
Age and treatment disparities in survival of primary malignant cardiac tumors: an analysis of over 40 years and 500 patients
Highlight box
Key findings
• Early surgical treatment is the best choice for patients with primary malignant cardiac tumors (PMCTs).
What is known and what is new?
• Older ages have been shown to be associated with poor survival of many malignant tumors.
• This is the first and most complete report exploring the relationship between patient age, treatment, and survival of PMCTs.
What is the implication, and what should change now?
• We found a significantly better survival in patients aged <20 years old compared with any other age group after being diagnosed with sarcomas, which may be associated with the tumor being diagnosed in its early stages, no metastasis, better general condition and hence, lower surgical risk.
Introduction
Primary malignant cardiac tumors (PMCTs) are rare and tend to have a poor prognosis, due to their aggressive biological behavior and the inadequate clinical expertise with the disease (1). Over the past 40 years, the incidence and survival of PMCTs appear to have increased in the United States (US) (2,3). The most common histopathologic type of PMCTs is sarcoma, with lymphoma and mesotheliomas accounting for a smaller fraction of cases (4).
When a primary cardiac tumor is highly suspected, surgical resection is the mainstay of treatment for both benign and malignant behaviors (5). Even though many patients refuse surgery or other treatment due to their poor general condition or the presence of significant comorbidities, the available retrospective data suggest that multimodality treatment (including surgery, chemotherapy, and radiation) planned by a multidisciplinary team and patient-tailored may improve the relapse-free survival (RFS) and, in some cases, the overall survival (OS) (6). However, due to the lower incidence and the difficulty of early diagnosis of PMCTs, the data are limited and are mostly based on single center studies, multicenter studies with small sample sizes, case reports, and autopsy studies (7-11). Nevertheless, almost nothing is known on the mortality and survival rates of PMCTs currently, particularly in different age and treatment groups. A small number of other studies which have examined PMCTs survival in the US have not reported death rates by either age or therapy (12-15). Older ages are often associated with higher mortality and poor prognosis. Although malignant cardiac tumors are more common in young adults, the association between age and survival in patients diagnosed with PMCTs remains unclear (15-17).
In this report, we described the survival differences between different ages and treatment strategies in specific PMCTs subtypes using data from the population-based US Surveillance, Epidemiology, and End Results (SEER) cancer registries. We present this article in according with the STREGA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1054/rc).
Methods
Study population
All data were extracted from 18 registries of SEER through SEER*Stat software (version 8.3.4; https://seer.cancer.gov/seerstat/) on 25 October 2018. The 18 cancer registries in the US are as follows: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles and San Jose-Monterey, Rural Georgia, the Alaska Native, Greater California, Kentucky, Louisiana, New Jersey, and Greater Georgia. We used SEER 18-Registry Research Data with Hurricane Katrina-Impacted Louisiana Cases, November 2015 Submission (1973–2013 varying) - Linked to County Attributes - Total U.S., 1969–2014 Counties, National Cancer Institute, Division of Cancer Control and Population Sciences (DCCPS), Surveillance Research Program, released in April 2016, based on the November 2015 submission. The selection criteria were as follows: case selection (site and morphology. primary site-labeled) = ‘C38.0-Heart’. A total of 696 patients with PMCTs diagnosed from 1973 to 2013 were identified, among whom 52 patients were excluded as they were lost to follow-up; 101 patients had secondary malignancies or multiple primary tumors; data of 14 patients were unavailable as to whether they received radiation therapy (RT) or surgical treatment (Figure S1).
PMCTs subtypes
All PMCTs subtypes were selected and differentiated using the International Classification of Childhood Cancer (ICCC) site recode extended International Classification of Diseases for Oncology, third edition (ICD-0-3)/World Health Organization (WHO) 2008. We used Codes IX, II, and XII (a.5) for sarcomas, lymphomas, and mesotheliomas, respectively. Other PMCTs were defined as Codes I (e), III (c.2), IV (b), VII (b), VIII (d.2), X (b.2), X (b.4), XI (f.10), XII (b), and tumors which were not classified by ICCC or in situ (Table S1).
Treatment
Patients who received RT and surgery were identified based on SEER variables. Receipt of RT was defined as beam radiation or radiation (NOS method or source not specified); patients who did not receive RT treatment were defined as none or refused; cancer-directed surgery was defined as definitive in nature using SEER surgical codes. Receipt of surgery was defined as surgery performed; patients who did not receive surgery were defined as not recommended, patient died prior to recommended surgery and recommended but not performed (patient refused or unknown reason). Other patients, where it was unclear whether they had received surgery or RT, were excluded due to limited numbers (n=14). However, since SEER does not include information on receipt of chemotherapy and other treatment modalities, we could not gather any information on the role of chemotherapy on specific histopathologic types of PMCTs.
Outcomes and study cohort
Survival was defined as the time between diagnosis and death till 31 December 2013. The SEER registries calculated survival time in months based on active follow-up for vital status. The cohort was divided into 4 age groups: <20, 20–50, 51–80, and >80 years. We compared these 4 groups on the basis of the following patient demographic variables: gender, race (white, black, or other), ethnicity (Hispanic or non-Hispanic), subtype, year of diagnosis, and therapy. Treatments cohorts were divided into the following groups: surgery, radiation, surgery and radiation, and no treatment. In this cohort, gender, age, race (white, black, or other), ethnicity (Hispanic or non-Hispanic), subtypes, and year of diagnosis were also compared respectively.
Statistical analysis
Chi-squared test was used to assess significance of the difference between proportions in demographic and tumor characteristics by age and treatment. Kaplan-Meier (KM) product-limit estimates of OS probability were calculated for all PMCTs and for each subtype by age and treatment and compared with global log-rank tests. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of death from any cause, comparing each age group to the 20–50 years group and comparing each treatment group to the surgery group respectively. A P value of <0.05 was defined as statistically significant. All standard statistical tests were performed with SPSS 19.0 (IBM Corp., Armonk, NY, USA).
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All data were extracted from SEER database of the USA. We obtained the data application license on the SEER official website on 23 October, and signed a data use agreement.
Results
Population characteristics
A total of 529 patients were finally included in this study. The different age categories were <20 years old (34 patients; 6.4%), 20–50 years old (230 patients; 43.5%), 51–80 years old (221 patients; 41.8%), and >80 years old (44 patients; 8.3%) (Table 1). There was male predominance for patients diagnosed with PMCTs at <50 years old and female predominance for patients diagnosed at >50 years old (P=0.001). In all age groups, most patients were white (72.1%) and non-Hispanic (82.8%). The majority of PMCTs subtypes were sarcomas (n=315, 64.9%) in patients ≥80 years old, but lymphomas (n=25, 56.8%) in patients aged >80 years old (P<0.001). More diagnosed patient cases were recorded during later years of the study as compared to the earlier years.
Table 1
| Variable | Age group, n (%) | P value | ||||
|---|---|---|---|---|---|---|
| Total (n=529) | <20 years (n=34) | 20–50 years (n=230) | 51–80 years (n=221) | >80 years (n=44) | ||
| Gender | 0.001 | |||||
| Male | 288 (54.4) | 20 (58.2) | 144 (62.6) | 105 (47.5) | 19 (43.2) | |
| Female | 241 (45.6) | 14 (41.2) | 86 (37.4) | 116 (52.5) | 25 (56.8) | |
| Race | 0.019 | |||||
| White | 413 (78.1) | 25 (73.5) | 175 (76.1) | 175 (79.2) | 38 (86.4) | |
| Black | 55 (10.4) | 7 (20.6) | 30 (13.0) | 18 (8.1) | NA | |
| Others | 61 (11.5) | 2 (5.9) | 25 (10.9) | 28 (12.7) | 6 (13.6) | |
| Ethnicity | 0.002 | |||||
| Non-Hispanic | 438 (82.8) | 22 (64.3) | 187 (81.3) | 192 (86.9) | 37 (84.1) | |
| Hispanic | 91 (17.2) | 12 (35.3) | 43 (18.7) | 29 (13.1) | 7 (15.9) | |
| Subtype | <0.001 | |||||
| Sarcoma | 324 (61.2) | 18 (52.9) | 181 (78.7) | 116 (52.5) | 9 (20.5) | |
| Lymphoma | 122 (23.1) | 7 (20.6) | 24 (10.4) | 66 (29.9) | 25 (56.8) | |
| Mesothelioma | 32 (6.1) | 1 (3.0) | 13 (5.7) | 17 (7.7) | 1 (2.3) | |
| Others | 51 (9.6) | 8 (23.5) | 12 (5.2) | 22 (9.9) | 9 (20.5) | |
| Year of diagnosis | 0.25 | |||||
| 1973–1981 | 32 (6.1) | 3 (8.8) | 17 (7.4) | 11 (49.8) | 1 (2.3) | |
| 1982–1990 | 47 (8.9) | 1 (3.0) | 24 (10.4) | 20 (9.0) | 2 (4.5) | |
| 1991–1999 | 82 (15.5) | 5 (14.7) | 33 (14.4) | 38 (17.2) | 6 (13.6) | |
| 2000–2008 | 210 (39.6) | 13 (38.2) | 92 (40.0) | 86 (38.9) | 19 (43.2) | |
| 2009–2013 | 158 (29.9) | 12 (35.3) | 64 (27.8) | 66 (29.9) | 16 (36.4) | |
| Surgery | <0.001 | |||||
| Performed | 250 (42.3) | 20 (58.8) | 128 (55.7) | 93 (42.1) | 9 (20.5) | |
| Not performed | 279 (52.7) | 14 (41.2) | 102 (44.3) | 128 (57.9) | 35 (79.5) | |
| Radiation | 0.006 | |||||
| Performed | 106 (20.0) | 5 (14.7) | 60 (26.1) | 38 (17.2) | 3 (6.8) | |
| Not performed | 423 (80.0) | 29 (85.3) | 170 (73.9) | 183 (82.8) | 41 (93.2) | |
PMCTs, primary malignant cardiac tumors; SEER, Surveillance, Epidemiology, and End Results; NA, not applicable.
In Table 2, all patients were grouped according to different treatments. Of these patients, 193 (36.5%) received surgery, 49 (9.4%) received RT, 57 (10.8%) received a combination of surgery and RT, and 230 (43.5%) received no treatment. No significant difference was observed across gender, race, ethnicity, and year of diagnosis among treatment groups. Patients younger than 50 years or diagnosed with cardiac sarcomas were more willing to accept active treatment (P<0.001), whereas most patients older than 50 years or diagnosed with lymphomas received no treatment (P<0.001).
Table 2
| Variable | Treatment group, n (%) | P value | ||||
|---|---|---|---|---|---|---|
| Total (n=529) | Surgery (n=193) | Radiation (n=49) | Surgery and radiation (n=57) | No treatment (n=230) | ||
| Gender | 0.184 | |||||
| Male | 288 (54.4) | 100 (51.8) | 29 (59.2) | 28 (49.1) | 131 (57.0) | |
| Female | 241 (45.6) | 93 (48.2) | 20 (40.8) | 29 (50.9) | 99 (43.0) | |
| Race | 0.775 | |||||
| White | 413 (78.1) | 149 (77.2) | 38 (77.5) | 44 (77.2) | 182 (79.1) | |
| Black | 55 (10.4) | 19 (9.8) | 4 (8.2) | 5 (8.8) | 27 (11.7) | |
| Others | 61 (11.5) | 25 (13.0) | 7 (14.3) | 8 (14.0) | 21 (9.2) | |
| Ethnicity | 0.772 | |||||
| Non-Hispanic | 438 (83.0) | 157 (81.3) | 43 (87.8) | 46 (80.7) | 192 (83.5) | |
| Hispanic | 91 (17.0) | 36 (18.7) | 6 (12.2) | 11 (19.3) | 38 (16.5) | |
| Age at diagnosis, years | <0.001 | |||||
| <20 | 34 (6.4) | 18 (9.3) | 3 (6.1) | 2 (3.5) | 11 (4.8) | |
| 20–50 | 230 (43.5) | 96 (49.7) | 28 (57.1) | 32 (56.1) | 74 (32.2) | |
| 51–80 | 221 (41.8) | 71 (36.8) | 16 (32.7) | 22 (38.6) | 112 (48.7) | |
| >80 | 44 (8.3) | 8 (4.2) | 2 (4.1) | 1 (1.8) | 33 (14.3) | |
| Subtype | <0.001 | |||||
| Sarcoma | 324(61.2) | 141 (73.0) | 25 (51.0) | 55 (96.5) | 103 (44.8) | |
| Lymphoma | 122 (23.1) | 20 (10.4) | 19 (38.8) | 2 (3.5) | 81 (35.2) | |
| Mesothelioma | 32 (6.1) | 11 (5.7) | 3 (6.1) | NA | 18 (7.8) | |
| Others | 51(9.6) | 21 (10.9) | 2 (4.1) | NA | 28 (12.2) | |
| Year of diagnosis | 0.356 | |||||
| 1973–1981 | 32 (6.1) | 9 (4.7) | 4 (8.2) | 7 (12.3) | 12 (5.2) | |
| 1982–1990 | 47 (8.9) | 12 (6.2) | 7 (14.3) | 6 (10.5) | 22 (9.6) | |
| 1991–1999 | 82 (15.5) | 28 (14.5) | 8 (16.3) | 7 (12.3) | 39 (17.0) | |
| 2000–2008 | 210 (39.6) | 88 (45.6) | 18 (36.7) | 19 (33.3) | 85 (36.9) | |
| 2009–2013 | 158 (29.9) | 56 (29.0) | 12 (24.5) | 18 (31.6) | 72 (31.3) | |
PMCTs, primary malignant cardiac tumors; SEER, Surveillance, Epidemiology, and End Results; NA, not applicable.
Age-based survival
Survival data were available for 529 patients with PMCTs of known age and treatment. A total of 436 deaths occurred over a median follow-up of 80 months. Survival curves showed significant variation by age; median survival time of patients with all PMCTs was 22.5, 11, 5, and 1 months for patients aged <20 years, 20–50 years, 51–80 years, and >80 years, respectively (global log-rank P=0.0026; Figure 1). Separately, survival after sarcomas and lymphomas diagnosis varied significantly by age, with median survival of 17.5, 11, 4, and 1 months, respectively (P<0.0001; Figure 1) and 126, 15.5, 16, and 1 months, respectively (P=0.0073; Figure 1) for <20 years, 20–50 years, 51–80 years, and >80 years. In contrast, no significant difference was observed in mesothelioma and others (P=0.62 and P=0.12).

After adjusting for gender, race, year of diagnosis, ethnicity, subtype, and treatment, survival was longer for patients aged <20 years (HR 0.49; 95% CI, 0.30 to 0.78; P=0.002) compared with the 20–50 years group, but shorter for patients aged >80 years (HR 1.83; 95% CI, 1.25 to 2.68; P=0.002) when all PMCTs were considered together (Table 3). However, there was no difference in survival between the 20–50 years and 51–80 years age groups (HR 1.10; 95% CI, 0.89 to 1.37; P=0.36). Separately, for sarcomas, younger age was associated with better survival, with HR 0.54 (95% CI, 0.30 to 0.98; P=0.043), 1.49 (95% CI, 1.16 to 1.92; P=0.002), and 3.90 (95% CI, 1.95 to 7.79; P<0.001) for <20 years, 51–80 years, and >80 years when we used patients aged 20–50 years as the reference category. Similarly, patients aged <20 years had better survival after being diagnosed with lymphomas (HR 0.07; 95% CI, 0.01 to 0.58; P=0.014), patients aged >80 years had worse survival after being diagnosed with mesothelioma (HR 19.70; 95% CI, 1.57 to 247.05; P=0.021), and patients aged 51–80 years had worse survival after being diagnosed with any other PMCTs (HR 2.26; 95% CI, 1.02 to 5.02; P=0.046) (Table 3).
Table 3
| Age at diagnosis | All PMCTs | Sarcoma | Lymphoma | Mesothelioma | Others | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR† (95% CI) | P value | HR‡ (95% CI) | P value | HR‡ (95% CI) | P value | HR‡ (95% CI) | P value | HR‡ (95% CI) | P value | |||||
| 20–50 years | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| <20 years | 0.49 (0.30 to 0.78) |
0.002 | 0.54 (0.30 to 0.98) |
0.043 | 0.07 (0.01 to 0.58) |
0.014 | 0.76 (0.08 to 6.99) |
0.808 | 0.86 (0.28 to 2.60) |
0.787 | ||||
| 51–80 years | 1.10 (0.89 to 1.37) |
0.36 | 1.49 (1.16 to 1.92) |
0.002 | 0.67 (0.35 to 1.28) |
0.223 | 0.87 (0.35 to 2.15) |
0.761 | 2.26 (1.02 to 5.02) |
0.046 | ||||
| >80 years | 1.83 (1.25 to 2.68) |
0.002 | 3.90 (1.95 to 7.79) |
<0.001 | 2.04 (0.97 to 4.31) |
0.06 | 19.70 (1.57 to 247.05) |
0.021 | 1.20 (0.39 to 3.71) |
0.75 | ||||
†, adjusted by sex, race, year of diagnosis, ethnicity, subtype, therapy; ‡, adjusted by sex, race, year of diagnosis, ethnicity, therapy. Others includes III (c.2) PNET, IV (b) other peripheral nervous cell tumors, I (e) unspecified and other specified leukemias, VII (b) hepatic carcinomas, VIII (d.2) malignant chordomas, X (b.2) malignant teratomas: extracranial/extragonadal, X (b.4) yolk sac tumor: extracranial/extragonadal, XI (f.10) carcinomas of other specified sites, XII (b) other unspecified malignant tumors, not classified by ICCC or in situ. HR hazard ratio; PMCTs, primary malignant cardiac tumors; SEER, Surveillance, Epidemiology, and End Results; Ref, referent; CI, confidence interval.
Treatment-based survival
In all PMCTs, there was no significant difference in survival curves by treatment, with median survival times of 12, 12, 11, and 2 months for only surgery, only RT, surgery and RT, and no treatment, respectively (global log-rank P=0.25; Figure 2). However, for sarcomas, a significant variation in survival by treatment was observed, with median survival times of 12, 9, 11, and 2 months for only surgery, only RT, surgery and RT, and no treatment, respectively (global log-rank P=0.0057; Figure 2).
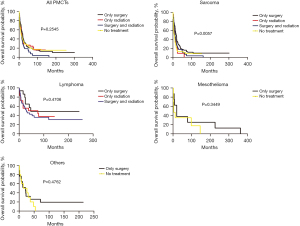
After controlling for age, sex, race, year of diagnosis, ethnicity, and subtype, for all PMCTs, we found that patients who had no treatment had a worse survival (HR 1.52; 95% CI, 1.22 to 1.89; P<0.001) compared to those who received surgery only (Table 4). By subtype and treatment, patients who received only RT had a 1.49-fold increased risk of death (95% CI, 0.95 to 2.34; P<0.046), and those who received no treatment had a 1.99-fold increased risk of death (95% CI, 1.51 to 2.62; P<0.001) compared to the only surgery group after being diagnosed with sarcomas. Interestingly, in other specific histologic types of PMCTs, patients who received no treatment also had a worse survival (HR 2.24; 95% CI, 1.15 to 4.34; P=0.017) compared to those who received surgery only. However, no significant association was observed between treatments and survival for patients diagnosed with lymphomas and mesothelioma (Table 4).
Table 4
| Treatment | All PMCTs | Sarcoma | Lymphoma | Mesothelioma | Others | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR† (95% CI) | P value | HR‡ (95% CI) | P value | HR‡ (95% CI) | P value | HR‡ (95% CI) | P value | HR‡ (95% CI) | P value | |||||
| Only surgery | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Surgery and radiation | 1.05 (0.76 to 1.46) |
0.765 | 1.10 (0.79 to 1.55) |
0.571 | NA | 0.966 | NA | NA | NA | NA | ||||
| Only radiation | 0.92 (0.65 to 1.31) |
0.642 | 1.49 (0.95 to 2.34) |
0.046 | 1.05 (0.44 to 2.48) |
0.916 | 1.70 (0.38 to 7.55) |
0.485 | 0.75 (0.13 to 4.18) |
0.74 | ||||
| No treatment | 1.52 (1.22 to 1.89) |
<0.001 | 1.99 (1.51 to 2.62) |
<0.001 | 1.61 (0.81 to 3.23) |
0.177 | 1.34 (0.47 to 3.83) |
0.591 | 2.24 (1.15 to 4.34) |
0.017 | ||||
†, adjusted by sex, race, age, year of diagnosis, ethnicity, subtype; ‡, adjusted by sex, race, age, year of diagnosis, ethnicity. Others includes III (c.2) PNET, IV (b) other peripheral nervous cell tumors, I (e) unspecified and other specified leukemias, VII (b) hepatic carcinomas, VIII (d.2) malignant chordomas, X (b.2) malignant teratomas: extracranial/extragonadal, X (b.4) yolk sac tumor: extracranial/extragonadal, XI (f.10) carcinomas of other specified sites, XII (b) other unspecified malignant tumors, not classified by ICCC or in situ. HR, hazard ratio; PMCTs, primary malignant cardiac tumors; SEER, Surveillance, Epidemiology, and End Results; Ref, referent; CI, confidence interval; NA, not applicable.
Discussion
PMCTs has a very poor prognosis and continues to pose a therapeutic challenge to cardiac surgeons and oncologists because of the technical difficulty involved in extensive cardiac resections and the aggressive biological nature of the tumors (1,6). In this study, we analyzed the proportions and survival of PMCT subtypes by age and treatment using data from the largest cancer registry in the US. To our knowledge, this is the first and most complete report exploring the relationship between patient age, treatment, and survival of PMCT. Several striking features are evident in our results which have received limited prior attention.
Older ages have been shown to be associated with poor survival of many malignant tumors such as colorectal cancer (18), breast cancer (19), and prostate cancer (20), which may be related to poor health status, greater surgical risk, and advanced cancer status. Not surprisingly, our analysis confirmed that increasing age was also associated with worse survival for patients diagnosed with PMCTs. After adjustment for patient clinical and treatment characteristics, age older than 80 years old was still associated with significantly higher all-cause mortality, particularly in patients with cardiac mesotheliomas and sarcomas. Moreover, this result was also consistent with our previous finding that age older than 80 years is a major risk factor for early death in PMCTs.
As the most frequent PMCTs, sarcoma can occur at any age with no gender predominance (21). In this report, we found a significantly better survival in patients aged <20 years old compared with any other age group after being diagnosed with sarcomas, which may be associated with the tumor being diagnosed in its early stages, no metastasis, better general condition and hence, lower surgical risk. Similar results were also observed in patients with cardiac lymphomas. Clearly, variations of the treatment approach in young versus elderly patients with malignant tumors are present. In this study, evaluation of PMCTs in the SEER registry indicates that the percentage of patients that receive surgery drops from 56% to 9% for patients aged <50 years versus >80 years and that of RT drops from 25% to 7% for patients aged <50 years versus >80 years. In our opinion, young patients should undergo surgery to remove the tumor and restore the function of the heart as soon as possible after a clear diagnosis, and for elderly patients and in particular, patients aged over 70 years, individual life expectancy has to be weighed against the potential harms and benefits from aggressive treatment.
Radical surgery remains the mainstay of treatment in PMCTs without infiltrated margins, so as to both prevent local relapse and improve the survival. Currently, chemotherapy, RT, or a combination of both are being used as adjuvant therapy to decrease tumor size and facilitate surgical resection of PMCTs (22,23). The Cleveland Clinic has reported that patients who received multimodality treatment had a better survival than patients treated with surgery, RT, or chemotherapy only (24). Herein, for the first time, we compared OS among multiple subtypes of PMCTs, and we found that individuals who received no treatment have worse survival. However, across all subtypes of PMCTs, no significant difference was observed in OS between the surgery only group, RT only group, and combined surgery and RT group. RT plays a minor role in the management of patients with cardiac sarcoma, primarily due to radiation-related cardiac toxicity and technical limitation. Consistently, our findings showed a significant higher risk of death in patients diagnosed with sarcomas who received RT only as compared to the surgery only group. Another possibility is that ionizing radiation may cause acute inflammation which ultimately leads to pericarditis, and both coronary and valvular heart disease (25).
Primary cardiac lymphoma is an extranodal type of non-Hodgkin’s lymphoma exclusively located in the heart and/or pericardium (26). For cardiac lymphomas, chemotherapy is the main treatment, and surgery might be considered only as a rescue treatment in a case of severe hemodynamic impairment by a large mass. Herein, we have also provided evidence that surgery has no significant effect in the treatment of lymphoma. Similar results were also observed in patients with cardiac mesothelioma. Although the no treatment group did not achieve statistical significance when compared with the surgical group, it still carries higher mortality. A greater number of randomized clinical trials and multicenter retrospective study with larger sample size will be needed for further investigation.
Limitations
The study has the following limitations that need to be considered while evaluating these results. Due to the nature of a retrospective study and because the data in this study were collected over 5 decades, there are unavoidable confounding factors even after adjustments, such as histopathologic classifications, diagnostic, treatment modalities, and so on. Importantly, due to the low incidence of PMCTs, it was difficult to collect a larger number of cases, so this study had to rely on public database data. In addition, the SEER database does not publicize data on chemotherapy and other treatment modalities, so this study did not explore the role of chemotherapy on PMCTs survival. Lastly, lack of information on the details of surgery or RT and the mode of death also limits our understanding of the natural course of PMCTs and thus are potential confounders influencing survival analyses. Despite the limitations of the research, no other data source can provide such high numbers of PMCTs patients.
Conclusions
In conclusion, this study confirms the poor survival of patients diagnosed with PMCTs at any age and with or without treatment over the past 5 decades. However, significant differences were also observed in the proportions of PMCTs subtypes and survival between age and treatments subpopulations in the US. Patients who received no treatment or of older ages often have worse survival. Compared to RT or combination of radiotherapy and surgery, only surgery appears to be a better choice for patients with sarcomas. Future research is still needed to better understand how these poor outcomes can be improved.
Acknowledgments
We acknowledge the contributions of all doctors from the Department of Thoracic Surgery, Affiliated Hospital of Nantong University to the study, who also gave written permission for the publication of data and conclusions. We also thank Dr. Valdano Manuel (Complexo Hospitalar de Doenças Cardio-Pumonares Cardeal Dom Alexandre do Nascimento, Luanda, Angola) for the critical comments and valuable advice on this study.
Funding: This study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STREGA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1054/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1054/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1054/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sultan I, Bianco V, Habertheuer A, et al. Long-Term Outcomes of Primary Cardiac Malignancies: Multi-Institutional Results From the National Cancer Database. J Am Coll Cardiol 2020;75:2338-47. [Crossref] [PubMed]
- Lestuzzi C, Reardon MJ. Primary Cardiac Malignancies: The Need for a Multidisciplinary Approach and the Role of the Cardio-Oncologist. J Am Coll Cardiol 2020;75:2348-51. [Crossref] [PubMed]
- Burazor I, Aviel-Ronen S, Imazio M, et al. Primary malignancies of the heart and pericardium. Clin Cardiol 2014;37:582-8. [Crossref] [PubMed]
- Basso C, Rizzo S, Valente M, et al. Cardiac masses and tumours. Heart 2016;102:1230-45. [Crossref] [PubMed]
- Bruce CJ. Cardiac tumours: diagnosis and management. Heart 2011;97:151-60. [Crossref] [PubMed]
- Lestuzzi C, De Paoli A, Baresic T, et al. Malignant cardiac tumors: diagnosis and treatment. Future Cardiol 2015;11:485-500. [Crossref] [PubMed]
- Aloysius MM, Shrivastava S, Rojulpote C, et al. Racial and ethnic characteristics and cancer-specific survival in Primary Malignant Cardiac Tumors. Front Cardiovasc Med 2022;9:961160. [Crossref] [PubMed]
- Garcia Brás P, Branco LM, Galrinho A, et al. Malignant Primary and Metastatic Cardiac Tumors: A Single-Center 27-Year Case Review. Oncology 2023;101:292-302. [Crossref] [PubMed]
- Bui Q, Ngo TNM, Mazur J, et al. Long-term outcomes of primary cardiac malignant tumors: Difference between African American and Caucasian population. Cancer Med 2021;10:8838-45. [Crossref] [PubMed]
- Fang X, Zheng S. Primary cardiac angiosarcoma: a case report. J Int Med Res 2021;49:3000605211033261. [Crossref] [PubMed]
- Rahouma M, Baudo M, Dabsha A, et al. Outcomes of Octogenarians with Primary Malignant Cardiac Tumors: National Cancer Database Analysis. J Clin Med 2022;11:4899. [Crossref] [PubMed]
- Isogai T, Yasunaga H, Matsui H, et al. Factors affecting in-hospital mortality and likelihood of undergoing surgical resection in patients with primary cardiac tumors. J Cardiol 2017;69:287-92. [Crossref] [PubMed]
- Gaisendrees C, Gerfer S, Schröder C, et al. Benign and malignant cardiac masses: long-term outcomes after surgical resection. Expert Rev Anticancer Ther 2022;22:1153-8. [Crossref] [PubMed]
- Hoffmeier A, Sindermann JR, Scheld HH, et al. Cardiac tumors--diagnosis and surgical treatment. Dtsch Arztebl Int 2014;111:205-11. [Crossref] [PubMed]
- Matteucci M, Ferrarese S, Mantovani V, et al. Surgical treatment of primary cardiac tumors in the contemporary era: A single-centre analysis. J Card Surg 2021;36:3540-6. [Crossref] [PubMed]
- Di Bari N, D'Errico Ramirez A, Nasso G. Primary malignant cardiac tumors: Sex-related therapy and multidisciplinary approach as a new challenge for the future. J Card Surg 2022;37:1287-9. [Crossref] [PubMed]
- Thomas-de-Montpréville V, Nottin R, Dulmet E, et al. Heart tumors in children and adults: clinicopathological study of 59 patients from a surgical center. Cardiovasc Pathol 2007;16:22-8. [Crossref] [PubMed]
- Leung WK, Ho KY, Kim WH, et al. Colorectal neoplasia in Asia: a multicenter colonoscopy survey in symptomatic patients. Gastrointest Endosc 2006;64:751-9. [Crossref] [PubMed]
- Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014;311:1327-35. [Crossref] [PubMed]
- Drazer MW, Huo D, Eggener SE. National Prostate Cancer Screening Rates After the 2012 US Preventive Services Task Force Recommendation Discouraging Prostate-Specific Antigen-Based Screening. J Clin Oncol 2015;33:2416-23. [Crossref] [PubMed]
- Rosenkranz ER, Murphy DJ Jr. Diagnosis and neonatal resection of right atrial angiosarcoma. Ann Thorac Surg 1994;57:1014-5. [Crossref] [PubMed]
- Blackmon SH, Patel A, Reardon MJ. Management of primary cardiac sarcomas. Expert Rev Cardiovasc Ther 2008;6:1217-22. [Crossref] [PubMed]
- Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol (R Coll Radiol) 2007;19:748-56. [Crossref] [PubMed]
- Randhawa JS, Budd GT, Randhawa M, et al. Primary Cardiac Sarcoma: 25-Year Cleveland Clinic Experience. Am J Clin Oncol 2016;39:593-9. [Crossref] [PubMed]
- Basavaraju SR, Easterly CE. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med Phys 2002;29:2391-403. [Crossref] [PubMed]
- Lestuzzi C. Primary tumors of the heart. Curr Opin Cardiol 2016;31:593-8. [Crossref] [PubMed]


