Efficacy of subpleural continuous infusion of local anesthetics after thoracoscopic pulmonary resection for primary lung cancer compared to intravenous patient-controlled analgesia
Introduction
Thoracic surgery can cause severe postoperative pain that results from the surgical incisions, disrupted intercostal nerves, retracted ribs, chest wall inflammation, injured pulmonary parenchyma, and placement of chest tubes (1). The postoperative pain can adversely interfere with the respiratory function, performance, outcome and quality of life of patients (2). Uncontrolled postoperative pain can lead to respiratory compromise, resulting in increased morbidity and a prolonged hospital stay (3). Although thoracoscopic surgery reduces postoperative pain compared with open thoracotomy, patients can also experience moderate to severe pain, especially during the first few hours after thoracoscopic surgery (4,5). The analgesic options for postoperative pain control in thoracic surgery include thoracic epidural anesthesia, thoracic paravertebral blockade, intravenous patient-controlled analgesia (IV-PCA), and local anesthetic infusion in the wound or extrapleural space as an intercostal nerve block (6). Several studies have compared the effectiveness of these pain control options, but no gold standard for pain control after thoracic surgery has been established, especially for thoracoscopic surgery.
Typically, IV-PCA is used after thoracoscopic surgery, but it has side effects that include dizziness, nausea and vomiting due to the systemic effects of analgesics (7). The subpleural continuous infusion of local anesthesia (ON-Q system; Kimberly-Clark, Atlanta, GA, USA) can apply analgesics locally along the intercostal nerves without systemic effects. We hypothesized that the ON-Q system would control pain effectively compared with IV-PCA, with minimal side effects. Therefore, this retrospective study compared the effectiveness and side effects of the ON-Q system and IV-PCA in patients undergoing thoracoscopic pulmonary resection for primary lung cancer.
Methods
This study was approved by the Institutional Review Board of our institution (IRB-No.: AJIRB-MED-MDB-15-446). We retrospectively reviewed 66 patients who underwent thoracoscopic pulmonary resection for primary lung cancer in Ajou University Hospital (Suwon, Korea) from January 2014 to August 2015. The exclusion criteria were cases of thoracotomy conversion, not a primary lung cancer, and use of a rib spreader or another pain control method, such as thoracic epidural PCA or continuous IV opioid infusion without a patient-controlled device. Of the 66 patients, 36 used IV-PCA and 30 used the ON-Q system.
Surgical methods and catheter placement
All operations were performed under general anesthesia, which was typically induced using 1.5–2.0 mg/kg of propofol (Fresofol; Fresenius Kabi, Seoul, Korea), 0.5–1.5 µg/kg of remifentanil (ULTIVA; GlaxoSmithKline, Seoul, Korea), and 0.6 mg/kg rocuronium (Rocumeron; Ilsung Pharmaceuticals, Seoul, Korea). After inducing anesthesia, a double-lumen endotracheal tube was inserted for single-lung ventilation during video-assisted thoracic surgery (VATS) pulmonary resection and a radial arterial line and central venous catheter were placed in the appropriate locations. Anesthesia was maintained using sevoflurane (1–2 minimal alveolar concentration) and remifentanil (0.1–0.3 µg/kg/min) in 50% oxygen and air. The tidal volume during single-lung ventilation was 6 mL/kg with 100% oxygen. The patient was positioned in the lateral decubitus position with the affected side up and the skin was prepared preoperatively with 10.0% povidone iodine topical solution. The utility port was made in the 4th intercostal space (ICS) for upper lobe or right middle lobe resection and in the 5th ICS for lower lobe resection. Two 10-mm trocar ports were made below the 2nd and 3rd ICS as utility windows for the thoracoscope and surgical device. All operations were performed by a single surgeon using the same technique. After performing the pulmonary resection, the mediastinal lymph nodes were dissected completely.
Fentanyl citrate (Fentanyl; Hana, Seoul, Korea) and an antiemetic (Nasea, ramosetron HCl; Astellas, Seoul, Korea) were used for IV-PCA (AutoMed 3200, Ace Medical, Seoul, Korea) using 100 mL of normal saline containing 1,000 µg fentanyl and antiemetic; the basal continuous infusion rate was 2 mL/h, and the bolus PCA dose was 2 mL. The ON-Q system catheter, which has several side holes for infusion, was placed in the extrapleural space. It was typically placed from the 9th to the 3rd ICS alongside the thoracic sympathetic chain to cover all of the VATS incisions (Figure 1). The ON-Q system was filled with 250 mL of 0.6% ropivacaine (Rocaine, Reyon Pharmaceuticals, Seoul, Korea) and the continuous infusion rate was 4 mL/h. If pleural adhesions were identified or there was notable pleural damage during the thoracoscopic lobectomy, IV-PCA was used instead of the ON-Q system.
Assessment of pain
Pain was assessed using a numeric pain intensity scale (NPIS). The score was recorded by nurses every 8 hours and after interventions for pain control with IV analgesia (8). The highest NPIS score from the day of surgery to the day of discharge, number of additional IV analgesic injections, side effects associated with IV-PCA or the ON-Q system, and early discontinuation of pain control devices due to side effects were abstracted from the medical records of all patients. Early discontinuation was defined as suspension of the infusion using pain control devices before complete infusion of the initial amount of fluid. This usually meant suspending the infusion using pain control devices before the second postoperative day.
Statistical methods
Statistical analysis was performed using SPSS for Windows software (ver. 20.0; IBM Corp., Somers, NY, USA). The patients’ general characteristics and pain scores are provided as means ± standard deviation for continuous variables, and as the number of cases with frequencies (%) for categorical variables. The groups were compared using the chi-square test or Fisher’s exact test for discrete variables and independent samples t-test for continuous variables. The highest NPIS scores on each postoperative day in the two groups were compared using repeated measures analysis of variance (ANOVA). A P value less than 0.05 indicated statistical significance.
Results
General characteristics
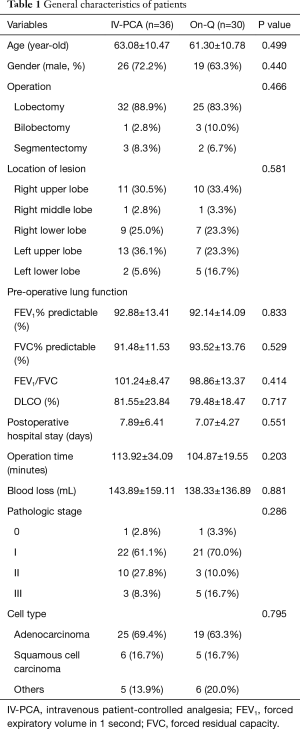
The general characteristics of the patients are summarized in Table 1. The mean age was 63.08±10.47 and 61.30±10.78 years in the IV-PCA and ON-Q groups, respectively (P=0.499). There were 26 (72.2%) and 19 (63.3%) males in the IV-PCA and ON-Q groups, respectively (P=0.440). To treat the primary lung cancer, we performed 57 (86.3%) lobectomies, 4 (6.1%) bilobectomies, and 5 (7.6%) segmentectomies. The right upper lobe was the most common location of the lesions in this study (21/66, 31.8%), followed by the left upper lobe. The preoperative lung functions did not differ between the two groups, including the forced expiratory volume in 1 second (FEV1), forced residual capacity (FVC), FEV1/FVC (%), and diffusing capacity of the lungs for carbon monoxide (DLCO). There was no difference between the groups in terms of the postoperative hospital stay (7.89±6.41 vs. 7.07±4.27 days; P=0.551), operating time (113.92±34.09 vs. 104.87±19.55 min; P=0.203), blood loss during the operation (143.89±159.11 vs. 138.33±136.89 mL; P=0.881), pathological stage (P=0.286), or cell type (P=0.795) (Table 1).
Full table
Pain control
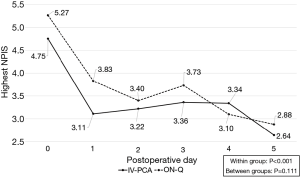
The highest NPIS scores with or without each pain control device and the number of additional IV analgesic injections did not differ between the two groups. The average highest daily NPIS scores after VATS from the day of surgery to postoperative day 2 in the IV-PCA group were 4.75±2.35, 3.11±1.35, and 3.22±1.15, respectively, and those in the ON-Q group were 5.27±1.87, 3.83±1.80, and 3.40±1.38, respectively. Repeated-measures ANOVA showed that NPIS scores decreased gradually with time (P<0.001), but there were differences in the patterns of the NPIS scores between the two groups (P=0.111, Figure 2), with P values of 0.334, 0.067, and 0.570, respectively (independent sample t-test). There were 0.72±0.94 additional IV analgesic injections in the IV-PCA group and 0.83±0.65 in the ON-Q group (P=0.587) from the operation day to the second postoperative day.
Side effects and discontinuations
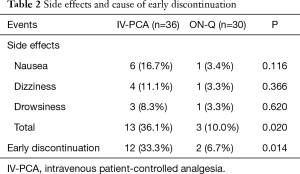
Side effects are summarized in Table 2 and were noted in 13 of 36 (36.1%) IV-PCA patients and 3 of 30 (10.0%) ON-Q patients. The rate of side effects was significantly higher in the IV-PCA group (P=0.020). The most frequent side effect of IV-PCA was nausea on the day of surgery, which was usually accompanied by dizziness or drowsiness. In the ON-Q group, there was one case each of nausea, dizziness, and drowsiness (Table 2). Early discontinuation of the pain control device was required for 12 (33.3%) patients in the IV-PCA group versus 2 (6.7%) in the ON-Q group, and the difference was significant (P=0.014). Nausea was the main cause of early discontinuation of the pain control device in the IV-PCA group, while two patients in the ON-Q group discontinued pain control because of drowsiness and nausea (Table 2). The side effects disappeared after discontinuing the pain control devices. There was no catheter-related infection in any patient.
Full table
Discussion
Pain control after thoracic surgery is important for postoperative recovery. Postoperative pain can reduce bronchial clearance and is associated with mucous plugging, hypoxia, atelectasis, and pulmonary infection (6). Effective control of postoperative pain can lead to a better postoperative outcome after thoracoscopic pulmonary resection. In addition, proper pain control can prevent chronic post-thoracotomy pain syndrome (9). There are several options for analgesia after thoracic surgery, including thoracic epidural anesthesia, thoracic paravertebral blockade, IV-PCA, local anesthetic infusion in the wound or extrapleural space, and combinations of these methods (6,10). Epidural PCA has proven to be effective for pain control (6,11,12), but thoracic epidural catheterization has risks of dural puncture, epidural hematoma formation, nerve damage, hypotension, and unsuccessful catheter placement (7). The catheterization also takes time and the success rate usually depends on operator experience. Compared with epidural PCA, IV-PCA can be applied easily with fewer procedure-related complications and equivalent efficacy (7). However, IV-PCA administers analgesics via a systemic route, which can result in side effects such as dizziness, somnolence, pruritus, nausea and vomiting, even when delivered with a PCA system (7). In our series, the incidence of side effects in the IV-PCA group was 36.1%, and most patients who developed side effects in the IV-PCA group could not maintain the pain control device because of the side effects (12/13, 92.3%), although all of the side effects subsided after stopping IV-PCA. We believe that these systemic side effects originated from the type of infusion agent and route of infusion. Therefore, we sought another pain control system as an alternative to IV-PCA.
Some studies reported notable pain control with the subpleural injection of local anesthetics (10,13), while other studies found this to be less effective (12,14). Local anesthetics such as bupivacaine, which is similar to ropivacaine, have shorter analgesic durations (10), so they require continuous infusion postoperatively to be effective. We hypothesized that the continuous infusion of local anesthetics along the intercostal nerve would reduce the postoperative pain. Compared with IV-PCA, our data showed that the ON-Q system resulted in equivalent pain control with fewer side effects compared with systemic analgesics. The ON-Q pain control system continuously infused 0.6% ropivacaine at 4 mL/h. The total volume of the chamber is 250 mL, enabling pain control for up to 60 hours. The ON-Q system can provide sufficient analgesia in the immediate postoperative period because the NPIS score postoperatively is usually highest on the day of surgery and decreases gradually over time (11). Our results are supported by previous reports; Ried et al. (1) compared the ON-Q system and thoracic epidural analgesia after thoracic surgery and reported effective pain control after thoracotomy with the ON-Q system.
When inserting the On-Q system catheter, several precautions are required. The operator needs to make a careful approach to prevent pleural tearing during catheter and sheath placement. If the pleura are injured during the procedure, the local anesthetics will leak into the pleural cavity, which may lead to an insufficient analgesic effect. We used a blunt-end tunneler to prevent pleural injury, and achieved direct visualization during the procedure using a thoracoscope. If there were severe inter-pleural adhesions, massive inter-pleural adhesiolysis was performed during VATS pulmonary resection; in cases of significant pleural damage, especially to the costal pleura due to any operative or patient-related factors, we felt that the On-Q system was inappropriate. The On-Q system catheter should cover all intercostal levels that were used for operative incisions for the VATS pulmonary resection. Finally, the On-Q system should be avoided in patients with a significant bleeding tendency.
This study had several limitations. First, it was a retrospective study and the assignment of the pain control device to each patient was not randomized, resulting in selection bias. IV-PCA was performed mainly in patients with pleural adhesions or incidental injury of the parietal pleura during the operation. Second, the study had a relatively small number of cases. Third, the additional IV injection for analgesia was not standardized and the potency of the pain control differed with the drugs used. The additional intervention for pain control was measured according to the number of injections because we could not quantify each IV agent. Despite these limitations, our study is the first to compare IV-PCA and the ON-Q system in the context of thoracoscopic surgery only. Unlike other studies, the consistency of patients is an advantage of our study. Previous studies enrolled patients who underwent different surgical procedures, such as lobectomy, wedge resection, or pleurodesis (11). We limited the study group to patients with primary lung cancer who underwent thoracoscopic pulmonary resections, and a single surgeon performed all of the operations using a standard operative technique. A randomized prospective study of various pain-control devices after thoracoscopic surgery should be performed to improve postoperative pain control.
In conclusion, the efficacy of the ON-Q system was equivalent to IV-PCA in postoperative pain control after thoracoscopic pulmonary resection for primary lung cancer, with fewer side effects and less early discontinuation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of our institution (No. AJIRB-MED-MDB-15-446).
References
- Ried M, Schilling C, Potzger T, et al. Prospective, comparative study of the On-Q® PainBuster® postoperative pain relief system and thoracic epidural analgesia after thoracic surgery. J Cardiothorac Vasc Anesth 2014;28:973-8. [Crossref] [PubMed]
- Kolettas A, Lazaridis G, Baka S, et al. Postoperative pain management. J Thorac Dis 2015;7:S62-72. [PubMed]
- Deneuville M, Bisserier A, Regnard JF, et al. Continuous intercostal analgesia with 0.5% bupivacaine after thoracotomy: a randomized study. Ann Thorac Surg 1993;55:381-5. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Perttunen K, Nilsson E, Kalso E. I.v. diclofenac and ketorolac for pain after thoracoscopic surgery. Br J Anaesth 1999;82:221-7. [Crossref] [PubMed]
- Gebhardt R, Mehran RJ, Soliz J, et al. Epidural versus ON-Q local anesthetic-infiltrating catheter for post-thoracotomy pain control. J Cardiothorac Vasc Anesth 2013;27:423-6. [Crossref] [PubMed]
- Kim JA, Kim TH, Yang M, et al. Is intravenous patient controlled analgesia enough for pain control in patients who underwent thoracoscopy? J Korean Med Sci 2009;24:930-5. [Crossref] [PubMed]
- Pasero C, McCaffery M, editors. Pain: clinical manual. St. Louis, MO: Mosby Incorporated, 1999.
- Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg 2009;36:170-80. [Crossref] [PubMed]
- Wheatley GH 3rd, Rosenbaum DH, Paul MC, et al. Improved pain management outcomes with continuous infusion of a local anesthetic after thoracotomy. J Thorac Cardiovasc Surg 2005;130:464-8. [Crossref] [PubMed]
- Yie JC, Yang JT, Wu CY, et al. Patient-controlled analgesia (PCA) following video-assisted thoracoscopic lobectomy: comparison of epidural PCA and intravenous PCA. Acta Anaesthesiol Taiwan 2012;50:92-5. [Crossref] [PubMed]
- Kanazi GE, Ayoub CM, Aouad M, et al. Subpleural block is less effective than thoracic epidural analgesia for post-thoracotomy pain: a randomised controlled study. Eur J Anaesthesiol 2012;29:186-91. [Crossref] [PubMed]
- Esme H, Apiliogullari B, Duran FM, et al. Comparison between intermittent intravenous analgesia and intermittent paravertebral subpleural analgesia for pain relief after thoracotomy. Eur J Cardiothorac Surg 2012;41:10-3. [PubMed]
- Helms O, Mariano J, Hentz JG, et al. Intra-operative paravertebral block for postoperative analgesia in thoracotomy patients: a randomized, double-blind, placebo-controlled study. Eur J Cardiothorac Surg 2011;40:902-6. [PubMed]


