
The role of CD38 in ischemia reperfusion injury in cardiopulmonary bypass and thoracic transplantation: a narrative review
Introduction
Cardiothoracic surgeons specialize in supporting the body through cardiopulmonary bypass (CPB) and mechanical circulatory support in order to perform a variety of life-saving operations. This includes stopping and starting blood flow to the heart during CPB, and to the donor heart or lungs during procurement and subsequent organ transplant. The ischemia caused by the lack of blood flow, as well as the reestablishment of blood flow, results in an injury known as ischemia reperfusion injury (IRI). IRI ultimately results in dysregulation of cellular homeostasis, the breakdown of the endothelial barrier and ultimately cell death (1-3). Although this injury can affect all organs, some of the most devastating consequences arise from IRI in the heart and lungs (4,5).
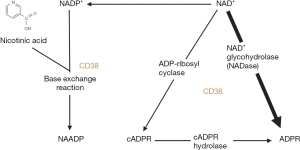
Although many molecular pathways lead to endothelial cellular injury, a common pathway leading to injury revolves around cluster of differentiation 38 (CD38). CD38 is an evolutionarily conserved multifunctional enzyme found in a wide variety of tissues and was originally discovered as an antigen marker on lymphocytes (6). It was later found to have adenosine diphosphate (ADP) ribosyl cyclase, cyclic ADP ribose (cADPR) hydrolase, nicotine adenine dinucleotide (NAD)+ glycohydrolase (NADase) and nicotinamide adenine dinucleotide phosphate (NADP)+ phosphatase (NADPase) function (Figure 1) (3). Today its primary and most widely recognized function is to serve as a major NADase in the body, which generates crucial secondary messengers for calcium mobilization: cADPR and nicotinic acid adenine dinucleotide phosphate (NAADP) (7,8). cADPR and NAADP are involved in the mobilization and equilibration of intracellular calcium, a prerequisite for maintaining cellular function, metabolism, and signaling (7). The NAD+/CD38/cADPR/Ca2+ axis is intimately involved in the production of inflammatory cytokines and signals, and indirectly regulates cell survival (2,3,9-12).
CD38 exists in two main typologies: type II, which is mainly extracellularly bound on the plasma membrane or inside endosomes or vacuoles, or type III, which is within the cytosol on the membrane of organelles such as the endoplasmic reticulum. This creates a typological paradox as CD38’s substrates (NAD+/NADP+) and its products (cADPR/NAADP) are located intracellularly (13-16). This notion is important to understand when determining which CD38 typology is activated, as this can lead to an increase or decrease in the inflammatory response and thus cell survival and longevity (5,17,18). By intervening on the fundamental molecular mechanisms that affect IRI, rather than mitigating its side-effects, CD38 modulation is a promising novel therapeutic target during CBP or thoracic transplantation.
The objective of this narrative review is to provide: (I) a basic overview of the NAD+/CD38/cADPR/Ca2+ axis; (II) the current understandings of the role of CD38 in IRI as it relates to CPB and transplantation; (III) CD38 modulation, and future directions of novel therapies to improve IRI-related injury and; (IV) explore the potential interaction of CD38 with CD39 in cardiovascular stress. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-725/rc) (19).
Methods
We conducted a review of the literature by performing a search of the PubMed database on 2 April 2023. To find relevant publications on CD38 we utilized the MeSH terms: “CD38” AND “Ischemia” OR “CD38” AND “Transplant” OR “CD38” AND “Heart” from 1990–2023. We included all papers that discussed CD38 in the context of IRI, thoracic transplantation, cardiac surgery, cardiac related IRI or could provide underlying mechanisms of CD38 that would provide historical or mechanistic background to the reader. Exclusion criteria included papers that discussed CD38 in the context of non-relatable disease states or treatments (i.e., cancer, bone marrow transplant, kidney or liver disease) and pathophysiology not related to IRI. Additionally, due to the novelty of this topic, papers were included that were referenced in papers from the original PubMed search (but for reasons unknown did not yield a search result PubMed) if they met the inclusion criteria. Using this search method, a total of 146 articles were found, and after inclusion and exclusion, and final selection 36 papers were included (Figure S1). The selection process was conducted amongst the authors: Gouchoe DA, Vijayakumar A, Aly AH and Cui EY. Consensus was obtained if a majority felt that it should be included or if the paper was needed to provide adequate background information to the reader. Full details on the search strategy can be seen in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 4/2/2023 |
| Databases and other sources searched | PubMed |
| Search terms used | “CD38” AND “Ischemia” OR “CD38” AND “Transplant” OR “CD38” AND “Heart” |
| Timeframe | 1990–2023 |
| Inclusion and exclusion criteria | Inclusion: papers that discussed CD38 in the context of IRI, thoracic transplantation, cardiac surgery, cardiac related IRI or could provide underlying mechanisms of CD38 that would provide historical or mechanistic background to the reader |
| Exclusion: papers that discussed CD38 in the context of non-relatable disease states or treatments (i.e., cancer, bone marrow transplant, kidney or liver disease) and pathophysiology not related to IRI | |
| Selection process | The selection process was conducted amongst the authors: Gouchoe DA, Vijayakumar A, Aly AH and Cui EY. Consensus was obtained if a majority felt that it should be included or if the paper was needed to provide adequate background information to the reader |
| Any additional considerations | Additionally, due to the novelty of this topic, papers were included that were referenced in papers from the original PubMed search (but for reasons unknown did not result in PubMed) if they met the inclusion criteria |
CD38, cluster of differentiation 38; IRI, ischemia reperfusion injury.
NAD+/CD38/cADPR/Ca2+ axis
A critical event and sequelae in IRI is the breakdown of the endothelial barrier due to the underlying injury or insult. The NAD+/CD38/cADPR/Ca2+ axis is a common pathway leading to injury and endothelial barrier dysfunction (2,3). When cells are injured, there is an activation of CD38 and resulting consumption of NAD+ and NADP+, and the production of secondary messengers: cADPR and NAADP (2,3).
NAD+ is a crucial coenzyme, serving a significant role in oxidation-reduction reactions and participating in the regulation of inflammatory and metabolic homeostasis (10,20). NADP+ is another essential coenzyme involved in mitochondrial and metabolic pathways, and its dysregulation causes increased oxidative stress (5). Furthermore, consumption of NAD+/NADP+ prevents these cofactors from being available to other NAD+-dependent deacetylases, which are important in cell signaling and cell metabolism (10). The generation of cADPR and NAADP causes a surge in intracellular Ca2+ (Figure 2). cADPR regulates intracellular Ca2+ homeostasis by acting as the signal for Ca2+ release via ryanodine receptor activation (21,22), while NAADP maintains intracellular Ca2+ homeostasis by signaling Ca2+ release from the two-pore channel receptor located in lysosomes, endolysosomes, or the ryanodine receptor (18-20). Because it controls many cellular functions, dysregulation of Ca2+ results in the elaboration of inflammatory cytokines, reactive oxygen species (ROS), and overall skewing of the cell towards an inflammatory phenotype (23,24). Therefore, the NAD+/CD38/cADPR/Ca2+ axis can directly regulate inflammation and indirectly regulate cell survival.

The CD38 typological paradox
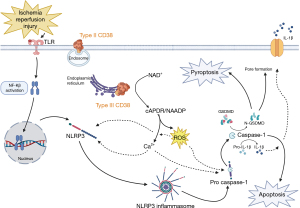
The CD38 exists in two main typologies, i.e., the orientation of a molecule in 3D space: type II and type III. CD38 has traditionally been characterized as a glycosylated cell surface protein (type II) protein with an extracellular catalytic domain (25). Type II CD38 functions to maintain NAD+ turnover in the extracellular environment, as well as regulate precursors of its synthesis (26). As has been described, this creates a typological paradox. The substrates (Ca2+, NAD+ and NADP+) and their products (cADPR, NAADP) are located primarily in the cytosol and (13,27), while the site of primary CD38 action was thought to be extracellular. However, a recent study has identified that CD38 also exists in a non-glycosylated type III typology (Figure 3) with the catalytic domain oriented to the cytoplasm (27). Type III CD38 is present on the membranes of cytosolic organelles including the nucleus, mitochondria, endoplasmic reticulum, and lysosomes; where it can regulate its substrates (Ca2+, NAD+ and NADP+) (13-16). This principle is important to understand when determining which typology of CD38 is activated, as this can lead to an increase or decrease cytokine production, as well as a protective or harmful result for the cell (5,17,18).
CD38’s role in programmed cellular death
IRI is a complex process mediated by many damage-associated molecular patterns (DAMPs) and inflammatory signaling cascades that lead to impaired cell function, ultimately leading to endothelial cell death (28). One such factor is nuclear factor-Kβ (NF-Kβ) (29), which translocates to the nucleus and increases the transcription levels of pro-interleukin (IL)-1β, pro-IL-18 and NOD-like receptor protein-3 (NLRP3) (30,31). Additionally, NF-Kβ serves as a transcription factor for CD38, promoting its production (32,33). As discussed previously, CD38 activation increases intracellular Ca2+ and ROS production (23,24). This is important as both Ca2+ and ROS are the activators of NLRP3, which allows the formation of the NLRP3-inflammasome (34-38). Once this is complete, the NLRP3-inflammasome cleaves pro-caspase-1 into its active form, caspase-1.
Caspase-1 has a diverse set of functions, but most importantly can trigger two kinds of programmed cell death: apoptosis and pyroptosis. Apoptosis is a form of programmed cell death characterized by a controlled dismantling of cellular architecture that leads to cell death and prepares cells for removal by phagocytes without triggering an unwanted immune response. Cells induce death through proteolysis ultimately from caspase-3/6/7 (39,40). Several studies have highlighted that caspase-1 activates caspase-3, although exact mechanisms are yet to be elucidated (41-44). Furthermore, caspase-1 activates caspase-8, which serves as the activator of caspase-3/6/7 (40,42,45,46). Pyroptosis is another form of programmed cell death and is more often associated with IRI than apoptosis (47). It differs from apoptosis in that cell death arises from plasma membrane disruption, organelle swelling, and mitochondria dysfunction. Caspase-1 most commonly is associated with cleaving gasdermin-D (GSDMD) into N-GSDMD, pro-IL-1β to IL-1β and pro-IL-18 to IL-18 and subsequently initiates pyroptosis. During pyroptosis, N-GSDMD interacts with the cell membrane to form transmembrane pores which allows IL-1β, IL-18 and additional signals to be released promoting further inflammation and cell death (40,47). A summary of this cascade can be seen in Figure 3.
Clinical applications of CD38 mediated IRI and modulation
Reducing CPB associated IRI
Inherent to cardiac surgery and CPB, is the cessation and resumption of blow flood. Additionally, the blood-bypass circuit interaction activates inflammatory cascades. This pause and resumption of blood flow causes IRI in heart tissue during any cardiac operation that requires arresting the heart. IRI in the heart begins with oxidative stress, inflammation, and intracellular calcium overload and rapidly progresses to irreversible cell death by apoptosis and pyroptosis (48-51). IRI was first described in the heart by Jennings et al. when canine hearts underwent temporary coronary occlusion, resulting in visible histological changes (52). Clinically, after arresting the heart during CPB, IRI can result in arrhythmia, myocardial stunning, low cardiac output syndrome, and perioperative myocardial infarction (53).
In patients with perioperative mortality soon after coronary artery bypass surgery, histological evidence of IRI can be seen in 25–45% of patients (54,55). One potential significant contributor to the CPB associated inflammatory response is the IRI associated with cessation of ventilation and the associated development of atelectasis during CPB. Apnea, atelectasis, and hyperoxia during CPB create a pro-inflammatory state and foster IRI, and invariably increase pulmonary vascular resistance, impair right ventricular performance, and cause low cardiac output syndrome (56-58). Although few studies, such as the MECANO trial, have indicated a potential benefit to continued ventilation during CPB, the ideal ventilatory strategy and the fraction of inspired oxygen remain to be determined (59,60). Besides the PROVECS trial, which reported lung damage from alveolar distention is associated with an increase in soluble receptor advanced glycation end-products (sRAGE), other markers of molecular injury have not been studied in depth (61,62). In order to further study the role of ventilatory strategy, inflammation, and outcomes on CPB and the interaction of CD38 at large—the FOCUS trial is being used to look at this relationship specifically at select centers. The large, randomized, prospective, multicenter FOCUS trial (NCT04978636, “The eFfect of cOntinuous Low Tidal Volume Ventilation With Hyperoxia Avoidance During CardiopUlmonary Bypass”). The primary objective of the study is to investigate the effect of continuous low-tidal volume ventilation with hyperoxia avoidance (using FiO2 of 0.21) during CPB on postoperative pulmonary complications (PPCs) and 30-day mortality. Secondly, the FOCUS trial will determine the effects of these ventilatory approaches on lung injury and serum markers of IRI [sRAGE (63) and 8-isoprostaglandin F2α (64)], in addition to tracking levels of CD38 to determine the activity levels and its association with PPCs. The main hypothesis is that PPCs and mortality will be reduced in cardiac surgical patients receiving low-tidal volume ventilation with FiO2 =0.21 during CPB, and that serum markers of inflammation, such as CD38, will be reduced.
IRI has been shown to upregulate CD38 activity in the heart when exposed to injury (3). Subsequently, this increased activity leads to the depletion of NAD+/NADP+. At the endothelial level, the reduction of NAD+/NADP+ limits the function of oxidoreductase endothelial nitric oxide synthase (eNOS), increases the production of ROS and overall leads to poor cell function (5,65,66). Globally, impaired eNOS function limits nitric oxide (NO) production leading to vasoconstriction, poor myocardial perfusion, and ultimately decreased cardiac activity (3,67). In addition, upregulation leads to an increase in intracellular calcium. Dysregulation of calcium homeostasis within the cytoplasm results in delayed after depolarizations and ultimately arrhythmias for the patient (68-70). Finally, the consumption of NAD+ by CD38 impacts many other NAD+ dependent pathways. Decreasing levels of NAD+ leads to diminished activity of sirtuins, which are important mediators of antioxidants and inactivity leads to an increase in oxygen stress on cardiomyocytes (71,72). Thus, CD38 plays a vital role in facilitating cardiac dysregulation, damage, and arrhythmias and ultimately contributes to poor function and outcomes for patients suffering from IRI after CBP.
Several studies have examined CD38 genetic ablation and its effect on IRI in hearts. In general, cardiac cells that have genetically ablated CD38 show increased resistance to oxidative stress caused by IRI and, furthermore, suppress Ca2+ overload offering additional protection (71). Additionally, the absence of CD38 leads to sustained NAD+/NADP+ levels, which overall lead to sustained endothelial protection and functionally reduce arrhythmias during IRI (69). In addition to genetic ablation, there are several potent inhibitors of the CD38 pathway. 78c is the most common CD38 inhibitor and has had similar results to CD38 genetic ablation in that it maintains NAD+/NADP+ levels, reduces injury, and preserves cardiac function (67,73). Furthermore, a recent study examined the CD38 inhibitor MK-0159 (5). This inhibitor, as opposed to 78c, is available orally and has been shown to restore NAD+, decrease secondary messengers that generate calcium, and most importantly protect the heart against IRI.
While we have discussed the possible benefits of CD38 inhibition, there are also detrimental effects. Deactivation of CD38 in the heart for a prolonged period of time can also have detrimental effects. Genetic CD38 deficiency can cause dysregulation of autophagy, leading to impaired clearance of type I collagen in the coronary arteries, and will ultimately cause plaque build-up and lead to atherosclerotic disease (17). Furthermore, due to the lack of calcium-generating secondary messengers, cholesterol transport is also dysregulated and leads to accumulation of cholesterol in lysosomes, further facilitating atherosclerosis (74). Finally, it has been shown that a CD38 deficiency suppresses nuclear factor erythroid-2-related factor-2, promoting smooth muscle cell dedifferentiation, enhancing cell perforation, and again promoting atherosclerosis (75).
In future endeavors, it will be important to balance the function of CD38, as it appears to have a dual function: (I) mediating cell injury and elaborating inflammatory pathways to promote damage; and (II) ensuring that coronary arteries and vessels remain clear of plaque. To date, there have been no large animal studies or clinical trials evaluating the efficacy of CD38 inhibition and its effect on the heart during CPB. The efficacy of CD38 inhibition during IRI will need to be further substantiated in additional preclinical experiments as well as in human tissues.
Improving donor viability in thoracic transplantation
Transplantation is often the only cure for end-stage lung and heart disease. However, transplantation is limited by the available donor pool. Limited donor availability for lung and heart transplantation has led providers to explore the use of marginal donors, with promising results in both organs (76-78). However, these organs have significant limitations due to the substantial tissue injury that can result from various degrees of warm and cold ischemia before and during procurement. If not carefully controlled and accounted for, damage sustained from prolonged ischemic time and the subsequent IRI can lead to disastrous post-operative complications. In the heart, IRI manifests clinically as primary graft dysfunction (PGD) in heart allografts (79). Patients present with ventricular dysfunction (left, right, or both) with low cardiac output and hypotension (80,81). PGD affects around 20% of heart transplantation patients, with 1-year mortality rates ranging from 15–40% depending on severity (82). In the lungs, IRI also manifests clinically as PGD in lung allografts (83). Patients often have an increased oxygen demand, decreasing PaO2:FiO2 ratio, and interstitial opacities on imaging (84,85). The development of PGD has profound short- and long-term impacts on patient outcomes (86,87). Unfortunately, PGD is a relatively common adverse event that occurs in up to 40% of patients and significantly impacts recipient mortality and morbidity (86,88,89). Ultimately, the modulation of CD38 activity may help extend the viability of transplanted organs by reducing IRI in both the donor population during procurement and the recipient population during the transplant operation, and furthermore plays a role in immune modulation in general.
Immune modulation and antibody-mediated rejection
CD38’s broad role in inflammation and cytokine release have obvious connections to immune modulation (90). CD38’s role of NAD+ modulation could have direct impacts on immune modulation leading to improved allograft survival and integrity. When supplemented with additional NAD+in vivo, Elkhal et al. were able to demonstrate mice undergoing transplantation were able to have prolonged allograft survival by promoting a robust systemic IL-10 response originating from CD4+ T-helper cells (91). Inhibition of CD38 during the post-operative period following transplantation could have similar effects and promote allograft survival and decrease cell-mediated rejection. However, optimal dosing and treatment during are yet not known with the available CD38 inhibitors, nor effect in the post-transplantation period. This will be an area of future study and concern. In addition to the possibility of CD38 inhibition in the post-operative period, CD38 inhibition could have profound impacts on antibody mediated rejection. CD38 is expressed on the surface of a variety of immune cells to include plasma cells, and recently targeting these cells has been explored in transplantation (92). By using daratumumab (monoclonal CD38 inhibitor), Kwun et al. were able to show that pre-treating the donors with CD38 inhibitors significantly reduced anti-human leukocyte antigen (HLA) antibodies and lead to prolonged allograft survival. Overall, the modulation of CD38 could have profound impacts as an immunomodulator and future studies should be conducted in pre-clinical and clinical experiments to future validate these findings.
Heart transplantation
During heart transplantation it will be essential to focus on CD38’s main effects: consumption of NAD+, production of ROS and potent calcium secondary messengers. In addition to the studies already discussed, as well as important pathways to inhibit, the NLRP3-inflammasome will be particularly important to inhibit. As discussed previously, CD38’s byproducts serve to activate the NLRP3-inflammasome (34-38), which leads to both pyroptosis and apoptosis. NLRP3 activation has been shown to significantly contribute to cardiac IRI (93,94). Specifically, in donation after circulatory death (DCD) heart transplantation models, inhibiting the NLRP3-inflammasome has been shown to protect the donor allograft against pyroptosis and apoptosis alike (95). CD38 inhibition also has the potential to prevent the NLRP3-inflammasome formation and have similar effects on mechanisms of programmed cell-death. Clinically, this effect is also apparent as IL-18 (by-product of NLRP3 activation) concentrations at 24 hours post-transplant have been correlated with increasing severity of PGD (96).
During heart transplantation, the activation of certain types of CD38 could also be beneficial. Type II CD38 regulates extracellular adenosine from the consumption of NAD+ (18). Purine synthesis and cell signaling is an important component of IRI as well as rejection during heart transplantation (97). Adenosine, in addition to vasodilatory and anti-inflammatory effects, has been shown to have several cardioprotective effects during IRI and transplant (98-100). Type II CD38 might exert a complementary effect to another known ectoenzyme that synthesizes adenosine, CD39 (18). CD39 overexpression has been shown to be effective in mitigating cardiac injury during IRI (101,102), and specifically in heart transplantation it has the ability to prevent platelet aggregation and improve the survival of heart allografts (103-105). The possible synergistic effects of CD38 and CD39 remain an underexplored area of research. During heart transplantation, it will be important to distinguish inhibition of type II and type III CD38. Inhibition of type III CD38 mitigates inflammatory cascades, while modulation of type II CD38 could offer protection to heart allografts.
Lung transplantation
Although CD38’s role in heart-specific IRI has been firmly established, there is little data on its role in lung injury and insult. However, lung capillary networks are vast and CD38 is known to be highly conserved and expressed in the endothelium (3,12). Previous work examining the underlying molecular mechanisms of lung transplantation has highlighted several key pathways that overlap with the NAD+/CD38/cADPR/Ca2+ axis. As the endothelium senses the cessation and resumption of blood flow, CD38 activates the resulting ROS production, intracellular Ca2+ influx, and ultimately the production and release of IL-1β. As discussed previously, excessive levels of intracellular Ca2+, as well as ROS production, lead to pyroptosis and apoptosis, contributing to allograft damage, alveolar edema, and overall poor respiratory exchange (106). IL-1β plays an important role in recruiting recipient immune cells, most notably host classical monocytes (107). Monocytes are recruited to the donor allograft, break down the tight junctions of the endothelium, and perpetuate the recipient’s immune response by attracting neutrophils that infiltrate the alveolar space to cause more damage and edema (108,109). Furthermore, CD38 is an important modulator of endothelin-1 (ET-1) activity, which is a vasoactive protein and marker of acute lung injury (110,111). During lung transplantation, high levels of ET-1 have been correlated with the development of PGD as well. Increased activity of CD38 leads to increased responsiveness to ET-1, while absence leads to decreased response and ultimately improved blood flow (112,113). Inhibition of CD38 can decrease the effects of ET-1 on potential allografts, and therefore improve blood flow and potentially mitigate PGD. The loss of endothelial protection, and thus the breakdown of this important barrier, leads to extravasation of neutrophils, edema, injury, and overall poor allograft function. Inhibition of CD38 would offer novel means of endothelial protection to prevent against IRI-related damage and rescue allografts during lung transplantation.
Therapeutic delivery
While modulation of CD38 could have vast clinical implications, the proper delivery method of these agents, pending application, is yet to be determined. Because CD38 exists as both as an endo- and ecto-form it can be advantageous to be able to inhibit both types. While type II can be more readily targeted due to its inherent nature, type III poses unique challenges. 78c (10) is a potent inhibitor of CD38, however due to its molecular structure is insoluble in water, and is difficult to deliver orally or intravenously at large quantities. Targeted drug delivery using nano-particles or extracellular vesicles (114) to house CD38 inhibitors could overcome this difficultly and effectively deliver therapeutics to inhibit type III CD38. These solutions could be delivered during CPB as part of the cardioplegia solution, or as an aerosolized solution in the ventilator. During transplantation, they could again be delivered in the cold-flush solution or in the preservation solution during cold storage. The most excited application in transplantation would be during machine perfusion (MP) of the heart or lung (115-118). MP is a dynamic assessment platform that offers transplant physicians the opportunity to evaluation potential allograft’s suitability for transplantation. While the main advantage of MP currently is extended evaluation—therapeutic delivery and the possibility of resuscitation, repair and modifying allografts is the future of MP (119).
Limitations
There are limitations to this review. Since only PubMed indexed studies were included, invariably there were studies that perhaps met our inclusion criteria, however were not included in this work. In addition, because CD38 in the context of thoracic transplantation is a relatively new field of study, some of the inferences made must be further substantiated in pre-clinical as well as clinical studies.
Conclusions
CD38 is an important enzyme that is involved in many cellular functions. Intracellularly, it is most notably a NADase and a generator of potent Ca2+ generating secondary messengers. Ultimately, the release of these Ca2+ generating secondary messengers leads to the production of ROS, as well as activation of important mediators of programmed cell death. In the heart and during thoracic transplantation, this pathway is intimately involved in a wide variety of injury; namely the endothelium. In the heart, activation generally results in vasoconstriction, poor myocardial perfusion, and ultimately poor cardiac function. Parenthetically, however, CD38 activation prevents the accumulation of atherosclerotic disease. During transplantation, intracellular activation leads to infiltration of recipient innate immune cells, tissue edema, and ultimately PGD. Specifically, in heart transplantation, extracellular activation could be protective and improve allograft survival. Modulation of CD38 is a novel means to protect the heart and lung against IRI-related damage during CPB, as well as thoracic transplantation. These relationships are a promising area of research, and further study is needed in pre-clinical and clinical experiments in order to delineate a definitive connection between CD38 modulation and its use in thoracic transplant and CPB.
Acknowledgments
Funding: This research was generously supported through
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-725/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-725/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-725/coif). BAW is supported through the National Institutes of Health (NIH) National Heart Lung and Blood Institute grant R01HL143000. SMB is supported by the National Diabetes and Digestive and Kidney Diseases R01DK123475. RJG is supported through the NIH Grant U54CA260582, The Robert J. Anthony Fund for Cardiovascular Research and The JB Cardiovascular Research Fund. ME has served as a consultant for Boston Scientific in the past 36 months. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siddique A, Urban M, Strah H, et al. Controlled DCD lung transplantation: Circumventing imagined and real barriers-time for an international taskforce? J Heart Lung Transplant 2022;41:1198-203. [Crossref] [PubMed]
- Haffner CD, Becherer JD, Boros EE, et al. Discovery, Synthesis, and Biological Evaluation of Thiazoloquin(az)olin(on)es as Potent CD38 Inhibitors. J Med Chem 2015;58:3548-71. [Crossref] [PubMed]
- Reyes LA, Boslett J, Varadharaj S, et al. Depletion of NADP(H) due to CD38 activation triggers endothelial dysfunction in the postischemic heart. Proc Natl Acad Sci U S A 2015;112:11648-53. [Crossref] [PubMed]
- Chacon-Alberty L, Fernandez R, Jindra P, et al. Primary Graft Dysfunction in Lung Transplantation: A Review of Mechanisms and Future Applications. Transplantation 2023;107:1687-97. [Crossref] [PubMed]
- Lagu B, Wu X, Kulkarni S, et al. Orally Bioavailable Enzymatic Inhibitor of CD38, MK-0159, Protects against Ischemia/Reperfusion Injury in the Murine Heart. J Med Chem 2022;65:9418-46. [Crossref] [PubMed]
- Reinherz EL, Kung PC, Goldstein G, et al. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A 1980;77:1588-92. [Crossref] [PubMed]
- Oliva-Vilarnau N, Hankeova S, Vorrink SU, et al. Calcium Signaling in Liver Injury and Regeneration. Front Med (Lausanne) 2018;5:192. [Crossref] [PubMed]
- Aksoy P, White TA, Thompson M, et al. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun 2006;345:1386-92. [Crossref] [PubMed]
- Becherer JD, Boros EE, Carpenter TY, et al. Discovery of 4-Amino-8-quinoline Carboxamides as Novel, Submicromolar Inhibitors of NAD-Hydrolyzing Enzyme CD38. J Med Chem 2015;58:7021-56. [Crossref] [PubMed]
- Roboon J, Hattori T, Ishii H, et al. Inhibition of CD38 and supplementation of nicotinamide riboside ameliorate lipopolysaccharide-induced microglial and astrocytic neuroinflammation by increasing NAD J Neurochem 2021;158:311-27. [Crossref] [PubMed]
- Cockayne DA, Muchamuel T, Grimaldi JC, et al. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood 1998;92:1324-33.
- Li JP, Wei W, Li XX, et al. Regulation of NLRP3 inflammasome by CD38 through cADPR-mediated Ca(2+) release in vascular smooth muscle cells in diabetic mice. Life Sci 2020;255:117758. [Crossref] [PubMed]
- Wu Y, Zhang J, Fang L, et al. A cytosolic chaperone complex controls folding and degradation of type III CD38. J Biol Chem 2019;294:4247-58. [Crossref] [PubMed]
- Zhao YJ, Lam CM, Lee HC. The membrane-bound enzyme CD38 exists in two opposing orientations. Sci Signal 2012;5:ra67. [Crossref] [PubMed]
- Liu J, Zhao YJ, Li WH, et al. Cytosolic interaction of type III human CD38 with CIB1 modulates cellular cyclic ADP-ribose levels. Proc Natl Acad Sci U S A 2017;114:8283-8. [Crossref] [PubMed]
- Sun L, Adebanjo OA, Koval A, et al. A novel mechanism for coupling cellular intermediary metabolism to cytosolic Ca2+ signaling via CD38/ADP-ribosyl cyclase, a putative intracellular NAD+ sensor. FASEB J 2002;16:302-14. [Crossref] [PubMed]
- Bao JX, Zhang QF, Wang M, et al. Implication of CD38 gene in autophagic degradation of collagen I in mouse coronary arterial myocytes. Front Biosci (Landmark Ed) 2017;22:558-69. [Crossref] [PubMed]
- Horenstein AL, Chillemi A, Zaccarello G, et al. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology 2013;2:e26246. [Crossref] [PubMed]
- Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med 2006;5:101-17. [Crossref] [PubMed]
- Covarrubias AJ, Perrone R, Grozio A, et al. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol 2021;22:119-41. [Crossref] [PubMed]
- Gul R, Park DR, Shawl AI, et al. Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) and Cyclic ADP-Ribose (cADPR) Mediate Ca2+ Signaling in Cardiac Hypertrophy Induced by β-Adrenergic Stimulation. PLoS One 2016;11:e0149125. [Crossref] [PubMed]
- Lin WK, Bolton EL, Cortopassi WA, et al. Synthesis of the Ca(2+)-mobilizing messengers NAADP and cADPR by intracellular CD38 enzyme in the mouse heart: Role in β-adrenoceptor signaling. J Biol Chem 2017;292:13243-57. [Crossref] [PubMed]
- Benson JC, Trebak M. Too much of a good thing: The case of SOCE in cellular apoptosis. Cell Calcium 2023;111:102716. [Crossref] [PubMed]
- Bagur R, Hajnóczky G. Intracellular Ca(2+) Sensing: Its Role in Calcium Homeostasis and Signaling. Mol Cell 2017;66:780-8. [Crossref] [PubMed]
- Jackson DG, Bell JI. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol 1990;144:2811-5.
- Camacho-Pereira J, Tarragó MG, Chini CCS, et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab 2016;23:1127-39. [Crossref] [PubMed]
- Park DR, Nam TS, Kim YW, et al. Oxidative activation of type III CD38 by NADPH oxidase-derived hydrogen peroxide in Ca(2+) signaling. FASEB J 2019;33:3404-19. [Crossref] [PubMed]
- Kalogeris T, Baines CP, Krenz M, et al. Ischemia/Reperfusion. Compr Physiol 2016;7:113-70. [Crossref] [PubMed]
- Matsui N, Kasajima K, Hada M, et al. Inhibiton of NF-kappaB activation during ischemia reduces hepatic ischemia/reperfusion injury in rats. J Toxicol Sci 2005;30:103-10. [Crossref] [PubMed]
- Dowling JK, O’Neill LA. Biochemical regulation of the inflammasome. Crit Rev Biochem Mol Biol 2012;47:424-43. [Crossref] [PubMed]
- Ulland TK, Ferguson PJ, Sutterwala FS. Evasion of inflammasome activation by microbial pathogens. J Clin Invest 2015;125:469-77. [Crossref] [PubMed]
- Shu B, Feng Y, Gui Y, et al. Blockade of CD38 diminishes lipopolysaccharide-induced macrophage classical activation and acute kidney injury involving NF-κB signaling suppression. Cell Signal 2018;42:249-58. [Crossref] [PubMed]
- Kang BN, Tirumurugaan KG, Deshpande DA, et al. Transcriptional regulation of CD38 expression by tumor necrosis factor-alpha in human airway smooth muscle cells: role of NF-kappaB and sensitivity to glucocorticoids. FASEB J 2006;20:1000-2. [Crossref] [PubMed]
- Ming SL, Zeng L, Guo YK, et al. The Human-Specific STING Agonist G10 Activates Type I Interferon and the NLRP3 Inflammasome in Porcine Cells. Front Immunol 2020;11:575818. [Crossref] [PubMed]
- Ismael S, Ahmed HA, Adris T, et al. The NLRP3 inflammasome: a potential therapeutic target for traumatic brain injury. Neural Regen Res 2021;16:49-57. [Crossref] [PubMed]
- Paik S, Kim JK, Silwal P, et al. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol 2021;18:1141-60. [Crossref] [PubMed]
- Zhong Z, Zhai Y, Liang S, et al. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun 2013;4:1611. [Crossref] [PubMed]
- Jo EK, Kim JK, Shin DM, et al. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 2016;13:148-59. [Crossref] [PubMed]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 2008;9:231-41. [Crossref] [PubMed]
- Denes A, Lopez-Castejon G, Brough D. Caspase-1: is IL-1 just the tip of the ICEberg? Cell Death Dis 2012;3:e338. [Crossref] [PubMed]
- Benchoua A, Guégan C, Couriaud C, et al. Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci 2001;21:7127-34. [Crossref] [PubMed]
- Zhang WH, Wang X, Narayanan M, et al. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci U S A 2003;100:16012-7. [Crossref] [PubMed]
- Tsuchiya K, Nakajima S, Hosojima S, et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat Commun 2019;10:2091. [Crossref] [PubMed]
- Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem Biol 2017;24:507-14.e4. [Crossref] [PubMed]
- Guégan C, Vila M, Teismann P, et al. Instrumental activation of bid by caspase-1 in a transgenic mouse model of ALS. Mol Cell Neurosci 2002;20:553-62. [Crossref] [PubMed]
- Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta 2011;1813:558-63. [Crossref] [PubMed]
- Burdette BE, Esparza AN, Zhu H, et al. Gasdermin D in pyroptosis. Acta Pharm Sin B 2021;11:2768-82. [Crossref] [PubMed]
- Neri M, Riezzo I, Pascale N, et al. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediators Inflamm 2017;2017:7018393. [Crossref] [PubMed]
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92-100. [Crossref] [PubMed]
- Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol 2005;14:170-5. [Crossref] [PubMed]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121-35. [Crossref] [PubMed]
- Jennings RB, Reimer KA. Lethal myocardial ischemic injury. Am J Pathol 1981;102:241-55.
- Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol 2010;106:360-8. [Crossref] [PubMed]
- Weman SM, Karhunen PJ, Penttilä A, et al. Reperfusion injury associated with one-fourth of deaths after coronary artery bypass grafting. Ann Thorac Surg 2000;70:807-12. [Crossref] [PubMed]
- Moore GW, Hutchins GM. Coronary artery bypass grafts in 109 autopsied patients. Statistical analysis of graft and anastomosis patency and regional myocardial injury. JAMA 1981;246:1785-9.
- Beer L, Warszawska JM, Schenk P, et al. Intraoperative ventilation strategy during cardiopulmonary bypass attenuates the release of matrix metalloproteinases and improves oxygenation. J Surg Res 2015;195:294-302. [Crossref] [PubMed]
- Beer L, Szerafin T, Mitterbauer A, et al. Continued mechanical ventilation during coronary artery bypass graft operation attenuates the systemic immune response. Eur J Cardiothorac Surg 2013;44:282-7. [Crossref] [PubMed]
- den Hengst WA, Gielis JF, Lin JY, et al. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol 2010;299:H1283-99. [Crossref] [PubMed]
- Nguyen LS, Estagnasie P, Merzoug M, et al. Low Tidal Volume Mechanical Ventilation Against No Ventilation During Cardiopulmonary Bypass in Heart Surgery (MECANO): A Randomized Controlled Trial. Chest 2021;159:1843-53. [Crossref] [PubMed]
- Boussion K, Tremey B, Gibert H, et al. Efficacy of maintaining low-tidal volume mechanical ventilation as compared to resting lung strategy during coronary artery bypass graft cardiopulmonary bypass surgery: A post-hoc analysis of the MECANO trial. J Clin Anesth 2023;84:110991. [Crossref] [PubMed]
- Lagier D, Velly LJ, Guinard B, et al. Perioperative Open-lung Approach, Regional Ventilation, and Lung Injury in Cardiac Surgery. Anesthesiology 2020;133:1029-45. [Crossref] [PubMed]
- Lagier D, Fischer F, Fornier W, et al. Effect of open-lung vs conventional perioperative ventilation strategies on postoperative pulmonary complications after on-pump cardiac surgery: the PROVECS randomized clinical trial. Intensive Care Med 2019;45:1401-12. [Crossref] [PubMed]
- Xiong X, Dou J, Shi J, et al. RAGE inhibition alleviates lipopolysaccharides-induced lung injury via directly suppressing autophagic apoptosis of type II alveolar epithelial cells. Respir Res 2023;24:24. [Crossref] [PubMed]
- Li J, Li H, Li H, et al. Amelioration of PM(2.5)-induced lung toxicity in rats by nutritional supplementation with fish oil and Vitamin E. Respir Res 2019;20:76. [Crossref] [PubMed]
- Diguet N, Trammell SAJ, Tannous C, et al. Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation 2018;137:2256-73. [Crossref] [PubMed]
- Boslett J, Hemann C, Christofi FL, et al. Characterization of CD38 in the major cell types of the heart: endothelial cells highly express CD38 with activation by hypoxia-reoxygenation triggering NAD(P)H depletion. Am J Physiol Cell Physiol 2018;314:C297-309. [Crossref] [PubMed]
- Boslett J, Hemann C, Zhao YJ, et al. Luteolinidin Protects the Postischemic Heart through CD38 Inhibition with Preservation of NAD(P)(H). J Pharmacol Exp Ther 2017;361:99-108. [Crossref] [PubMed]
- Fozzard HA. Afterdepolarizations and triggered activity. Basic Res Cardiol 1992;87:105-13. [Crossref] [PubMed]
- Agorrody G, Peclat TR, Peluso G, et al. Benefits in cardiac function by CD38 suppression: Improvement in NAD(+) levels, exercise capacity, heart rate variability and protection against catecholamine-induced ventricular arrhythmias. J Mol Cell Cardiol 2022;166:11-22. [Crossref] [PubMed]
- Liu MB, de Lange E, Garfinkel A, et al. Delayed afterdepolarizations generate both triggers and a vulnerable substrate promoting reentry in cardiac tissue. Heart Rhythm 2015;12:2115-24. [Crossref] [PubMed]
- Guan XH, Liu XH, Hong X, et al. CD38 Deficiency Protects the Heart from Ischemia/Reperfusion Injury through Activating SIRT1/FOXOs-Mediated Antioxidative Stress Pathway. Oxid Med Cell Longev 2016;2016:7410257. [Crossref] [PubMed]
- Wang LF, Cao Q, Wen K, et al. CD38 Deficiency Alleviates D-Galactose-Induced Myocardial Cell Senescence Through NAD(+)/Sirt1 Signaling Pathway. Front Physiol 2019;10:1125. [Crossref] [PubMed]
- Boslett J, Reddy N, Alzarie YA, et al. Inhibition of CD38 with the Thiazoloquin(az)olin(on)e 78c Protects the Heart against Postischemic Injury. J Pharmacol Exp Ther 2019;369:55-64. [Crossref] [PubMed]
- Xu X, Yuan X, Li N, et al. Lysosomal cholesterol accumulation in macrophages leading to coronary atherosclerosis in CD38(-/-) mice. J Cell Mol Med 2016;20:1001-13. [Crossref] [PubMed]
- Xu M, Li XX, Wang L, et al. Contribution of Nrf2 to Atherogenic Phenotype Switching of Coronary Arterial Smooth Muscle Cells Lacking CD38 Gene. Cell Physiol Biochem 2015;37:432-44. [Crossref] [PubMed]
- Bobba CM, Whitson BA, Henn MC, et al. Trends in Donation After Circulatory Death in Lung Transplantation in the United States: Impact Of Era. Transpl Int 2022;35:10172. [Crossref] [PubMed]
- Chew HC, Iyer A, Connellan M, et al. Outcomes of Donation After Circulatory Death Heart Transplantation in Australia. J Am Coll Cardiol 2019;73:1447-59. [Crossref] [PubMed]
- Messer S, Cernic S, Page A, et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant 2020;39:1463-75. [Crossref] [PubMed]
- Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1037-46. [Crossref] [PubMed]
- Singh SSA, Dalzell JR, Berry C, et al. Primary graft dysfunction after heart transplantation: a thorn amongst the roses. Heart Fail Rev 2019;24:805-20. [Crossref] [PubMed]
- Iyer A, Kumarasinghe G, Hicks M, et al. Primary graft failure after heart transplantation. J Transplant 2011;2011:175768. [Crossref] [PubMed]
- Buchan TA, Moayedi Y, Truby LK, et al. Incidence and impact of primary graft dysfunction in adult heart transplant recipients: A systematic review and meta-analysis. J Heart Lung Transplant 2021;40:642-51. [Crossref] [PubMed]
- Gelman AE, Fisher AJ, Huang HJ, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part III: Mechanisms: A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017;36:1114-20. [Crossref] [PubMed]
- Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34. [Crossref] [PubMed]
- Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med 2005;171:1312-6. [Crossref] [PubMed]
- Whitson BA, Nath DS, Johnson AC, et al. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg 2006;131:73-80. [Crossref] [PubMed]
- Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004-11. [Crossref] [PubMed]
- Balsara KR, Krupnick AS, Bell JM, et al. A single-center experience of 1500 lung transplant patients. J Thorac Cardiovasc Surg 2018;156:894-905.e3. [Crossref] [PubMed]
- Schwarz S, Benazzo A, Dunkler D, et al. Ventilation parameters and early graft function in double lung transplantation. J Heart Lung Transplant 2021;40:4-11. [Crossref] [PubMed]
- Antonelli A, Ferrannini E. CD38 autoimmunity: recent advances and relevance to human diabetes. J Endocrinol Invest 2004;27:695-707. [Crossref] [PubMed]
- Elkhal A, Rodriguez Cetina Biefer H, Heinbokel T, et al. NAD(+) regulates Treg cell fate and promotes allograft survival via a systemic IL-10 production that is CD4(+) CD25(+) Foxp3(+) T cells independent. Sci Rep 2016;6:22325. [Crossref] [PubMed]
- Kwun J, Matignon M, Manook M, et al. Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use. J Am Soc Nephrol 2019;30:1206-19. [Crossref] [PubMed]
- Jun JH, Shim JK, Oh JE, et al. Protective Effect of Ethyl Pyruvate against Myocardial Ischemia Reperfusion Injury through Regulations of ROS-Related NLRP3 Inflammasome Activation. Oxid Med Cell Longev 2019;2019:4264580. [Crossref] [PubMed]
- Zuo W, Tian R, Chen Q, et al. miR-330-5p inhibits NLRP3 inflammasome-mediated myocardial ischaemia-reperfusion injury by targeting TIM3. Cardiovasc Drugs Ther 2021;35:691-705. [Crossref] [PubMed]
- Xu L, Zeng Z, Niu C, et al. Normothermic ex vivo heart perfusion with NLRP3 inflammasome inhibitor Mcc950 treatment improves cardiac function of circulatory death hearts after transplantation. Front Cardiovasc Med 2023;10:1126391. [Crossref] [PubMed]
- Holmström EJ, Syrjälä SO, Dhaygude K, et al. Severe primary graft dysfunction of the heart transplant is associated with increased plasma and intragraft proinflammatory cytokine expression. J Heart Lung Transplant 2023;42:807-18. [Crossref] [PubMed]
- Zeiser R, Robson SC, Vaikunthanathan T, et al. Unlocking the Potential of Purinergic Signaling in Transplantation. Am J Transplant 2016;16:2781-94. [Crossref] [PubMed]
- Saemann L, Korkmaz-Icöz S, Hoorn F, et al. Reconditioning of circulatory death hearts by ex-vivo machine perfusion with a novel HTK-N preservation solution. J Heart Lung Transplant 2021;40:1135-44. [Crossref] [PubMed]
- Yang C, Xu H, Cai L, et al. Donor pretreatment with adenosine monophosphate-activated protein kinase activator protects cardiac grafts from cold ischaemia/reperfusion injury. Eur J Cardiothorac Surg 2016;49:1354-60. [Crossref] [PubMed]
- Korkmaz-Icöz S, Radovits T, Loganathan S, et al. Prolonging hypothermic ischaemic cardiac and vascular storage by inhibiting the activation of the nuclear enzyme poly(adenosine diphosphate-ribose) polymerase. Eur J Cardiothorac Surg 2017;51:829-35. [Crossref] [PubMed]
- Wheeler DG, Joseph ME, Mahamud SD, et al. Transgenic swine: expression of human CD39 protects against myocardial injury. J Mol Cell Cardiol 2012;52:958-61. [Crossref] [PubMed]
- Cai M, Huttinger ZM, He H, et al. Transgenic over expression of ectonucleotide triphosphate diphosphohydrolase-1 protects against murine myocardial ischemic injury. J Mol Cell Cardiol 2011;51:927-35. [Crossref] [PubMed]
- Dwyer KM, Robson SC, Nandurkar HH, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest 2004;113:1440-6. [Crossref] [PubMed]
- Huttinger ZM, Milks MW, Nickoli MS, et al. Ectonucleotide triphosphate diphosphohydrolase-1 (CD39) mediates resistance to occlusive arterial thrombus formation after vascular injury in mice. Am J Pathol 2012;181:322-33. [Crossref] [PubMed]
- Covarrubias R, Chepurko E, Reynolds A, et al. Role of the CD39/CD73 Purinergic Pathway in Modulating Arterial Thrombosis in Mice. Arterioscler Thromb Vasc Biol 2016;36:1809-20. [Crossref] [PubMed]
- Wu MY, Yiang GT, Liao WT, et al. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem 2018;46:1650-67. [Crossref] [PubMed]
- Chiu S, Bharat A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr Opin Organ Transplant 2016;21:239-45. [Crossref] [PubMed]
- Kurihara C, Lecuona E, Wu Q, et al. Crosstalk between nonclassical monocytes and alveolar macrophages mediates transplant ischemia-reperfusion injury through classical monocyte recruitment. JCI Insight 2021;6:e147282. [Crossref] [PubMed]
- Hsiao HM, Fernandez R, Tanaka S, et al. Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1β. J Clin Invest 2018;128:2833-47. [Crossref] [PubMed]
- Machuca TN, Cypel M, Zhao Y, et al. The role of the endothelin-1 pathway as a biomarker for donor lung assessment in clinical ex vivo lung perfusion. J Heart Lung Transplant 2015;34:849-57. [Crossref] [PubMed]
- Hamilton BC, Kukreja J, Ware LB, et al. Protein biomarkers associated with primary graft dysfunction following lung transplantation. Am J Physiol Lung Cell Mol Physiol 2017;312:L531-41. [Crossref] [PubMed]
- Thai TL, Arendshorst WJ. Mice lacking the ADP ribosyl cyclase CD38 exhibit attenuated renal vasoconstriction to angiotensin II, endothelin-1, and norepinephrine. Am J Physiol Renal Physiol 2009;297:F169-76. [Crossref] [PubMed]
- Lee JH, Zhang J, Massmann GA, et al. Antenatal betamethasone increases vascular reactivity to endothelin-1 by upregulation of CD38/cADPR signaling. J Dev Orig Health Dis 2014;5:56-62. [Crossref] [PubMed]
- Chung JJ, Kim ST, Zaman S, et al. Therapeutic Efficacy of Cryopreserved, Allogeneic Extracellular Vesicles for Treatment of Acute Myocardial Infarction. Int Heart J 2021;62:381-9. [Crossref] [PubMed]
- Sanchez PG, Cantu E, Hartwig M, et al. The NOVEL Study. A Multi-Center Clinical Trial Studying the Safety of Ex Vivo Lung Perfusion. J Heart Lung Transplant 2020;39:S110.
- Peterson DM, Beal EW, Reader BF, et al. Electrical Impedance as a Noninvasive Metric of Quality in Allografts Undergoing Normothermic Ex Vivo Lung Perfusion. ASAIO J 2022;68:964-71. [Crossref] [PubMed]
- Nelson K, Bobba C, Eren E, et al. Method of isolated ex vivo lung perfusion in a rat model: lessons learned from developing a rat EVLP program. J Vis Exp 2015;52309. [Crossref] [PubMed]
- Mallea JM, Hartwig MG, Keller CA, et al. Remote ex vivo lung perfusion at a centralized evaluation facility. J Heart Lung Transplant 2022;41:1700-11. [Crossref] [PubMed]
- Whitson BA, Black SM. Organ assessment and repair centers: The future of transplantation is near. World J Transplant 2014;4:40-2. [Crossref] [PubMed]


