
Safe and efficient 2-step implementation of totally minimally invasive esophagectomy
Highlight box
Key findings
• Hybrid minimal invasive esophagectomy can serve as a step towards totally minimally invasive esophagectomy in carefully selective patients without risking learning curve associated complications.
What is known and what is new?
• As a consequence of the recent evidence on learning curve associated increased risk of postoperative complications including anastomotic leakage when transiting to totally minimal invasive esophagectomy, it is of great importance to establish safe and efficient implementation strategies to avoid unnecessary complications.
• We present a such strategy and results of a stepwise implementation in carefully selected patients with no increase of postoperative complications during the implementation period. Laparoscopic assisted hybrid minimal invasive esophagectomy served as first step towards totally minimal invasive esophagectomy, and not until this was mastered, the thoracoscopic phase was included in carefully selected patients.
What is the implication, and what should change now?
• This study presents an example to follow for surgeons performing open esophagectomy aiming to implement totally minimal invasive esophagectomy.
Introduction
In patients with esophageal cancer, radical esophagectomy is a key component for curative treatment, and for decades, open esophagectomy has been the standard procedure.
The introduction of minimal invasive surgery, in terms of laparoscopy and thoracoscopy, has paved the way for surgical treatment of these cancers. Open esophagectomy is now increasingly being replaced by minimal invasive esophagectomy (MIE), either hybrid minimal invasive esophagectomy (HMIE) or totally minimal invasive esophagectomy (TMIE) (1). HMIE is usually performed as laparoscopic-assisted esophagectomy with the intrathoracic anastomosis performed through thoracotomy while TMIE includes both laparoscopic and thoracoscopic approach. TMIE is associated with reduced postoperative morbidity, shorter hospital stay and a better short-term quality of life compared to open esophagectomy and HMIE (1-4).
Implementation of TMIE is challenging, has a long learning curve and is associated with a considerable learning curve-related morbidity (5). In a multicenter study of 646 patients, it was claimed that as many as 36 patients experienced learning curve related anastomosis leakage (5).
A learning curve associated risk of anastomotic leakage of 18% before the learning curve was completed and leakage rate was reduced to 4% emphasizes the need for a safe implementation strategy without compromising the quality of the surgery (5).
The first TMIE in our institution was performed in September 2016. Prior to this, HMIE with laparoscopic gastric mobilization and intrathoracic anastomosis through a right thoracotomy, was the standard procedure for gastric pull-up procedures for 10 years, starting in 2006.
Simultaneously with increasing experience and standardization of HMIE, the thoracic surgeons introduced video-assisted thoracoscopic surgery (VATS), leading to surgical and practical familiarization of the minimal invasive procedures of the entire surgical team. In this way, HMIE and VATS served as a step towards TMIE.
The aim of this study was to evaluate the complication rate during implementation of TMIE, after 10 years of HMIE as standard procedure. The hypothesis of the study was that a 2-step implementation strategy and carefully selection of the patients provides a safe implementation of the TMIE procedure from the HMIE procedure without compromising the postoperative outcomes and oncological outcome in terms of surgical radicality and number of lymph nodes taken out assessed in pathological examination. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-462/rc).
Methods
The study was carried out as a retrospective observational single center cohort study including all patients undergoing totally minimally invasive Ivor Lewis esophagectomy due to cancer or high-grade dysplasia from September 1st, 2016 to August 31st, 2021 in a single high-volume tertiary institution (Aarhus University Hospital). Patients undergoing other esophagectomy than TMIE was excluded. Data was collected from patient files and included patient and tumor characteristics, pre-operative neoadjuvant oncologic treatment details, intraoperative details, postoperative complications, final histopathology and details about length of stay (LOS), readmission and mortality.
Registration of complications within 30 days from surgery, or during the in-hospital stay after surgery, followed International Consensus on standardization of data collection of complications associated with esophagectomy proposed by the Esophagectomy Complication Consensus Group (ECCG) (6). The Clavien-Dindo classification was used to rank complications (7). The procedure was evaluated using the updated nine variables for textbook outcome for esophageal cancer surgery, presented by Kalff et al. (8) in 2021. The nine variables include tumor-negative resection margins, ≥20 lymph nodes retrieved, no intraoperative complication, no complication of ≥ Clavien-Dindo III, no anastomotic leakage, no intensive care unit admission, no hospital stay ≥14 days, no in-hospital mortality and no readmission related to surgical procedure.
Registration of pneumonia followed the criteria defined by the American Thoracic Society and Infectious Diseases Society of America as the presence of new lung infiltrate plus clinical evidence that the infiltrate is of an infectious origin, which include new onset of fever, purulent sputum, leukocytosis, and decline in oxygenation (9).
Operative technique
Prior to surgery, all diagnosed esophageal cancer patients were discussed in multidisciplinary team (MDT) meetings. Eligible patients for surgery were referred to surgery preceded by neoadjuvant chemo(radio)therapy.
All procedures were performed in collaboration with experienced minimal invasive general and thoracic surgeons. The general surgeon was the operating surgeon in the laparoscopic part with assistance from the thoracic surgeon and the thoracic surgeon was the operating surgeon in the thoracic part with assistance from the general surgeon.
Prior to surgery, all patients received a thoracic epidural catheter and a double lumen endotracheal tube. The laparoscopic part was performed using 5 laparoscopic ports with the operating surgeon placed between the patient’s leg. When the gastric tube conduit was constructed using laparoscopic staplers (Endo GiaTM universal, Covidien, Medtronic, Minneapolis, USA), the wounds were closed, and the patient was repositioned on the left side in a 90-degree angle. Single-lung ventilation was established and five 12-mm thoracoscopic ports were placed in the right side of the thorax with the operating surgeon placed on the back side of the patient. The esophagus was mobilized and resected using ultrasonic and bipolar energy (Thunderbeat, Olympus Medical Systems, Tokyo, Japan) to the desired level of resection. Usually, the esophagus was resected from cardia to the level of the azygos vein. The thoracic duct was not ligated unless iatrogenic injury occurred. The azygos vein was spared unless the anastomosis was intended cranially to the vein. The resected esophagus was pulled out through a mini thoracotomy (12-mm thoracoscopic port was extended and a wound protector retractor was placed (Alexis wound protector-retractor, Applied Medical, California, USA) without using a rib spreader. The anvil of the circular stapler (EEATM Circular Stapler with Tri-StapleTM Technology, Covidien, Medtronic) in the oral part of esophagus was secured by purse-string sutures using the endoscopic suturing device (Endo StitchTM, Medtronic). Intrathoracic anastomosis was performed end-to-side using the circular stapler introduced trough the gastric conduit tube. The anastomosis was covered with an omentum/pleural flap. A chest tube was inserted and carefully placed parallel to the conduit. The lung was inflated, and two-lung ventilation was again established. At the end of the procedure, pylorus was dilated with a 20-mm balloon and a triple lumen nasojejunal tube was placed under flexible gastroscopic vision.
Postoperative care
The recovery after surgery took place on a special thoracic recovery unit, and the patient was transferred to the thoracic ward on the first postoperative day. The patient started enteral feeding through the nasojejunal tube on the first night after surgery, with increasing dosage until full dosage on the third postoperative day. The first 5 days the patients were restricted to an oral intake of 200 mL water, with increasing volume the following days until oral liquid diet was initiated on the 8th postoperative day where the chest tube was removed if no change in drainage output. The patient was discharged when the intake reaches 75% of the calculated required intake for the patient.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the hospital administration of Aarhus University Hospital and Central Denmark Region. This approval allowed data extraction without informed consent according to The Danish Health Care Act Section 42d Subsection 2.
Results
A total of 369 patients underwent esophagectomy due to cancer or multifocal high-grade dysplasia in the study period from September 1st, 2016 to August 31st, 2021. Of those, 120 patients underwent TMIE corresponding to 32.5% of the cases (see Figure 1).
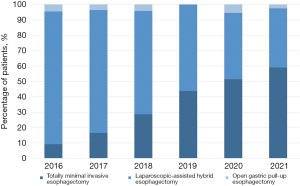
With the first TMIE performed in September 2016 the percentage of TMIE has increased from 10% of the cases in 2016 to approximately 50% of all gastric pull-up esophagectomies performed in 2020 and 2021 until August 31st (see Figure 2).
Patient and tumor characteristics are shown in Table 1.
Table 1
| Patient characteristics | Values |
|---|---|
| Gender, n (%) | |
| Male | 100 (83.3) |
| Female | 20 (16.7) |
| Age (years), mean [range] | 68 [39–84] |
| BMI (kg/m2), mean ± SD | 26.2±5.2 |
| Tumor location, n (%) | |
| Esophagus | 31 (25.8) |
| Gastroesophageal junction | 89 (74.2) |
| Histology, n (%) | |
| Adenocarcinoma | 94 (78.3) |
| Squamous cell carcinoma | 19 (15.8) |
| Other | 7 (5.8) |
| cTNM—T-disease, n (%) | |
| T1 | 4 (3.3) |
| T2 | 17 (14.2) |
| T3 | 94 (78.3) |
| T4 | 1 (0.8) |
| Unknown (entire cTNM is missing) | 4 (3.3) |
| Neoadjuvant therapy, n (%) | |
| Completed | 65 (54.2) |
| Interrupted | 15 (12.5) |
| No neoadjuvant therapy | 39 (32.2) |
| Unknown | 1 (0.8) |
| Type of neoadjuvant therapy, n (%) | |
| Chemotherapy | 27 (33.8) |
| Chemoradiotherapy | 52 (65.0) |
| Unknown | 1 (1.2) |
BMI, body mass index; SD, standard deviation; cTNM, clinical TNM staging; TNM, tumor, node, metastasis.
Conversion did not occur in the abdominal procedure but occurred in five patients in the thoracic phase, three of them needed additional suturing on the anastomosis or stapler line, the remaining two due to visible tumor in the resection margin and hereby further dissection to obtain macroscopic free margins was performed using open surgery. Mean number of retrieved lymph nodes was 29 lymph nodes and in 113 patients (94.2%) ≥16 lymph nodes were retrieved. Tumor-negative resection margins (R0) was achieved in 118 (98.3%). In the remaining two patients, tumor was present in the anal resection margin.
Seven patients (5.8%) experienced postoperative pneumonia. Anastomotic leakage occurred in 9 patients (7.5%) during the study period. Seven out of the nine leakages were type I or II leakage (see Table 2). The 90-day survival was 99.2%. One patient died 31 days after surgery, due to irreversible severe brain damage after resuscitation from cardiac arrest. There was no 30-day mortality.
Table 2
| Complications | N (%) |
|---|---|
| Pulmonary, n (%) | |
| Pneumonia | 7 (5.8) |
| Pleural effusion requiring additional drainage procedure | 16 (13.3) |
| Pneumothorax requiring treatment | 4 (3.3) |
| Atelectasis mucous plugging requiring treatment | 5 (4.2) |
| Cardiac, n (%) | |
| Cardiac arrest | 1 (0.8) |
| Dysrhythmia atrial requiring treatment | 20 (16.7) |
| Gastrointestinal, n (%) | |
| Esophagoenteric leak from anastomosis or staple line | 9 (7.5) |
| Type I—local defect requiring no change in therapy or treated medically or with dietary modification | 1 |
| Type II—localized defect requiring interventional but not surgical therapy, for example interventional radiology drain or stent | 6 |
| Type III—Localized defect requiring surgical therapy | 2 |
| Conduit necrosis/failure† | 2 (1.7) |
| Need for dilatation of pylorus | 13 (10.8) |
| Thromboembolic, n (%) | |
| Stroke | 1 (0.8) |
| Neurologic/psychiatric, n (%) | |
| Recurrent nerve injury | 1 (0.8) |
| Acute delirium | 4 (3.3) |
| Infection, n (%) | |
| Wound infection requiring opening wound or antibiotics | 9 (7.5) |
| Other, n (%) | |
| Chyle leak‡ | 1 (0.8) |
†, conduit necrosis: type II, focal necrosis identified endoscopically and not associated with free anastomotic or conduit leak; ‡, type IIA chyle leak, output <1 L/day, treated with total parenteral nutrition in 10 days.
A total of 88 (73.3%) patients experienced no complications or ≤ Clavien-Dindo grade II complication. Figure 3 shows the distribution of complications according to the Clavien-Dindo classification among all patients each year, except 2016 and 2017 which are combined due to small number of patients. The difference from shown percentage distribution of complications to 100% represents the patients without any complication.
To evaluate the relevant outcomes beyond anastomotic leakages, textbook outcome for esophageal cancer surgery was calculated. A total of 55 (45.8%) patients achieved textbook outcome for esophageal cancer surgery based on the nine variables included in the updated variables from 2021 (8).
Discussion
This study included all patients from the implementation phase of TMIE in a single high-volume center. With the first patients undergoing TMIE in fall 2016, the implementation was considered succeeded during 2020 and 2021 where more cases were performed totally minimal invasive than hybrid minimal invasive. The study presents a safe transition to totally minimally invasive esophagectomy without compromising postoperative complications using a 2-step implementation procedure in carefully selective patients.
There are some limitations in this study. The results presented in this study was retrospective and there was no comparison of complication rates due to the lack of a control group. The patients undergoing HMIE did not serve as control group since these patients are a different type of patients with bigger tumors and more comorbidity, especially pronounced in the beginning of the implementation phase. However, the aim of the study was not to compare the complication rates to other type of esophagectomies, merely to evaluate the complication rates only in patients undergoing TMIE during the implementation period. Another major limitation is selection bias, where only the most suitable patients were offered TMIE especially in the start of the study period. The possibility of the complication rates to be affected by selection bias is one of the central messages of this study, meaning that carefully selection of patients when implementing TMIE may be crucial to avoid learning curve-related increase of complications. When reporting the actual complication rates after TMIE in our institution, the distribution of complications in 2020 and 2021 where the majority of the patients were offered TMIE probably show the true distribution of complications. Since the presented implementation strategy took place in a single high-volume tertiary institution by dedicated esophageal cancer specialists with advanced laparoscopic and thoracoscopic skills, it might not be the same results in another institution with another setup. In addition to that, the importance of centralization of advanced upper gastrointestinal cancer surgery has been shown in several studies (10-12), and in Denmark, the centralization occurred in 2003 to 2006 where the numbers of centres treating esophageal cancer was reduced from 26 centres to just four centres. This improved the quality of care with lower anastomotic leakage and lower 30- and 90-day mortality (13).
This study showed a low rate of anastomotic leakage during the implementation period starting from September 2016. The highest rate was in 2019 where 10.7% of the patients who underwent TMIE experienced anastomotic leakage. A leakage rate above this level, did not occur in any year of the implementation period in contradiction to the findings in the Dutch multicenter center study including 646 patients, where learning curve associated leakage reached 18.8% during the implementation period (14). In our center, postoperative computer tomographic (CT) scan was not performed routinely, hereby, only patients with clinical suspicion of anastomotic leakages had CT scan performed, and silent leaks may have been overlooked. However, these would probably have been without any clinical relevance. The majority of the patients in this study with anastomotic leakage was endoscopic managed using a stent, however, two of the nine patients required surgical treatment. This is in accordance with data from the Society of Thoracic Surgeons National Database and Online Esodata database showing that a significant lower number of anastomotic leakages after minimally invasive esophagectomy required surgical treatment compared to anastomotic leakages after open esophagectomy (1,4).
Pneumonia rate was low (5.8%) during the study period, this may be due to a restrictive definition from the American Thoracic Society and Infectious Diseases Society of America used in the ECCG definition. In a large database evaluation of 6,022 esophageal cancer patients, the pneumonia rate was 12.8% when using ECCG definitions (1). Defining and reporting pneumonia rates are two very different things, hence, the comparison of pneumonia rate in studies and trials should be done with caution. However, two different definitions were used in two randomized controlled trials on minimal invasive esophagectomy with pneumonia rates on 12.8% and 12%, respectively (15,16) suggesting that the pneumonia rate after minimal invasive esophagectomy is approximately 12%. Numbers of retrieved lymph nodes was ≥16 in 94% of the patients. This cut off number was set according to national and international guidelines for lymph nodes harvest and accurate staging during esophagectomy (17).
This study displays a strategy for implementing TMIE, and the study comments only on the implementation of laparoscopic and thoracoscopic assisted Ivor Lewis esophagectomy and not robotic assisted esophagectomy or other minimally invasive techniques. This study suggests a 2-step approach where the thoracoscopy is added when the laparoscopic part is mastered and the thoracic surgeons are trained in thoracoscopic lung surgery, other set-ups where the thoracoscopic part is the first minimal invasive technique to be mastered followed by the laparoscopic part can possibly provide a similar safe implementation with no reduction of the quality of the surgery and without compromising the locoregional control of the cancer disease. The reduction in pulmonary complication after transition to minimally invasive esophagectomy compared to open esophagectomy can be seen after both HMIE (15) and TMIE (16). Nonetheless, the MIRO trial compared laparoscopic-assisted HMIE to open esophagectomy and showed significantly less pulmonary complication after hybrid esophagectomy suggesting that the minimal invasive abdominal phase attribute to this reduction and proves the benefit of laparoscopic-assisted HMIE as the first step for surgeons performing open esophagectomy aiming to implement TMIE.
Prior to implementation of TMIE, the esophageal cancer team consisting of general- and thoracic surgeons with advanced laparoscopic and thoracoscopic skills and dedicated surgical nurses visited a high volume minimally invasive esophageal cancer center in Helsinki, Finland. Here the team was introduced to the procedures and challenging aspects before introducing TMIE. Approximately 500 esophagectomies was performed by the surgical team of two general surgeons and two thoracic surgeons in the 10 years prior to implementation of TMIE in 2016. The first patients offered TMIE was carefully selected and only patients with small tumors in the gastroesophageal junction and no comorbidity met the criteria for TMIE in the beginning of the implementation. Only a few cases suitable for TMIE was offered TMIE in the beginning, and as the implementation went on without any increase of postoperative complications including leakage, more patients with bigger tumors and more comorbidity was offered TMIE. Now TMIE is the standard offer for all patients with tumors in distal esophagus or gastrointestinal junction regardless of their comorbidity. If patients are offered open Ivor Lewis esophagectomy or HMIE, it is usually due to frozen section on specific lymph nodes before proceeding the operation e.g., lymph node near the adrenal gland or lower paratracheal lymph nodes.
Surgical simulation training was not used in our institution, though it is widely used to improve operative performance and practice complex procedures in a realistic artificial setting, and for esophageal and foregut surgery, a few simulators are designed (18-21) where the procedures can be trained and the quality of the surgery can be measured. Surgical simulation training can have significant effect on operative performance (22) and may have beneficially effect on implementation of complex procedures like TMIE.
Conclusions
HMIE can serve as a step towards totally minimally invasive esophagectomy. We found that learning curve associated complications was minimized and a high level of cancer control was achieved by a 2-step implementation strategy in carefully selected patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-462/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-462/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-462/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-462/coif). TDC has been on the speaker bureaus for AstraZeneca and Bristol-Myers Squibb and has been in an Advisory Board for Sanofi and AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the hospital administration of Aarhus University Hospital and Central Denmark Region. This approval allowed data extraction without informed consent according to The Danish Health Care Act Section 42d Subsection 2.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kuppusamy MK, Low DEInternational Esodata Study Group (IESG). Evaluation of International Contemporary Operative Outcomes and Management Trends Associated With Esophagectomy: A 4-Year Study of >6000 Patients Using ECCG Definitions and the Online Esodata Database. Ann Surg 2022;275:515-25. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of Life and Late Complications After Minimally Invasive Compared to Open Esophagectomy: Results of a Randomized Trial. World J Surg 2015;39:1986-93. [Crossref] [PubMed]
- Sihag S, Kosinski AS, Gaissert HA, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Comparison of Early Surgical Outcomes From The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101:1281-8; discussion 1288-9. [Crossref] [PubMed]
- van Workum F, Stenstra MHBC, Berkelmans GHK, et al. Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann Surg 2019;269:88-94. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Kalff MC, van Berge Henegouwen MI, Gisbertz SS. Textbook outcome for esophageal cancer surgery: an international consensus-based update of a quality measure. Dis Esophagus 2021;34:doab011. [Crossref] [PubMed]
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [Crossref] [PubMed]
- Anderson O, Ni Z, Møller H, et al. Hospital volume and survival in oesophagectomy and gastrectomy for cancer. Eur J Cancer 2011;47:2408-14. [Crossref] [PubMed]
- Tol JA, van Gulik TM, Busch OR, et al. Centralization of highly complex low-volume procedures in upper gastrointestinal surgery. A summary of systematic reviews and meta-analyses. Dig Surg 2012;29:374-83.
- Dikken JL, van Sandick JW, Allum WH, et al. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg 2013;100:83-94. [Crossref] [PubMed]
- Kjaer DW, Larsson H, Svendsen LB, et al. Changes in treatment and outcome of oesophageal cancer in Denmark between 2004 and 2013. Br J Surg 2017;104:1338-45. [Crossref] [PubMed]
- Claassen L, Hannink G, Luyer MDP, et al. Learning Curves of Ivor Lewis Totally Minimally Invasive Esophagectomy by Hospital and Surgeon Characteristics: A Retrospective Multinational Cohort Study. Ann Surg 2022;275:911-8. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Zhan C, Shi Y, Jiang W, et al. How many lymph nodes should be dissected in esophagectomy with or without neoadjuvant therapy to get accurate staging? Dis Esophagus 2020;33:doz009. [Crossref] [PubMed]
- Schlottmann F, Murty NS, Patti MG. Simulation Model for Laparoscopic Foregut Surgery: The University of North Carolina Foregut Model. J Laparoendosc Adv Surg Tech A 2017;27:661-5. [Crossref] [PubMed]
- Fann JI, Feins RH, Hicks GL Jr, et al. Evaluation of simulation training in cardiothoracic surgery: the Senior Tour perspective. J Thorac Cardiovasc Surg 2012;143:264-72. [Crossref] [PubMed]
- Trehan K, Zhou X, Tang Y, et al. THE GooseMan: a simulator for transhiatal esophagectomy. J Thorac Cardiovasc Surg 2013;145:1450-2. [Crossref] [PubMed]
- Fabian T, Glotzer OS, Bakhos CT. Construct validation: simulation of thoracoscopic intrathoracic anastomosis. JSLS 2015;19:e2015.00001.
- Meling TR, Meling TR. The impact of surgical simulation on patient outcomes: a systematic review and meta-analysis. Neurosurg Rev 2021;44:843-54. [Crossref] [PubMed]




