
Comparison of percutaneous coronary intervention of native coronary artery versus bypass graft in patients with prior coronary artery bypass grafting
Highlight box
Key findings
• Compared with patients undergoing native coronary artery percutaneous coronary intervention (PCI), those undergoing bypass graft PCI had higher risk of slow-flow/no-reflow, peri-procedural stroke, and non-fatal myocardial infarction (MI) at follow-up.
What is known and what is new?
• There was insufficient evidence on PCI of native coronary artery being more beneficial to bypass graft. Some studies supported that a higher incidence of adverse cardiovascular events in graft group, but others found no difference between native and graft groups.
• In our study, we found that native coronary artery was preferred for PCI in patients with prior coronary artery bypass grafting (CABG), as native coronary artery PCI was correlated with lower rates of slow-flow/no-reflow, peri-procedural stroke, and non-fatal MI at follow-up.
What is the implication, and what should change now?
• Patients in graft group had worse outcomes compared to those in native group. Thus, it is prone to choose native coronary artery as the PCI target vessel in patients with prior CABG.
Introduction
Coronary artery disease (CAD) has been recognized as the leading cause of death worldwide. Coronary artery bypass grafting (CABG) surgery remains one of the main treatments for patients with three-vessel and/or left-main CAD globally (1). Because of high incidence of graft failure and the progression of atherosclerosis both in the native coronary artery and bypass graft, patients with prior CABG often develop symptom of recurrent angina pectoris or presentation with an acute coronary syndrome (ACS), and need for repeat revascularization (2,3). Compared with the initial CABG, repeat CABG has higher risk of mortality and worse outcome, therefore, percutaneous coronary intervention (PCI) is the preferred treatment strategy for revascularization in these patients (4).
The risk of bypass graft PCI, especially PCI of saphenous vein grafts (SVGs), was high, these patients most of the time had complex lesion in native coronary artery (5). Besides, accelerated progression of atherosclerosis of the native coronary artery after CABG, it is challenging to both native coronary artery PCI and bypass graft PCI (6). Several observational studies reported that native coronary artery was preferred for PCI in patients with prior CABG, and patients undergoing native coronary artery PCI had less procedural-related complications and adverse cardiovascular events compared with those undergoing bypass grafts PCI (5,7-10). Furthermore, the 2018 European Society of Cardiology (ESC) guidelines on myocardial revascularization recommended native coronary artery as the preferred interventional target-vessel in patients with failed bypass graft (11). However, it remains controversial whether native coronary artery PCI carries better outcomes than bypass graft PCI. Therefore, the purpose of the present study was to investigate the association between the PCI target-vessel (native coronary artery vs. bypass graft) and adverse cardiovascular events. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-473/rc).
Methods
Study design and patient population
This was an observational, retrospective study based on National Clinical Research Center of Cardiovascular Diseases (Beijing Anzhen Hospital, Beijing, China). From January 2010 to September 2020, 1,469 patients with prior CABG who underwent PCI were admitted in our cardiovascular center and consecutively enrolled in the study. The first PCI after CABG was defined as the index procedure, and the subsequent procedures were considered as outcomes. One hundred and thirty-eight patients did not undergo the index PCI and 55 patients were lost to follow-up. Ultimately, 1,276 patients were included in the study, which including 156 patients with emergency PCI and the rest received elective PCI. Patients were divided into native group and graft group according to whether they received native coronary artery or bypass graft PCI. Patients undergoing PCI of both a native coronary artery and a bypass graft were considered to be part of the graft group. No patient underwent PCI of both arterial grafts and SVGs.
The PCI procedures were performed according to standard preparation and technique via radial and/or femoral approach. The choice of target-vessel of bypass graft or native coronary artery was left to the discretion of the operators. The patient’s demographics, cardiovascular risk factors, medications, target vessel, coronary angiographic findings, procedural results and procedure-related complications were collected from electronic medical records and retrospectively studied. All patients were followed through clinic visit or telephone contact by independent personnel. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Beijing Anzhen Hospital, Capital Medical University (No. 2022084X) and individual consent for this retrospective analysis was waived.
Clinical outcomes
The primary study endpoint was major adverse cardiac and cerebrovascular events (MACCEs), a composite of all-cause death, non-fatal stroke, non-fatal MI, or target vessel revascularization (TVR). The secondary study endpoints included major adverse cardiac events (MACEs) (the composite of all-cause death, non-fatal MI and TVR, each individual component of the MACCE and the procedural-related complications. If stroke, MI or TVR occurred more than once, the first event was considered as the index outcome. The endpoints were assessed by at least two independent cardiologists.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median with interquartile range (IQR). Categorical variables were reported as counts and percentages. The baseline characteristics, procedural findings and outcomes were compared between groups. The two independent samples t-test or Mann-Whitney U test was applied to compare continuous variables between groups, and Chi-squared test or Fisher’s exact test was applied to compare categorical variables. Because the study patients were not randomized to receive native coronary artery or bypass graft PCI, we used inverse probability of treatment weighting (IPTW), a type of propensity score analysis, to adjust for confounders. Variables used to create these weights included demographics [age, gender, body mass index (BMI), systolic blood pressure (SBP)], risk factors [hypertension, dyslipidemia, diabetes mellitus (DM), current smoking, prior MI, prior PCI, heart failure (HF), cerebrovascular disease, peripheral arterial disease (PAD), chronic kidney disease (CKD), family history of CAD, anemia], clinical presentation [chronic coronary syndrome (CCS), non-ST-segment elevation ACS (NSTE-ACS) and ST-segment elevation myocardial infarction (STEMI)] and procedure findings [chronic total occlusion (CTO) lesions, thrombus lesions, LM/multi-vessel disease, number of lesions treated per patient, and number of drug eluting stents (DES) used per patient]. Survival analysis was demonstrated by Kaplan-Meier survival curves with or without IPTW, and compared the two groups with the log-rank test. Multivariable Cox proportional hazards regression analysis was conducted to estimate hazard ratio (HR) and 95% confidence interval (CI) with the native coronary artery PCI as the reference. Cox regression model was the same as the model of IPTW.
All P values were two sided, and P value <0.05 was considered statistical significance. Statistical analysis was performed with SPSS (version 24.0; IBM, Chicago, IL, USA) and R Programming Language (version 4.1.0; Vienna, Austria).
Results
Patient population
Between January 2010 to September 2020, 1,276 patients with prior CABG undergoing index PCI were consecutively enrolled in this study after excluding patients who did not undergo the index PCI and/or were lost to follow-up (Figure 1). In the study cohort, most target vessels in the patients included were the native coronary arteries (n=1,072, 84.0%) and with less were bypass grafts (n=204, 16.0%). There were 1,168 native coronary arteries underwent PCI with protected graft vessel. In graft group, 197 patients (96.6%) received SVG-PCI, 7 patients (3.4%) received LIMA-PCI. A total of 1,744 lesions were treated, including 1,529 lesions (87.7%) in native group and 215 (12.3%) in graft group. After 5 years from CABG, PCI of bypass graft increased. The proportion of graft group increased compared with native group with the time after CABG (Table 1).

Table 1
| Total patients | Post-CABG | |||
|---|---|---|---|---|
| 0–1 year (n=177) | >1–5 years (n=427) | >5–10 years (n=457) | >10 years (n=215) | |
| Native group | 156 (88.1) | 385 (90.2) | 377 (82.5) | 154 (71.6) |
| Graft group | 21 (11.9) | 42 (9.8) | 80 (17.5) | 61 (28.4) |
Values are n (%). CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Baseline characteristics
The age of the patients included in the study was 64.5±8.0 years, and 74.8% were males. The baseline characteristics of the patients classified according to the treated vessel are shown in Table 2. Compared with native group, patients in graft group had higher rates of hypertension, current smoking, PAD and use of angiotensin converting enzyme inhibitor (ACEI)/angiotensin II receptor blockers (ARBs) or Angiotensin receptor-neprilysin inhibitor (ARNI). Patients in graft group had higher levels of low-density lipoprotein-cholesterol (LDL-C) and fasting plasma glucose (FPG), more numbers of SVGs, more lesions treated per patient, and longer duration from CABG to PCI, but fewer number of DES implanted per patient. After adjusting for confounders by IPTW, we found that patients in graft group had more numbers of SVGs, longer time from CABG to PCI and higher proportion of Beta-blockers treated compared with native group.
Table 2
| Characteristics | Unweighted | Weighted | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n=1,276) | Native group (n=1,072) | Graft group (n=204) | P value | Native group | Graft group | P value | ||
| Demographics | ||||||||
| Age (years) | 64.5±8.0 | 64.6±7.9 | 63.7±8.8 | 0.122 | 64.5±7.9 | 64.4±8.9 | 0.995 | |
| Men | 955 (74.8) | 796 (74.3) | 159 (77.9) | 0.306 | 805 (74.9) | 150 (75.1) | 0.962 | |
| BMI (kg/m2) | 26.2±3.2 | 26.2±3.2 | 26.4±3.1 | 0.495 | 26.2±3.2 | 26.1±3.2 | 0.823 | |
| SBP (mmHg) | 130±17 | 130±16 | 131±19 | 0.464 | 130±17 | 131±19 | 0.437 | |
| Comorbidities | ||||||||
| Hypertension | 930 (72.9) | 769 (71.7) | 161 (78.9) | 0.042 | 784 (73.0) | 145 (72.6) | 0.935 | |
| Dyslipidemia | 1,270 (99.5) | 1,066 (99.4) | 204 (100.0) | 0.608 | 1,069 (99.5) | 200 (100.0) | 0.287 | |
| DM | 587 (46.0) | 488 (45.5) | 99 (48.5) | 0.476 | 499 (46.4) | 97 (48.6) | 0.611 | |
| Current smokers | 302 (23.7) | 239 (22.3) | 63 (30.9) | 0.011 | 255 (23.7) | 49 (24.4) | 0.856 | |
| Prior MI | 619 (48.5) | 522 (48.7) | 97 (47.5) | 0.823 | 523 (48.7) | 100 (50.0) | 0.702 | |
| Prior PCI | 275 (21.6) | 241 (22.5) | 34 (16.7) | 0.079 | 232 (21.6) | 44 (22.0) | 0.909 | |
| Heart failure | 112 (8.8) | 95 (8.9) | 17 (8.3) | 0.913 | 94 (8.8) | 17 (8.3) | 0.844 | |
| Cerebrovascular disease | 163 (12.8) | 134 (12.5) | 29 (14.2) | 0.577 | 137 (12.8) | 23 (11.7) | 0.698 | |
| PAD | 130 (10.2) | 98 (9.1) | 32 (15.7) | 0.007 | 110 (10.4) | 21 (10.5) | 0.887 | |
| CKD | 50 (3.9) | 42 (3.9) | 8 (3.9) | 1.000 | 42 (3.9) | 8 (3.8) | 0.964 | |
| Family history of CAD | 120 (9.4) | 96 (9.0) | 24 (11.8) | 0.259 | 101 (9.4) | 19 (9.5) | 0.959 | |
| Laboratory tests | ||||||||
| TG (mmol/L) | 1.8±1.4 | 1.8±1.5 | 1.7±1.3 | 0.713 | 1.8±1.5 | 1.7±1.1 | 0.309 | |
| TC (mmol/L) | 4.0±1.1 | 4.0±1.1 | 4.1±1.0 | 0.111 | 4.0±1.1 | 4.1±1.0 | 0.227 | |
| HDL-C (mmol/L) | 1.0±0.2 | 1.0±0.3 | 1.0±0.2 | 0.441 | 1.0±0.3 | 1.0±0.2 | 0.335 | |
| LDL-C (mmol/L) | 2.3±0.9 | 2.3±0.9 | 2.5±0.8 | 0.047 | 2.3±0.9 | 2.5±0.8 | 0.060 | |
| FPG (mmol/L) | 7.2±3.0 | 7.1±2.9 | 7.5±3.5 | 0.042 | 7.1±3.0 | 7.7±3.7 | 0.054 | |
| Glycosylated hemoglobin (%) | 6.7±1.3 | 6.7±1.3 | 6.9±1.5 | 0.150 | 6.7±1.3 | 6.9±1.5 | 0.343 | |
| LVEF (%) | 58±9 | 58±9 | 58±10 | 0.707 | 58±9 | 58±9 | 0.701 | |
| Emergency PCI | 156 (12.2) | 128 (11.9) | 28 (13.7) | 0.551 | 130 (12.1) | 27 (13.3) | 0.663 | |
| Symptoms | ||||||||
| CCS | 35 (2.7) | 29 (2.7) | 6 (2.9) | 1.000 | 29 (2.7) | 5 (2.4) | 0.836 | |
| NSTE-ACS | 1,203 (94.3) | 1,012 (94.4) | 191 (93.6) | 0.785 | 1,014 (94.4) | 190 (95.2) | 0.637 | |
| STEMI | 38 (3.0) | 31 (2.9) | 7 (3.4) | 0.849 | 32 (3.0) | 5 (2.4) | 0.638 | |
| Target vessel | ||||||||
| LM | 154 (12.1) | 142 (13.2) | 12 (5.9) | – | – | – | – | |
| LAD | 314 (24.6) | 291 (27.1) | 23 (11.3) | – | – | – | – | |
| LCX | 423 (33.2) | 396 (36.9) | 27 (13.2) | – | – | – | – | |
| RCA | 603 (47.3) | 573 (53.5) | 30 (14.7) | – | – | – | – | |
| LIMA-LAD | 7 (0.5) | – | 7 (3.4) | – | – | – | – | |
| SVG-LAD | 62 (4.9) | – | 62 (30.4) | – | – | – | – | |
| SVG-LCX | 82 (6.4) | – | 82 (40.2) | – | – | – | – | |
| SVG-RCA | 123 (9.6) | – | 123 (60.3) | – | – | – | – | |
| SVG-RI | 2 (0.2) | – | 2 (1.0) | – | – | – | – | |
| Number of LIMA | 0.408 | 0.276 | ||||||
| 0 | 259 (20.3) | 214 (20.0) | 45 (22.1) | 213 (19.8) | 44 (22.2) | |||
| 1 | 972 (76.2) | 815 (76.0) | 157 (77.0) | 819 (76.2) | 154 (77.2) | |||
| ≥2 | 11 (0.9) | 11 (1.1) | 0 (0.0) | 11 (1.0) | 0 (0.0) | |||
| Unknown | 34 (2.7) | 32 (3.0) | 2 (1.0) | 31 (2.9) | 1 (0.6) | |||
| Number of SVG | <0.001 | <0.001 | ||||||
| 0 | 74 (5.8) | 74 (6.9) | 0 (0.0) | 76 (7.0) | 0.0 (0.0) | |||
| 1 | 278 (21.8) | 247 (23.0) | 31 (15.2) | 247 (23.0) | 28 (14.0) | |||
| 2 | 515 (40.4) | 427 (39.8) | 88 (43.1) | 431 (40.1) | 94 (47.0) | |||
| 3 | 303 (23.7) | 234 (21.8) | 69 (33.8) | 233 (21.6) | 65 (32.4) | |||
| ≥4 | 64 (5.0) | 51 (4.7) | 13 (6.4) | 51 (4.7) | 11 (5.4) | |||
| Unknown | 42 (3.3) | 39 (3.6) | 3 (1.5) | 38 (3.5) | 3 (1.3) | |||
| Number of lesions treated per patient | 1 [1–2] | 1 [1–2] | 1 [1–2] | 0.001 | 1 [1–2] | 1 [1–2] | 0.992 | |
| Number of DES used per patient | 1 [1–2] | 2 [1–2] | 1 [1–2] | 0.015 | 2 [1–2] | 2 [1–2] | 0.750 | |
| Years from CABG to PCI | 6.4±4.4 | 6.1±4.3 | 8.0±4.6 | <0.001 | 6.1±4.3 | 7.8±4.7 | <0.001 | |
| Medication | ||||||||
| Aspirin | 1,267 (99.3) | 1,064 (99.3) | 203 (99.5) | 1.000 | 1,066 (99.2) | 198 (99.4) | 0.883 | |
| P2Y12 inhibitors | 1,267 (99.3) | 1,063 (99.2) | 204 (100.0) | 0.391 | 1,066 (99.2) | 200 (100.0) | 0.190 | |
| Statin | 1,263 (99.0) | 1,059 (98.8) | 204 (100.0) | 0.230 | 1,062 (98.9) | 200 (100.0) | 0.114 | |
| Beta-blockers | 1,067 (83.6) | 886 (82.6) | 181 (88.7) | 0.041 | 890 (83.0) | 180 (90.4) | 0.010 | |
| ACEI/ARBs or ARNI | 682 (53.4) | 556 (51.9) | 126 (61.8) | 0.012 | 568 (52.9) | 120 (60.2) | 0.102 | |
Values are mean ± standard deviation, median [interquartile range], or n (%). BMI, body mass index; SBP, systolic blood pressure; DM, diabetes mellitus; MI, myocardial infraction; PCI, percutaneous coronary intervention; PAD, peripheral artery disease; CKD, chronic kidney disease; CAD, coronary artery disease; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; FPG, fasting plasma glucose; LVEF, left ventricular ejection fraction; CCS, chronic coronary syndrome; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; STEMI, ST-segment elevation myocardial infarction; LM, left main; LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery; LIMA, left internal mammary artery; SVG, saphenous vein graft; RI, ramus intermedius; DES, drug eluting stent; CABG, coronary artery bypass grafting; ACEI, angiotensin converting enzyme inhibitor; ARBs, angiotensin II receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitor.
Procedural characteristics
Table 3 shows the procedural characteristics between the two groups. In the overall lesion treated, the rate of procedural success was 93.8%. Compared with native group, patients in graft group were more likely to select femoral approach, to undergo PCI of a thrombus and restenosis lesion, to have thrombolysis in myocardial infarction (TIMI) grade 3 flow post-PCI, to receive the therapy of Tirofiban, and more preferred to choose excimer laser coronary atherectomy (ELCA), but less likely to undergo PCI of a CTO lesion. Moreover, graft group had higher rate of procedure success, larger diameter and shorter length of the stents than native group. Most bypass graft target lesions (71.6%) were located at the body of the graft. Embolic protection devices (EPD) were used in 61 patients (28.4%) who underwent PCI of bypass graft, while none were used in native group (P<0.001). Large number of patients (90.4%) received DES implantation, whereas only 2 patients with 4 lesions received bare-metal stent (BMS) implantation during native coronary artery PCI.
Table 3
| Variables | Total (n=1,744) | Native group (n=1,529) | Graft group (n=215) | P value |
|---|---|---|---|---|
| Femoral approach | 940 (53.9) | 773 (50.6) | 140 (65.1) | <0.001 |
| PCIs success rate | 1,635 (93.8) | 1,425 (93.2) | 210 (97.7) | 0.017 |
| Lesion characteristics | ||||
| Chronic total occlusion | 418 (24.0) | 394 (25.8) | 24 (11.2) | <0.001 |
| Thrombus | 36 (2.1) | 20 (1.3) | 16 (7.4) | <0.001 |
| Restenosis lesion | 342 (19.6) | 127 (8.3) | 215 (100.0) | <0.001 |
| SVG lesion location | ||||
| Aortic anastomosis | 40 (2.3) | – | 40 (18.6) | – |
| Body | 154 (8.8) | – | 154 (71.6) | – |
| Distal anastomosis | 58 (3.3) | – | 58 (27.0) | – |
| Unknown | 5 (0.3) | – | 5 (2.3) | – |
| LIMA lesion location | 7 (0.4) | – | 7 (3.3) | – |
| Proximal | 2 (0.1) | – | 2 (0.9) | – |
| Distal anastomosis | 5 (0.3) | – | 5 (2.3) | – |
| Treatment | ||||
| Unfractionated heparin | 1,594 (91.4) | 1,402 (91.7) | 192 (89.3) | 0.298 |
| Tirofiban | 38 (2.2) | 27 (1.8) | 11 (5.1) | 0.004 |
| Multivessel PCI | 631 (36.2) | 559 (36.6) | 72 (33.5) | 0.423 |
| Use of IABP | 11 (0.6) | 11 (0.7) | 0 | 0.431 |
| Number of stents implanted per lesion | 1 [1–2] | 1 [1–2] | 1 [1–1] | 0.010 |
| Bare metal stent | 4 (0.2) | 4 (0.3) | 0 | 1.000 |
| Drug eluting stent | 1,576 (90.4) | 1,385 (90.6) | 191 (88.8) | 0.491 |
| Diameter of stents | 2.5±1.2 | 2.5±1.2 | 2.8±1.3 | <0.001 |
| Length of stents | 30.1±27.0 | 31.3±27.7 | 22.0±19.3 | <0.001 |
| Drug coated balloon | 52 (3.0) | 41 (2.7) | 11 (5.1) | 0.080 |
| Pre-PCI TIMI flow grade | <0.001 | |||
| 3 | 1,145 (65.7) | 1,009 (66.0) | 136 (63.3) | |
| 1–2 | 175 (10.0) | 121 (8.0) | 54 (25.1) | |
| 0 | 424 (24.3) | 399 (26.1) | 25 (11.6) | |
| Post-PCI TIMI flow grade | <0.001 | |||
| 3 | 1,601 (91.8) | 1,399 (91.5) | 202 (94.0) | |
| 1–2 | 33 (1.9) | 23 (1.5) | 10 (4.7) | |
| 0 | 110 (6.3) | 107 (7.0) | 3 (1.4) | |
| PTCA | 144 (8.3) | 125 (8.2) | 19 (8.8) | 0.843 |
| Excimer laser coronary atherectomy | 10 (0.6) | 6 (0.4) | 4 (1.9) | 0.029 |
| Use of embolic protection devices | 61 (3.5) | 0 | 61 (28.4) | <0.001 |
Values are mean ± standard deviation, median [interquartile range], or n (%). PCI, percutaneous coronary intervention; SVG, saphenous vein graft; LIMA, left internal mammary artery; IABP, intro-aortic balloon pump; TIMI, thrombolysis in myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty.
Short-term outcomes: procedural-related complications
Table 4 displays the procedural-related complications of the patients included in the study. Regardless of adjustment for confounders by IPTW, graft group had higher risk of slow-flow/no-reflow (1.5% vs. 0.1%, P=0.011 before IPTW, and 2.2% vs. 0.1%, P<0.001 after IPTW). Besides, graft group had higher incidence of peri-procedural stroke compared with native group (0.3% vs. 0, P=0.021 after IPTW). However, there was no significant difference in other complications.
Table 4
| Outcomes | Unweighted | Weighted | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n=1,276) | Native group (n=1,072) | Graft group (n=204) | P value | Native group | Graft group | P value | ||
| Procedural complications | 60 (4.7) | 45 (4.2) | 15 (7.4) | 0.077 | 47 (4.4) | 12 (6.1) | 0.385 | |
| In-hospital mortality | 1 (0.1) | 1 (0.1) | 0 | 1.000 | 1 (0.1) | 0 | 0.666 | |
| Peri-procedural MI | 1 (0.1) | 1 (0.1) | 0 | 1.000 | 1 (0.1) | 0 | 0.666 | |
| Peri-procedural stroke | 1 (0.1) | 0 | 1 (0.5) | 0.353 | 0 | 1 (0.3) | 0.021 | |
| Perforation of target vessel | 4 (0.3) | 3 (0.3) | 0 | 1.000 | 4 (0.4) | 0 | 0.461 | |
| Dissection of target vessel | 29 (2.3) | 23 (2.1) | 3 (1.5) | 0.723 | 24 (2.2) | 3 (1.3) | 0.405 | |
| Intramural hematoma of target vessel | 5 (0.4) | 3 (0.3) | 2 (1.0) | 0.392 | 3 (0.3) | 1 (0.5) | 0.491 | |
| Slow-flow/no-reflow | 4 (0.3) | 1 (0.1) | 3 (1.5) | 0.011 | 1 (0.1) | 5 (2.2) | <0.001 | |
| Temporary pacing | 2 (0.2) | 2 (0.2) | 0 | 1.000 | 2 (0.2) | 0 | 0.545 | |
| Cardiopulmonary resuscitation | 1 (0.1) | 1 (0.1) | 0 | 1.000 | 1 (0.1) | 0 | 0.666 | |
| Equipment loss or entrapment | 2 (0.2) | 2 (0.2) | 0 | 1.000 | 3 (0.3) | 0 | 0.559 | |
| Hemodynamic instability | 10 (0.8) | 6 (0.6) | 4 (2.0) | 0.100 | 6 (0.5) | 3 (1.2) | 0.209 | |
| Other complications | 4 (0.3) | 2 (0.2) | 2 (1.0) | 0.240 | 2 (0.2) | 1 (0.5) | 0.298 | |
Values are n (%). CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; MI, myocardial infarction.
Follow-up outcomes
During the 43-month (IQR, 24 to 66 months) follow-up period, 397 (31.1%) patients developed MACCE, which included 103 (8.1%) all-cause death, 39 (3.1%) non-fatal stroke, 57 (4.5%) non-fatal MI, and 198 (15.5%) TVR. Regardless of IPTW, the risk of MACCE in graft group was similar to that in native group. Patients in graft group had a significantly higher incidence of non-fatal MI than those in native group regardless of IPTW adjustment (7.8% vs. 3.8%, P=0.018 and 8.3% vs. 3.9%, P=0.030, separately). However, there was no difference in other outcomes (Table 5).
Table 5
| Outcomes | Unweighted | Weighted | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n=1,276) | Native group (n=1,072) | Graft group (n=204) | P value | Native group | Graft group | P value | ||
| All-cause death | 103 (8.1) | 87 (8.1) | 16 (7.8) | 1.000 | 88 (8.2) | 19 (9.7) | 0.542 | |
| Non-fatal stroke | 39 (3.1) | 36 (3.4) | 3 (1.5) | 0.225 | 36 (3.3) | 3 (1.2) | 0.088 | |
| Non-fatal MI | 57 (4.5) | 41 (3.8) | 16 (7.8) | 0.018 | 42 (3.9) | 17 (8.3) | 0.030 | |
| TVR | 198 (15.5) | 163 (15.2) | 35 (17.2) | 0.548 | 166 (15.4) | 34 (17.2) | 0.568 | |
| MACE | 358 (28.1) | 291 (27.1) | 67 (32.8) | 0.115 | 295 (27.4) | 70 (35.3) | 0.053 | |
| MACCE | 397 (31.1) | 327 (30.5) | 70 (34.3) | 0.320 | 331 (30.8) | 73 (36.5) | 0.167 | |
Values are n (%). CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; MI, myocardial infraction; TVR, target vessel revascularization; MACE, major adverse cardiac event; MACCE, major adverse cardiac and cerebrovascular event.
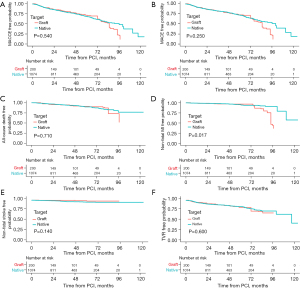
Kaplan-Meier analysis revealed that patients in graft group showed higher incidence of non-fatal MI (Log-rank P=0.017), while the incidence of MACCE, MACE, and other individual component of the MACCE was similar between the two groups (Figure 2). In addition, we found similar results after IPTW (Figure 3). Compared with native group, graft group was associated with a higher risk of non-fatal MI (HR: 2.091, 95% CI: 1.069–4.089; P=0.031), but similar in MACCE (HR: 1.077, 95% CI: 0.817–1.419; P=0.599) (Figure 4).


Discussion
This was a relatively large single-center retrospective study investigating the prognostic effect of different target vessels in prior CABG patients undergoing index PCI in China despite several large studies have been reported (5,9,12,13). This study demonstrated that, (I) most PCIs were performed in native coronary artery in prior CABG patients; (II) most bypass graft PCIs were done in SVGs; (III) patients in graft group had higher risk of slow-flow/no-reflow; and (IV) patients in graft group had higher incidence of non-fatal MI than native group.
In our study, native coronary arteries were the most frequent target vessels for intervention (84%), followed by bypass grafts (16%). Chen et al. reported that PCI was performed in native coronary arteries in 44% of the 95 patients, and first pointed that bypass graft PCI accounted for the majority with the time of CABG to PCI >10 years (2). However, Beerkens et al. found that native coronary artery PCI was performed in 73.5% of the 3,983 patients with prior CABG (13). Moreover, National Cardiovascular Data Registry (NCDR) Registry studies also supported that most PCIs were performed in the native coronary artery, but graft was more likely to be chosen as the PCI target vessel with the increasing time after CABG (5,9). In our study, we found that the proportion of bypass graft PCI increased with the time of CABG compared with native group, especially after 5 years of CABG, which was in line with the previous studies (5,9,10). One explanation for this finding is graft vessel, especially SVG, comes with accelerated pace of bypass graft failure in the late post-CABG, and another is progression atherosclerosis of native coronary artery, which may render native coronary artery unsuitable for PCI (3,14).
For patients studied, the difficulty and risk of PCIs were high, no matter in native coronary artery or bypass graft PCI (9,15). PCI of the native coronary artery is generally considered superior to bypass graft, and the ESC guidelines recommend the native coronary artery as the preferred target vessel for intervention since SVG-PCI carries higher incidence of adverse cardiovascular events (11). In clinical practice, the choice of the target vessel depends on the severity of native coronary artery or bypass graft lesions, and is left at the discretion of the operators. If there is diffusely degenerated lesion in bypass graft, native coronary artery may be chosen as the PCI target vessel. PCI of native coronary artery is not always possible to be performed when the presence of severely long, calcified, tortuous and CTO lesions in native coronary artery (9).
Our study found that the PCIs success rate in the native group was lower than that in the graft group. The main reason was the high number of patients with CTO lesions in the native group. Despite the emergence of novel techniques and advanced devices, the overall success rate of CTO-PCI remains low (16). It is a great challenge when performing CTO-PCI in prior CABG patients (17). Michael et al. found that patients with prior CABG had lower rate of technical success compared with those without prior CABG (18). Besides, history of prior CABG was independently associated with lower technical success rate. The RECHARGE study found that patients with prior CABG had higher CTO complexity and lower procedural success rate, and the procedural success rate was similar between CTO-PCI in graft vessel and native vessel (19). In our study, the overall success rate of CTO-PCI was 77.5% and the technical success rate was similar between two groups (76.6% vs. 91.7%, P=0.087), which was consistent with the RECHARGE study (19).
In our study, slow-flow/no-reflow phenomenon was considered as a relatively common complication of SVG-PCI compared to native group, which was consistent with the previous reports (5,12,20). One study reported that about 6–7% patients performed PCIs in SVG of all PCI patients with prior CABG (5,9). SVG-PCI has a higher incidence of no-reflow, which attributes to embolization of atheromatous material to the distal vasculature and intense arteriole vasospasm caused by microembolization of platelet-rich thrombi that releases vasoactive agents resulting in microvascular obstruction (5,21). Besides, Hong et al. demonstrated that patients with no-reflow had higher risk of death and MI compared with those without no-reflow (22).
To reduce the risk of no-reflow, advanced equipment and novel techniques are often used during SVG-PCI. In our study, EPD were used in SVG-PCI. A multicenter, retrospective cohort study showed that EPD was more common in SVG-PCI, about 38%, compared with 22% reported by the NCDR (23,24). The SAFER trial, the first multicenter randomized trial, found that EPD use was associated with lower rates of the primary end point, MI and no-reflow during stenting for SVG-PCI (25). In addition, a meta-analysis evaluating EPD in patients with SVG-PCI found that EPD had a consistent treatment benefit, reducing the incidence of adverse cardiac events at 30 days (26). Although the AHA/ACC guidelines recommend EPD use in SVG-PCI when technically feasible (Class I, Level of Evidence: B), the real-world usage remains limited (27).
With the improvement of ELCA and operator techniques, ELCA has been increasingly used for SVG intervention (28). It is able to vaporize the thrombus, reducing the risk of distal embolization (29). In the present study, ELCA was used more frequently in graft group compared to native group. “Staged revascularization”, a novel revascularization strategy, has been proposed for patients presenting with an ACS due to SVG failure, which is defined as the initial treatment of the culprit SVG followed by revascularization of the corresponding native coronary artery CTO a few weeks later. Of note, these patients undergoing staged revascularization may have better short and long-term outcomes (30).
It has been controversial whether native coronary artery PCI or bypass graft PCI is more beneficial in these patients. Garcia-Tejada et al. found that there was no difference in MACE, death and target lesion revascularization (TLR) between two groups during a median 2.5-year follow-up (7). Liu and his colleagues demonstrated that there was no difference in the incidence of MACE, cardiac death, non-fatal MI, or revascularization in diabetic patients between two groups during a median 45-month follow-up (10). However, several large registry studies have had different findings. VA-CART study, a national retrospective cohort study, found that graft group has higher incidence of mortality, MI, and repeat revascularization than native group during a 3.3-year follow-up (5). The Pan-London study further validated that graft group has significantly higher mortality compared with native group (12). In addition, several meta-analyses demonstrated that native coronary artery was the preferred target vessel, and patients in native group has lower incidence of long-term adverse cardiovascular events than bypass group (31,32).
As for long-term outcomes in our study, we found that patients in graft group carried a higher risk of non-fatal MI, but there was no difference in other study endpoints compared with native group with or without IPTW. What we found was not quite the same as before, and there were several possible explanations. First, atherosclerosis of SVG lesions often consists of vulnerable plaques and thrombus, which may embolize and cause myocardial necrosis (33). Progression of atherosclerosis in native coronary artery and graft overtime, which might be the major cause for the difference in non-fatal MI between the two groups. Besides, the SVG-PCI in the shaft lesions was correlated with a higher incidence of MI (34). In our study, most SVG-PCIs were performed in the body of SVG, which might be the possible reason for higher incidence of MI. Second, with the advent and improvement of interventional devices, such as EPD, ELCA, intravenous ultrasound (IVUS) and optical coherence tomography (OCT), which can help operators easily identify high-risk and complex lesions and further select more appropriate and safer strategies. Third, all patients studied were professionally evaluated by the interventional team, and then native coronary artery or bypass graft as the target vessel was determined.
In such patients, PCI was the safest and durable strategy for revascularization with a lower risk of in-stent restenosis, and should be preferred (32). If technically feasible, native coronary artery is preferred to be selected as target vessel, which is recommended in guidelines (11). Guidelines or consensus for coronary revascularization in prior CABG patients are limited. The Percutaneous Coronary Intervention of Native Coronary Artery Versus Venous Bypass Graft in Patients with Prior CABG (PROCTOR) trial (NCT03805048) is the first multi-center, randomized trial to investigate native coronary artery vs. bypass graft intervention in prior CABG patients, which will provide a paradigm for the selection of target vessels for such intervention in the future.
Limitations
There were several potential limitations of the study. First, this was a single-center, retrospective, observational study, and therefore subject to all the limitations of observational studies. Second, the sample size was relatively small, especially the number of CCS patients or patients in graft group. Although the study patients were enrolled consecutively, the majority of patients were male. Third, due to lack of randomization, the choice of target vessel was at the discretion of the operators. Although propensity score was performed, unmeasured confounders might explain some of the variability in procedural-related complications after PCI. Fourth, patients’ coronary anatomies were not assessed, such as the Synergy Between PCI With Taxus and Cardiac Surgery (SYNTAX) score. Fifth, the use of EPD for SVG-PCI was relatively low in our study. Possible reasons for low use of EPD include anatomic unsuitability, technical complexity and operator discretion.
Conclusions
This study found that native coronary artery might be preferred to be selected for PCI in patients with prior CABG because of lower rates of slow-flow/no-reflow phenomenon, peri-procedural stroke and non-fatal MI at follow-up. We are looking forward to the results of the PROCTOR randomized trial. More large-scale randomized trials are needed to provide more evidence for clinical practice.
Acknowledgments
We would like to express our gratitude sincerely to all those who helped us during the writing of this manuscript, and thanks to all the peer reviewers for their opinions and suggestions.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-473/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-473/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-473/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-473/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Beijing Anzhen Hospital, Capital Medical University (No. 2022084X) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629-38. [Crossref] [PubMed]
- Chen L, Théroux P, Lespérance J, et al. Angiographic features of vein grafts versus ungrafted coronary arteries in patients with unstable angina and previous bypass surgery. J Am Coll Cardiol 1996;28:1493-9. [Crossref] [PubMed]
- Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149-56. [Crossref] [PubMed]
- Morrison DA, Sethi G, Sacks J, et al. Percutaneous coronary intervention versus repeat bypass surgery for patients with medically refractory myocardial ischemia: AWESOME randomized trial and registry experience with post-CABG patients. J Am Coll Cardiol 2002;40:1951-4. [Crossref] [PubMed]
- Brilakis ES, O'Donnell CI, Penny W, et al. Percutaneous Coronary Intervention in Native Coronary Arteries Versus Bypass Grafts in Patients With Prior Coronary Artery Bypass Graft Surgery: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. JACC Cardiovasc Interv 2016;9:884-93. [Crossref] [PubMed]
- Hwang MH, Meadows WR, Palac RT, et al. Progression of native coronary artery disease at 10 years: insights from a randomized study of medical versus surgical therapy for angina. J Am Coll Cardiol 1990;16:1066-70. [Crossref] [PubMed]
- Garcia-Tejada J, Velazquez M, Hernandez F, et al. Percutaneous revascularization of grafts versus native coronary arteries in postcoronary artery bypass graft patients. Angiology 2009;60:60-6. [Crossref] [PubMed]
- Shoaib A, Johnson TW, Banning A, et al. Clinical outcomes of percutaneous coronary intervention for chronic total occlusion in native coronary arteries vs saphenous vein grafts. J Invasive Cardiol 2020;32:350-7.
- Brilakis ES, Rao SV, Banerjee S, et al. Percutaneous coronary intervention in native arteries versus bypass grafts in prior coronary artery bypass grafting patients: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv 2011;4:844-50. [Crossref] [PubMed]
- Liu D, Cui X, Luo X, et al. Long-term outcomes of percutaneous coronary intervention in grafts and native vessels in coronary artery bypass grafting patients with diabetes mellitus. J Thorac Dis 2019;11:4798-806. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Rathod KS, Beirne AM, Bogle R, et al. Prior Coronary Artery Bypass Graft Surgery and Outcome After Percutaneous Coronary Intervention: An Observational Study From the Pan-London Percutaneous Coronary Intervention Registry. J Am Heart Assoc 2020;9:e014409. [Crossref] [PubMed]
- Beerkens FJ, Singh R, Cao D, et al. Impact of target vessel choice on outcomes following percutaneous coronary intervention in patients with a prior coronary artery bypass graft. Catheter Cardiovasc Interv 2021;98:E785-95. [Crossref] [PubMed]
- Brilakis ES, Lee M, Mehilli J, et al. Saphenous vein graft interventions. Curr Treat Options Cardiovasc Med 2014;16:301. [Crossref] [PubMed]
- Gyenes G, Norris CM, Graham MM, et al. Percutaneous revascularization improves outcomes in patients with prior coronary artery bypass surgery. Catheter Cardiovasc Interv 2013;82:E148-54. [Crossref] [PubMed]
- Tajti P, Burke MN, Karmpaliotis D, et al. Update in the Percutaneous Management of Coronary Chronic Total Occlusions. JACC Cardiovasc Interv 2018;11:615-25. [Crossref] [PubMed]
- Thompson CA, Jayne JE, Robb JF, et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early U.S. experience. JACC Cardiovasc Interv 2009;2:834-42. [Crossref] [PubMed]
- Michael TT, Karmpaliotis D, Brilakis ES, et al. Impact of prior coronary artery bypass graft surgery on chronic total occlusion revascularisation: insights from a multicentre US registry. Heart 2013;99:1515-8. [Crossref] [PubMed]
- Budassi S, Zivelonghi C, Dens J, et al. Impact of prior coronary artery bypass grafting in patients undergoing chronic total occlusion-percutaneous coronary intervention: Procedural and clinical outcomes from the REgistry of Crossboss and Hybrid procedures in FrAnce, the NetheRlands, BelGium, and UnitEd Kingdom (RECHARGE). Catheter Cardiovasc Interv 2021;97:E51-60. [Crossref] [PubMed]
- Varghese I, Samuel J, Banerjee S, et al. Comparison of percutaneous coronary intervention in native coronary arteries vs. bypass grafts in patients with prior coronary artery bypass graft surgery. Cardiovasc Revasc Med 2009;10:103-9. [Crossref] [PubMed]
- Lee KW, Norell MS. Management of 'no-reflow' complicating reperfusion therapy. Acute Card Care 2008;10:5-14. [Crossref] [PubMed]
- Hong YJ, Jeong MH, Ahn Y, et al. Intravascular ultrasound findings that are predictive of no reflow after percutaneous coronary intervention for saphenous vein graft disease. Am J Cardiol 2012;109:1576-81. [Crossref] [PubMed]
- Aggarwal V, Stanislawski MA, Maddox TM, et al. Safety and effectiveness of drug-eluting versus bare-metal stents in saphenous vein bypass graft percutaneous coronary interventions: insights from the Veterans Affairs CART program. J Am Coll Cardiol 2014;64:1825-36. [Crossref] [PubMed]
- Mehta SK, Frutkin AD, Milford-Beland S, et al. Utilization of distal embolic protection in saphenous vein graft interventions (an analysis of 19,546 patients in the American College of Cardiology-National Cardiovascular Data Registry). Am J Cardiol 2007;100:1114-8. [Crossref] [PubMed]
- Baim DS, Wahr D, George B, et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation 2002;105:1285-90.
- Coolong A, Baim DS, Kuntz RE, et al. Saphenous vein graft stenting and major adverse cardiac events: a predictive model derived from a pooled analysis of 3958 patients. Circulation 2008;117:790-7. [Crossref] [PubMed]
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:e574-651. [Crossref] [PubMed]
- Fracassi F, Roberto M, Niccoli G. Current interventional coronary applications of excimer laser. Expert Rev Med Devices 2013;10:541-9. [Crossref] [PubMed]
- Tsutsui RS, Sammour Y, Kalra A, et al. Excimer laser atherectomy in percutaneous coronary intervention: a contemporary review. Cardiovasc Revasc Med 2021;25:75-85. [Crossref] [PubMed]
- Xenogiannis I, Tajti P, Burke MN, et al. Staged revascularization in patients with acute coronary syndromes due to saphenous vein graft failure and chronic total occlusion of the native vessel: A novel concept. Catheter Cardiovasc Interv 2019;93:440-4. [Crossref] [PubMed]
- Farag M, Gue YX, Brilakis ES, et al. Meta-analysis comparing outcomes of percutaneous coronary intervention of native artery versus bypass graft in patients with prior coronary artery bypass grafting. Am J Cardiol 2021;140:47-54. [Crossref] [PubMed]
- Farag M, Brilakis ES, Gasparini GL, et al. Percutaneous coronary intervention of native artery versus bypass graft in patients with prior coronary artery bypass graft surgery. Rev Cardiovasc Med 2022;23:232.
- Holmes DR Jr, Berger PB. Percutaneous revascularization of occluded vein grafts: is it still a temptation to be resisted? Circulation 1999;99:8-11. [Crossref] [PubMed]
- Hong YJ, Jeong MH, Ahn Y, et al. Impact of lesion location on intravascular ultrasound findings and short-term and five-year long-term clinical outcome after percutaneous coronary intervention for saphenous vein graft lesions. Int J Cardiol 2013;167:29-33. [Crossref] [PubMed]


