete; otherwise, the transinferior pulmonary ligament approach should be preferred.

Inferior pulmonary ligament approach
The sequence of the approach through the inferior pulmonary ligament was vein-bronchial-arterial. First, the inferior pulmonary vein was dissected from the surface of the lower lobe to the inside along the inferior pulmonary ligament. The vein of S6 was identified afterwards. Under the guidance of preoperative CT images, the vein of the target segment and its branches were clearly identified, clamped and divided by retracting the target segment to the other side while preserving the intersegmental veins. The lower pulmonary bronchus emerged immediately after the target segmental vein was dissected. Later, the basal segment bronchus and its bifurcations were dissected. According to the principle of the “stem-branch” method (15) and referring to the preoperative CT images, the main stem and bifurcations of the target basal bronchus were tracked and dissected. Then, the target bronchus was clamped subsequently, the lung on the operative side was inflated to further confirm the target bronchus. The accompanying feeding pulmonary artery of the target segment appeared after the target basal segmental bronchus was divided using a stapler. When the artery was clamped and divided, the final step was to process the intersegmental planes. The intersegmental demarcation line was marked with electrocautery after indocyanine green (18) intravenous injection or showed via the manner of inflation-deflation (19). Finally, the intersegmental pulmonary parenchyma was dissected with the aid of a stapler and energy device along with the intersegmental demarcation line. Finally, a single-direction thoracoscopic basal segmentectomy was completed.
Trans-interlobar fissure approach
The target artery, bronchus, vein, and intersegmental planes were sequentially accessed as they appeared, proceeding in a single direction.
Lymph node dissection
The tumor and 13 lymph nodes were sent for frozen-section pathological examination during the surgery. Once the primary cancer was diagnosed, the hilar and mediastinal lymph nodes were dissected. For GGOs with pathologically diagnosed lymph node-negative conditions, we sometimes performed systematic sampling (20) rather than further systemic lymph node dissection. A thoracic drainage tube was inserted from the posterior mediastinum to the apex of the chest, and then the incision was closed after ensuring proper hemostasis and passing the air leakage test.
Follow-up
All patients required regular pulmonary CT examination every 3 or 6 months postoperatively. Follow-up data were obtained from the medical center records or from patients or their relatives by telephone. If a patient was lost to follow-up, their survival information was taken from the National Death Registry as a substitute.
Data collection
The clinical and demographic data collected from patients included age, sex, smoking status, comorbidities, Eastern Cooperative Oncology Group (ECOG) score (21), tumor size, pathological tumor, node, and metastasis (TNM) stage, lymph node dissection or sampling, pathology subtypes, and number of target segments resected (according to the location of the target nodule, surgical methods were divided into single segmentectomies and combined dual- or tri-segmentectomies).
Outcome variables collected from patients included intraoperative conversion to thoracotomy, operative time, intraoperative blood loss, number of lymph nodes and lymph node stations harvested, duration days of chest tube drainage, postoperative hospital stay, incidence of postoperative complications (pulmonary infection, prolonged air leakage, persistent drainage, cerebrovascular accident), perioperative 30- and 90-day mortality, and survival data. The intraoperative blood loss was judged by the size of the collecting bottle connected to the negative pressure suction device. The duration of chest tube drainage could reflect the early postoperative recovery condition. Pulmonary infections were based on the following criteria: chest radiographs indicating pulmonary infection, at least one examination term (such as fever above 38 ℃ or an abnormal white blood cell count less than 4×109/L or greater than 12×109/L) or at least two symptoms (such as abnormal changes in respiratory secretions or a new or aggravated cough). Prolonged air leakage was defined as persistent pulmonary leakage for more than 5 days. Persistent drainage was defined as drainage time exceeding 7 days. Perioperative 30- and 90-day mortality were defined as any death within the first 30 or 90 days after the operation or hospitalization, respectively. Tumor recurrence in the ipsilateral lung, hilar, and mediastinal lymph nodes was defined as locoregional recurrence. Distant metastases were defined as tumor metastases in other organs (liver, bone, and brain). RFS was defined as the time interval from the date of surgery to the date of cancer-related recurrence or last follow-up. OS was calculated as the time interval from operation to any death or last follow-up.
Statistical analyses
Continuous variables following a normal distribution were presented as the mean ± standard deviation. Variables with nonnormal distributions were expressed as medians [interquartile ranges (IQRs)]. Data were presented as percentages and proportions for categorical variables. t-tests and U tests were used to compare continuous variables. Pearson χ2 test or Fisher’s exact test was used for categorical variables. To minimize potential selection bias, we performed 1:1 PSM. Propensity scores were calculated using a logistic regression model based on 10 variables: age, sex, smoking status, tumor size, TNM stage, pathology, lymph nodes, ECOG score (21), comorbidities, and the number of target segments resected. The matching tolerance was 0.02, and the matching method was nearest neighbor matching. We performed statistical analysis of the clinical characteristics of the overall and matched cohorts for all patients. Because cases in the uniport group were included since 2019, we chose the 3-year RFS rate and OS rate as the outcome indicators. Recurrence was observed only in the overall cohort and not in the matched cohort. No patient death was observed at the time of the follow-up cutoff. Therefore, the survival analysis was conducted only for RFS in the overall cohort. To ensure baseline comparability between groups, only nonmucinous adenocarcinoma cases (n=297) were included in the survival analysis. The RFS of the UTBS and TTBS groups was analyzed by the Kaplan-Meier (KM) method and the log-rank test. Univariate and multivariate Cox proportional hazards regression models were used to analyze variables for 3-year RFS. We applied Firth’s penalized partial likelihood to correct Cox regression models because the UTBS group had zero recurrence events and the partial likelihood converged to a finite value on survival analysis (22). Data analysis was performed using SPSS version 26.0. Survival curves were drawn by GraphPad Prism 8.0 software. A P<0.05 was considered statistically significant.
Subgroup analyses
In clinical practice, we believe that the number of lung segments resected could have an impact on operative time and intraoperative blood loss. Thus, subgroup analyses were conducted for the operative time and intraoperative blood loss between the single basal segmentectomy subgroup and the CBS subgroup.
Results
Clinical characteristics
From April 2015 to May 2022, 300 patients were included in this study (67 patients in the UTBS group and 233 patients in the TTBS group) (Figure 1). There were 101 males and 199 females, with an average age of 49.55±11.28 years and a median follow-up time of 17 months (IQR, 10–20) in the UTBS group and 30 months (IQR, 20–45) in the TTBS group. The frequency of CBS was 47.8% (32 cases) and 38.2% (89 cases) in the UTBS and TTBS groups, respectively. Postoperative pathology revealed that there were 43 patients with stage IA1 and 21 patients with stage IA2 in the UTBS, 141 patients with stage IA1 and 79 patients with stage IA2 in the TTBS. Pathological examination confirmed that none of the patients had cancerous cells involved in their surgical margins. Forty-three patients underwent lobe-specific hilar and mediastinal lymph node dissection in the UTBS, while in the TTBS group, 127 patients underwent the same procedure. Systemic hilar and mediastinal lymph node dissection was conducted for 19 cases in the UTBS group and 77 cases in the TTBS group. Systemic hilar and mediastinal lymph node sampling was performed for 5 patients in the UTBS group and 29 patients in the TTBS group. Two patients were confirmed lymph node invasion (1 case each of N1 and N2). Both patients declined adjuvant therapy and were followed regularly. The clinical characteristics of the 300 patients before and after PSM are shown in Table 1. After PSM, all baseline clinical variables were well balanced across the two groups. The details of single basal segmentectomy and CBS of the overall and matched cohorts can be found in Table S1.
Table 1
| Variables | Overall cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| UTBS (n=67) | TTBS (n=233) | P value | UTBS (n=64) | TTBS (n=64) | P value | ||
| Age (years) | 50.33±12.04 | 49.32±11.07 | 0.521 | 50.41±12.17 | 49.83±12.10 | 0.788 | |
| Sex, n (%) | 0.297 | 0.552 | |||||
| Male | 19 (28.4) | 82 (35.2) | 19 (29.7) | 16 (25.0) | |||
| Female | 48 (71.6) | 151 (64.8) | 45 (70.3) | 48 (75.0) | |||
| Smoking status, n (%) | 0.441 | 1.000 | |||||
| Yes | 13 (19.4) | 36 (15.5) | 11 (17.2) | 11 (17.2) | |||
| No | 54 (80.6) | 197 (84.5) | 53 (82.8) | 53 (82.8) | |||
| Comorbidities, n (%) | 0.793 | 0.122 | |||||
| Yes | 23 (34.3) | 76 (32.6) | 23 (35.9) | 15 (23.4) | |||
| No | 44 (65.7) | 157 (67.4) | 41 (64.1) | 49 (76.6) | |||
| ECOG score, n (%) | 0.163 | 0.713 | |||||
| 0 | 43 (64.2) | 121 (51.9) | 40 (62.5) | 42 (65.6) | |||
| 1 | 24 (35.8) | 110 (47.2) | 24 (37.5) | 22 (34.4) | |||
| ≥2 | 0 (0.0) | 2 (0.9) | |||||
| Tumor size | 0.517 | 0.699 | |||||
| ≤1 cm | 46 (68.7) | 150 (64.4) | 44 (68.8) | 46 (71.9) | |||
| >1 cm | 21 (31.3) | 83 (35.6) | 20 (31.3) | 18 (28.1) | |||
| Pathological tumor, node, and metastasis | 0.964 | 0.395 | |||||
| TNM stage (pTNM), n (%) | |||||||
| TisN0M0 | 3 (4.5) | 11 (4.7) | 3 (4.7) | 7 (10.9) | |||
| T1aN0M0 | 43 (64.2) | 141 (60.5) | 41 (64.1) | 40 (62.5) | |||
| T1bN0M0 | 21 (31.3) | 79 (33.9) | 20 (31.3) | 17 (26.6) | |||
| T1bN1or 2M0 | 0 (0.0) | 2 (0.9) | |||||
| Lymph nodes, n (%) | 0.063 | 1.000 | |||||
| Lobe-specific or Systemic dissection | 62 (92.5) | 204 (87.6) | 61 (95.3) | 60 (93.8) | |||
| Systemic sampling | 5 (7.5) | 29 (12.4) | 3 (4.7) | 4 (6.3) | |||
| Pathology, n (%) | 1.000 | ||||||
| Adenocarcinoma | 67 (100.0) | 230 (98.7) | 0.435 | 64 (100.0) | 64 (100.0) | 0.588 | |
| AIS or AAH | 4 (6.0) | 11 (4.8) | 4 (6.3) | 7 (10.9) | |||
| MIA | 39 (58.2) | 117 (50.9) | 38 (59.4) | 44 (48.9) | |||
| IA | 24 (35.8) | 102 (44.3) | 22 (34.4) | 23 (35.9) | |||
| Others | 0 (0.0) | 3 (1.3) | |||||
| Number of target segments resected, n (%) | 0.325 | 0.659 | |||||
| Single segment resection | 35 (52.2) | 144 (61.8) | 35 (54.7) | 40 (62.5) | |||
| Two segments resection | 27 (40.3) | 77 (33.0) | 26 (40.6) | 22 (34.4) | |||
| Three segments resection | 5 (7.5) | 12 (5.2) | 3 (4.7) | 2 (3.1) | |||
Data are presented as mean ± standard deviation for continuous variables and number (frequency)/No. (%) for categorical variables. VATS, video-assisted thoracic surgery; AIS, adenocarcinoma in situ; UTBS, uniport thoracoscopic basal segmentectomy; TTBS, triport thoracoscopic basal segmentectomy; AAH, atypical adenomatous hyperplasia; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma; ECOG, Eastern Cooperative Oncology Group.
Perioperative outcomes
Perioperative outcomes of the patients in the different groups before and after PSM are shown in Table 2. No cases in the UTBS group converted to TTBS, and no cases intraoperatively converted to thoracotomy. Perioperative 30- or 90-day mortality was not observed in either group. In the matched cohort, the median intraoperative blood loss (IBL) [20 (IQR, 10–20) mL] in the UTBS group was significantly less than that [30 mL (IQR, 20–50)] in the TTBS group (P=0.001). Stratified analysis showed that the median IBL of the UTBS group was also less than that of the TTBS group upon single basal segmentectomy (SBS) (P=0.009) and CBS (P=0.019) (Table 2). Subgroup analysis showed that the IBL were similar between SBS subgroup and CBS subgroup (P=0.110) (Table S2). Although there was no significant difference in the operative time between UTBS and TTBS (120 vs. 115 min, P=0.841), subgroup analysis showed that the operative time of SBS was less than that of CBS (110 vs. 120 min, P=0.002) (Table S2). There was no significant difference in the number of lymph nodes (P=0.856) and lymph node stations (P=0.561) harvested, duration of chest tube drainage (P=0.098) or postoperative hospital stay (P=0.330) between the two groups.
Table 2
| Variables | Overall cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| UTBS [n=67] | TTBS [n=233] | P value | UTBS [n=64] | TTBS [n=64] | P value | ||
| Operative time (min) | 120 [100–125] | 113 [90–132] | 0.415 | 120 [95–123.5] | 115 [95–125.5] | 0.841 | |
| Single segment resection | 115 [80–120] | 103 [85–125] | 0.851 | 112.5 [70–120] | 105 [90–125] | 0.872 | |
| Multiple segments resection* | 120 [117.5–142.5] | 120 [100–155] | 0.255 | 120 [113.75–126.25] | 120 [106–157.5] | 0.758 | |
| Intraoperative blood loss (mL) | 20 [12.5–20] | 20 [20–40] | 0.016 | 20 [10–20] | 30 [20–50] | 0.001 | |
| Single segment resection | 20 [20–20] | 20 [10–30] | 0.068 | 20 [17.5–20] | 30 [10–50] | 0.009 | |
| Multiple segments resection | 20 [10–30] | 20 [20–50] | 0.076 | 20 [10–30] | 30 [25–50] | 0.019 | |
| Number of LNs harvested | 5 [4–6] | 5 [4–6] | 0.473 | 5 [4–6] | 5 [4–6] | 0.856 | |
| Number of LN stations harvested | 4 [4–5] | 4 [3–5] | 0.399 | 4 [4–5] | 4 [3–5] | 0.561 | |
| Duration of chest tube drainage (day) | 2 [2–3] | 2 [2–3] | 0.085 | 2 [2–3] | 2 [2–3] | 0.098 | |
| Postoperative hospital stay (day) | 3 [3–4] | 4 [3–4] | 0.245 | 3 [3–4] | 4 [3–4] | 0.330 | |
| Postoperative complications, n (%) | 0.523 | 0.718 | |||||
| Yes | 4 (6.0) | 10 (4.3) | 3 (4.7) | 5 (7.8) | |||
| No | 63 (94.0) | 223 (95.7) | 61 (95.3) | 59 (92.2) | |||
Values are presented as median [interquartile range] or number (percentage). *, because the total number of three segments resected was too small, we combined the two segments and the three segments resected into one group. UTBS, uniport thoracoscopic basal segmentectomy; TTBS, triport thoracoscopic basal segmentectomy; LN, lymph node.
The postoperative complication rate of the overall cohort was 4.67% (14/300). In the matched cohort, the postoperative complication rate was 6.25% (6/128), as demonstrated in Table 3. The difference was not statistically significant (P=0.643).
Table 3
| Postoperative complication | Overall cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| UTBS (n=4) | TTBS (n=10) | P value | UTBS (n=3) | TTBS (n=5) | P value | ||
| Pulmonary infection | 3 | 7 | 1.000 | 2 | 4 | 0.643 | |
| Prolonged air leakage (>5 d) | 0 | 1 | 0 | 1 | |||
| Persistent drainage (>7 d) | 1 | 1 | 1 | 0 | |||
| Cerebrovascular accident | 0 | 1 | 0 | 0 | |||
UTBS, uniport thoracoscopic basal segmentectomy; TTBS, triport thoracoscopic basal segmentectomy.
Survival outcomes
The median follow-up times of the overall cohort and matched cohort in this study were 27 (IQR, 17–42) months and 20 (IQR, 15–35) months, respectively. The 3-year RFS of the overall cohort was 98.3%, and recurrence occurred in 0 and 5 patients in the UTBS group and TTBS group, respectively (HR: 0.306, 95% CI: 0.013–7.251, P=0.464, Figure 4). The recurrence pattern was composed of 3 locoregional recurrences and 2 distant metastases (one case of brain metastasis and the other of bone metastasis). Among the cohort of patients with local recurrence, there were two cases of stage IA2 lung cancer and one case of stage IA1 lung cancer. The recurrences were observed at 23, 9, and 80 months of follow-up, respectively. All the patients with recurrences were alive at the 3-year follow-up (the detailed results were presented in Table S3). The 3-year OS of the overall cohort was 100%. In univariate and multivariate analyses, no variables were calculated as independent prognostic factors for RFS, and the surgical approach (UTBS vs. TTBS) was not an independent risk factor for RFS (HR: 1.120, 95% CI: 0.342–13.051, P=0.879; HR: 1.399, 95% CI: 0.402–16.472, P=0.643) (Table 4).

Table 4
| Variables | Recurrence-free survival | ||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis* | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 1.063 (0.977, 1.158) | 0.157 | 1.072 (0.978, 1.186) | 0.138 | |
| Sex (male vs. female) | 6.707 (0.743, 885.926) | 0.101 | 7.340 (0.770, 980.876) | 0.090 | |
| Smoking status | 0.528 (0.004, 4.962) | 0.641 | |||
| Comorbidities | 1.359 (0.183, 10.115) | 0.764 | |||
| ECOG score | 0.255 | ||||
| 0 (ref) | |||||
| 1 | 2.866 (0.473, 28.808) | ||||
| ≥2 | 14.813 (0.541, 329.940) | 0.115 | |||
| Tumor size | 2.859 (0.306, 26.748) | 0.357 | 1.209 (0.103, 14.168) | 0.880 | |
| Surgical types (UTBS vs. TTBS) | 1.120 (0.342, 13.051) | 0.879 | 1.399 (0.402, 16.472) | 0.643 | |
| Lymph nodes (dissection vs. sampling) | 1.291 (0.137, 171.146) | 0.860 | 1.440 (0.145, 193.439) | 0.800 | |
| Histology subtypes | |||||
| AIS/AAH (ref) | |||||
| MIA | 0.143 (0.001, 5.175) | 0.344 | 0.378 (0.001, 1,304.87) | 0.759 | |
| IA | 1.791 (0.137, 578.068) | 0.756 | 2.983 (0.224, 24,397.2) | 0.495 | |
| Number of target segments resected | |||||
| Single segment resection (ref) | |||||
| Two segments resection | 3.201 (0.618, 19.352) | 0.160 | |||
| Three segments resection | 3.257 (0.023, 42.850) | 0.508 | |||
*, although the results of univariate analysis showed that none of the variables reached the P<0.1 threshold, these variables were clinically associated with lung cancer recurrence. Thus, multivariate analysis was still conducted for these variables. CI, confidence interval; HR, hazard ratio; UTBS, uniport thoracoscopic basal segmentectomy; TTBS, triport thoracoscopic basal segmentectomy; AIS, adenocarcinoma in situ; AAH, atypical adenomatous hyperplasia; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma.
Discussion
This study retrospectively included 300 patients to estimate the perioperative outcomes and survival results of UTBS and TTBS. Our study further filled the gap regarding the perioperative outcomes and oncological prognosis of UTBS via a single-direction approach. The results showed that the perioperative and mid-term oncological outcomes of UTBS and TTBS were comparable. Furthermore, UTBS was associated with less IBL.
Over the past decade, JCOG0802 (2), JCOG0804 (23), and JCOG1211 (24) have all reported excellent prognostic results of segmentectomy and wedge resection in mainly stage T1 (tumor size ≤2 cm) N0 lung cancer patients. Recently, another multicenter randomized controlled trial CALGB140503 (3), showed sublobar resection (segmentectomy accounting for 37.9%) had similar oncological results as lobar resection in lung cancer patients of stage T1 (tumor size ≤2 cm) N0. Although there are many studies on segmentectomy, only a few studies with small sample sizes (7,8,25,26) have focused on thoracoscopic basal segmentectomy. In 2015, Kikkawa et al. (25) studied complete thoracoscopic S9 or S10 segmentectomy in 23 patients using a pulmonary ligament approach. In 2017, Endoh et al. (26) reported the novel posterior approach to perform thoracoscopic S10 segmentectomy in 20 patients. Nevertheless, these reports were all completed under a multiport thoracoscopic strategy. At present, studies on thoracoscopic basal segmentectomy through a uniport approach are few (12,27). Moreover, studies comparing the oncological outcomes of uniport and triport thoracoscopic basal segmentectomy are lacking.
In the present study, we compared the perioperative and oncological outcomes between the UTBS and TTBS groups. Analysis from the overall cohort and the matched cohort together showed that the operative time was comparable between the two groups (P=0.415, P=0.841). The median IBL in the UTBS group was less than that in the TTBS group (P=0.016, P=0.001). A possible reason could be that one incision in UTBS oozes less blood than three incisions in TTBS.
Robotic anatomic segmentectomy is being increasingly performed, and previous study (28) showed that robotic segmentectomy achieves similar intraoperative blood loss and shorter median operative time comparing with our study. However, there is still a lack of literature specifically investigating robot-assisted basal segmentectomies, either single or combined with other lower lobe segments. Further research is required to compare oncological outcomes between robotic and thoracoscopic basal segmentectomy, regardless of uniportal or triport surgeries.
For the first time, the mid-term oncological outcomes of patients with peripheral GGO-predominant nodules ≤2 cm in size who underwent thoracoscopic basal segmentectomy were demonstrated. The local recurrence rate of our basal segmentectomy cohort stands at 1.0% (3/297), mirroring the comparable 1.8% (1/56) recurrence rate observed in the segmentectomy subgroup of JCOG0804 (23). However, the local recurrence rate in the segmentectomy group in JCOG 0802/WJOG 4607L (2) was as high as 10.5% (58/552), with surgical margin recurrence accounting for 19.0% (11/58) of those cases. Unfortunately, these studies did not separately report the recurrence date of the basal segmentectomy. The final data of our study showed that three locoregional recurrences, two distant metastases, and zero deaths were observed in our segmentectomy cohort. The segmentectomy group in our study had an overall (local plus distant) recurrence rate of only 1.7% (5/297), which was substantially lower than the 12.1% (67/552) of JCOG 0802/WJOG 4607L (2) and 30.4% (102/336) of CALGB140503 (3). The low recurrence rate in our study can be attributed to the following reasons. First, the 3-year follow-up period in our study was too short. Second, the vein-branch-artery proceeding sequence in the single-direction approach, which may reduce repeated turnover of lung and tumor cell dissemination (29). Third, the JCOG0802 and CALGB140503 primarily enrolled patients with predominantly solid pulmonary nodules, whereas our study focuses on nodules characterized by a predominant GGO pattern. It is noteworthy that an increased proportion of solid components within nodules is associated with a poorer prognosis (30). Local recurrence sites were all found in the ipsilateral lung, and there was no recurrence of the surgical margin. Literature (31,32) suggests that tumor spread through air space (STAS) is an independent prognostic factor for RFS and OS. The presence of STAS can partially explain why most of the recurrences in our study occurred within the ipsilateral lung rather than at the surgical margin. Further pathological studies on STAS are needed to investigate the reasons for tumor recurrence in our study. Another possible reason is that we used preoperative CT scans to locate the tumor and determine the anatomical relationships between surrounding tissues to guarantee adequate resection margins. The 3-year RFS and OS were 98.3% and 100.0%, respectively. The RFS of UTBS and TTBS was comparable. The surgical approach (UTBS vs. TTBS) was not associated with RFS. Therefore, we believe that UTBS could provide a similar oncological prognosis as TTBS for basal segmentectomy.
Our study found no significant difference in the number and station of lymph node harvested between UTBS and TTBS groups. However, the small sample size may limit the generalizability of our findings. Existing literature (33) suggests that the difference in lymph node detection between uniport and triport procedures remains controversial and requires further investigation. Although reduced lymph node detection may not affect the survival of T1a/T1b patients, it may be a significant issue in later stages.
According to previous studies (34,35), the rate of postoperative complications in segmentectomy ranged from 8% to 25%. In this study, the rate of postoperative complications in the overall cohort was 4.67% (14/300), which was less than that in previously published studies (34,35). After matching, the postoperative complication rates of the two groups were 4.69% and 7.81%, respectively, and the difference was not statistically significant (P=0.643). Therefore, the uniport procedure is safe compared to the triport procedure in basal segmentectomy. Pulmonary infection was the main complication of all postoperative complications in the overall cohort, occurring in 10 of 14 (71.4%) patients. Perioperative preparations for infection prevention should be performed when performing pulmonary segmental surgery.
Our study had four limitations. First, the choice of uniport and triport surgical options was up to the surgeon, which could lead to a bias. Although we balanced for confounding factors between the two groups by PSM, potential selection bias could not be completely eliminated because this study was retrospective. Second, in our center, the surgeons come from the same team. Before 2019, they mainly performed TTBS. After 2019, almost all surgeons gradually switched from TTBS to UTBS. Although all procedures were performed by experienced thoracic surgeons, there was unavoidable heterogeneity in the surgery. Additionally, there is also a lack of sufficient sample size to draw a learning curve to further explain the reasons for the differences in perioperative outcomes. Third, since the UTBS group was only included after 2019 and the outcome indicators we used were the 3-year RFS and 3-year OS, positive results were hardly found in such a short follow-up period. Thus, we will conduct further studies to update the survival results after a longer follow-up period. Fourth, as this study is retrospective, we were not able to evaluate the severity of postoperative pain, which is known benefit of uniport surgery. Finally, all results and dates were from one medical center, limiting the generalizability of the conclusions. Therefore, prospective, multicenter, large-scale randomized controlled trials are required to confirm these findings.
Conclusions
In conclusion, thoracoscopic anatomic segmentectomy performed by the uniport and triport approaches had comparable perioperative results and survival effects. Yet, the uniport procedure was associated with less intraoperative blood loss.
Acknowledgments
We would like to thank Director Liang Cai (ChuanChen Creative Brand Design) for providing technical guidance on image production for this article.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-477/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-477/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-477/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-477/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Xie D, Wu J, Hu X, et al. Uniportal versus multiportal video-assisted thoracoscopic surgery does not compromise the outcome of segmentectomy. Eur J Cardiothorac Surg 2021;59:650-7. [Crossref] [PubMed]
- Mu JW, Gao SG, Xue Q, et al. A propensity matched comparison of effects between video assisted thoracoscopic single-port, two-port and three-port pulmonary resection on lung cancer. J Thorac Dis 2016;8:1469-76. [Crossref] [PubMed]
- Ye Z, Zhang B, Chen Y, et al. Comparison of single utility port video-assisted thoracoscopic surgery (VATS) and three-port VATS for non-small cell lung cancer. Oncol Lett 2019;18:1311-7. [Crossref] [PubMed]
- Takamori S, Oizumi H, Suzuki J, et al. Thoracoscopic anatomical individual basilar segmentectomy. Eur J Cardiothorac Surg 2022;62:ezab509. [Crossref] [PubMed]
- Zhang M, Wu QC, Ge MJ. Thoracoscopic medial-basal segment segmentectomy. J Thorac Dis 2020;12:2820-3. [Crossref] [PubMed]
- Wang G, Wang Z, Sun X, et al. Uniportal video-assisted thoracoscopic anatomic segmentectomy for small-sized lung cancer. J Vis Surg 2016;2:154. [Crossref] [PubMed]
- Liu C, Liao H, Guo C, et al. Single-direction thoracoscopic basal segmentectomy. J Thorac Cardiovasc Surg 2020;160:1586-94. [Crossref] [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Tang Y, Liu C, Guo C, et al. Uniportal video-assisted thoracic surgery basal segmentectomy: a single-center retrospective cohort study. Transl Lung Cancer Res 2022;11:2125-35. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Pu Q, Liu C, Guo C, et al. Stem-Branch: A Novel Method for Tracking the Anatomy During Thoracoscopic S9-10 Segmentectomy. Ann Thorac Surg 2019;108:e333-5.
- Liu C, Liu L. Reappraise the advanced technique for tumor localization and sentinel lymph node assessment in clinical early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:1134. [Crossref] [PubMed]
- Zhu Y, Pu Q, Liu L. Trans-inferior-pulmonary-ligament VATS basal segmentectomy: application of single-direction strategy in segmentectomy of left S9+10. J Thorac Dis 2018;10:6266-8. [Crossref] [PubMed]
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [Crossref] [PubMed]
- Wang J, Xu X, Wen W, et al. Modified method for distinguishing the intersegmental border for lung segmentectomy. Thorac Cancer 2018;9:330-3. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55.
- Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics 2001;57:114-9. [Crossref] [PubMed]
- Suzuki K, Watanabe SI, Wakabayashi M, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]
- Aokage K, Suzuki K, Saji H, et al. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): a multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir Med 2023;11:540-9. [Crossref] [PubMed]
- Kikkawa T, Kanzaki M, Isaka T, et al. Complete thoracoscopic S9 or S10 segmentectomy through a pulmonary ligament approach. J Thorac Cardiovasc Surg 2015;149:937-9. [Crossref] [PubMed]
- Endoh M, Oizumi H, Kato H, et al. Posterior approach to thoracoscopic pulmonary segmentectomy of the dorsal basal segment: A single-institute retrospective review. J Thorac Cardiovasc Surg 2017;154:1432-9. [Crossref] [PubMed]
- Liu C, Wang W, Mei J, et al. Uniportal Thoracoscopic Single-Direction Basal Subsegmentectomy (Left S10a+ci): Trans-Inferior-Pulmonary-Ligament Approach. Ann Surg Oncol 2022;29:1389-91. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. [Crossref] [PubMed]
- Wei S, Guo C, He J, et al. Effect of Vein-First vs Artery-First Surgical Technique on Circulating Tumor Cells and Survival in Patients With Non-Small Cell Lung Cancer: A Randomized Clinical Trial and Registry-Based Propensity Score Matching Analysis. JAMA Surg 2019;154:e190972. [Crossref] [PubMed]
- Ujiie H, Kadota K, Chaft JE, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol 2015;33:2877-84. [Crossref] [PubMed]
- Suh JW, Jeong YH, Cho A, et al. Stepwise flowchart for decision making on sublobar resection through the estimation of spread through air space in early stage lung cancer. Lung Cancer 2020;142:28-33. [Crossref] [PubMed]
- Zhong Y, Xu Y, Deng J, et al. Prognostic impact of tumour spread through air space in radiological subsolid and pure solid lung adenocarcinoma. Eur J Cardiothorac Surg 2021;59:624-32. [Crossref] [PubMed]
- Sihoe ADL. Uniportal Lung Cancer Surgery: State of the Evidence. Ann Thorac Surg 2019;107:962-72. [Crossref] [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Donahue JM, Morse CR, Wigle DA, et al. Oncologic efficacy of anatomic segmentectomy in stage IA lung cancer patients with T1a tumors. Ann Thorac Surg 2012;93:381-7; discussion 387-8. [Crossref] [PubMed]


