
Clinicopathological characteristics correlated with programmed cell death-ligand 1 expression in advanced lung adenocarcinoma
Highlight box
Key findings
• The sex, clinical stage including T and M stage, white blood cell (WBC), carcinoembryonic antigen (CEA), and neuron specific enolase (NSE), commonly used clinicopathological parameters, are significantly related to the expression of PD-L1 in advanced lung adenocarcinoma patients. Multivariate logistic regression analysis showed that CEA, NSE, T stage and WBC are expected to become new biomarkers for predicting programmed cell death-ligand 1 (PD-L1) expression.
What is known and what is new?
• We even identified the optimal cut-off values for CEA and NSE to predict the PD-L1 expression of lung adenocarcinoma before treatment, and proposed the establishment of model CEA combined with NSE to predict PD-L1 expression.
What is the implication, and what should change now?
• We believe that this paper has successfully identified a more convenient alternative to the standard detection method to predict the expression of PD-L1, ensuring clinicians make an earlier diagnosis leading to more rapid treatment decisions.
Introduction
Lung cancer is the leading cause of cancer-related deaths globally (1,2). Among them, non-small cell lung cancer (NSCLC) is the most common histological type, accounting for more than 85% of the total number of patients (3,4), among which adenocarcinoma (ADC) accounts for nearly 60% of all NSCLC, making it the most common subtype of NSCLC. The vast majority of lung cancer patients are already in a locally advanced stage or with concurrent metastatic lesions at the time of diagnosis (5), thus losing the best opportunity for surgical resection. In the past several decades, despite significant breakthroughs of chemotherapy or targeted therapy against angiogenesis in the field of lung cancer (6), up to 90% of patients will inevitably relapse with a 5-year survival benefit of less than 20% (7).
With the application of immune checkpoint inhibitors (ICIs) targeting programmed cell death protein-1/programmed cell death-ligand 1 (PD-1/PD-L1), the therapeutic barriers of NSCLC have been greatly improved. Furthermore, there have been a large number of clinical trials confirming the effectiveness of ICIs (8-11). However, only a few subsets of patients can benefit from ICIs, which significantly limits its clinical applicability (12). Therefore, it is of great importance to develop biomarkers that can predict the therapeutic efficacy of ICIs in patients with advanced ADC, so as to accurately implement a therapeutic intervention.
PD-L1 expression and tumor mutation burden (TMB) have been explored as the most important predictors of clinical benefits in tumor histology for selecting NSCLC candidates suitable for immunotherapy (13-16). However, the detection of these biomarkers always requires an invasive procedure. Pathological detection can only be carried out to determine the expression levels of PD-L1 by obtaining pathological specimens through various biopsy methods, and sometimes even more complex and expensive measurement methods, such as next generation sequencing (NGS) (17,18). NGS has emerged as a molecular target detection technology in recent years, which can be used in the diagnostic process of tumor samples from advanced lung cancer patients. It is the most effective method for standard-of-care test and ideal actionable driving factor mutations and gene fusion (19). However, due to economic barriers and limited access to this new technology, the popularity of this detection method is relatively low. Therefore, a non-invasive and relatively low-cost method that produces sensitive and accurate predictors are urgently needed to estimate the expression levels of PD-L1.
It is reported that serum tumor markers (STMs) such as carcinoembryonic antigen (CEA), neuron specific enolase (NSE), and cytokeratin 19 fragment (CY21-1) have been widely used as predictors to reflect the efficacy of chemotherapy or targeted therapy in NSCLC patients (20-22) and may be related to the prognosis of NSCLC patients (23-25). However, few studies have been reported on biomarkers that can be used to predict the expression of PD-L1 in tumor tissues. According to the literature, only three studies (26-28) (Table 1) have reported the correlation between serum STMs concentration and PD-L1 expression; however, their results have not reached a consensus. Additionally, the study of combining several STMs to evaluate the expression of PD-L1 has also not been reported.
Table 1
| Study | No. of patients | Histology | STMs significantly related to PD-L1 expression | Other clinicopathological factors related to PD-L1 expression |
|---|---|---|---|---|
| Kato et al. (26) | 106 | ADC and SCC | CEA | Nodal metastasis and sample preservation time |
| Sun et al. (27) | 390 | ADC and SCC | None | Radiomics signature, histologic type, and histologic grade |
| Yang et al. (28) | 163 | ADC | None | Higher grade differentiation and vascular invasion |
STMs, serum tumor markers; PD-L1, programmed cell death-ligand 1; ADC, adenocarcinoma; SCC, squamous cell carcinoma; CEA, carcinoembryonic antigen.
Sarcopenia, characterized by a decrease of muscle mass and strength, is one of the signs of cancer (29). This is a recognized prognostic factor associated with poor prognosis of several cancers, including lung cancer (30,31), and survival (32). Furthermore, it shows a negative impact on most clinical outcomes of cancers. Skeletal muscle index (SMI) is a surrogate indicator for skeletal muscle quality evaluation based on cross-sectional images acquired by computed tomography (CT) scanning, which has been regarded as one of the diagnostic criteria of sarcopenia (33). SMI has been also proven to be a prognostic and predictive parameter for cancer patients (34).
In addition, the value of SMI in predicting the therapeutic effect of ICIs has also been confirmed. Wang et al. (35) found that the survival period of melanoma patients with sarcopenia who received anti-PD-(L)1 treatment was shorter. Li et al. (36) confirmed that the decrease of SMI was related to the shorter progression free survival and lower disease control rate of NSCLC receiving ICIs.
In view of the significance of SMI in the prognosis evaluation of NSCLC patients as well as the prediction of the therapeutic efficacy of ICIs, it was believed that it is valuable to confirm the relationship between SMI and PD-L1 expression. Furthermore, in this study it was also discussed the demographic parameters, including age, sex, body mass index (BMI), serum biochemical indicators, tumor-node-metastasis (TNM) stage, and the clinical and pathological data to further characterize the factors related to the expression of PD-L1 in the histopathological analysis of lung cancer in order to provide better treatment options for patients with NSCLC, especially during the late stages. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-523/rc).
Methods
Study design and patient selection
The clinical data of all advanced NSCLC patients with successful PD-L1 expression assessment was reviewed at the First Affiliated Hospital of Xi’an Jiaotong University in from May, 2018 to December, 2021. In view of the high prevalence, the histological type included in the study was ADC. A total of 136 patients were identified, and their clinicopathological characteristics were compared among different PD-L1 expression cohorts. The baseline clinicopathological covariates including age, sex, BMI, and clinical stage were collected from all consecutive participants. Routine blood test, biochemical test including serum albumin (Alb), lactic dehydrogenase (LDH), blood urea nitrogen (BUN), creatinine (Cr), SMI, and skeletal muscle density (SMD) were assessed by chest CT were routinely recorded within 48 hours upon first admission. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Review Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2021LSK-339). Written informed consent was waived due to retrospective nature of this study.
Detection of PD-L1 expression status
The biopsy or surgical specimens were formalin-fixed and paraffin-embedded. The lung tumors were sectioned for microscopic examination and stained with hematoxylin and eosin. Histological diagnosis and pathological characteristics, including histological type and differentiation degree, were recorded.
PD-L1 positive tumor cells were defined as complete or partial membrane staining. The staining of the cytoplasm and tumor related immune cells (such as macrophages) was excluded (37). Finally, the tumor proportion score (TPS) was calculated based on the percentage of PD-L1 positive tumor cells divided by the total tumor cells. Based on PD-L1 TPS, PD-L1 expression status was divided into positive and negative groups (≥1% vs. <1%; TPS 1–49% and ≥50% represented PD-L1+ low and PD-L1+ high expression, respectively) (38). Additionally, an immunohistochemistry assay was performed via the Ventana platform using a SP 263 antibody.
Statistical analysis
Quantitative variables are expressed as mean ± standard deviation (SD) or median [interquartile range (IQR)]. The qualitative variables are summarized as numbers and percentages. The normality of quantitative data was estimated using histograms and the Shapiro-Wilk test. Differences among continuous variables with normal distributions were tested by independent sample t-tests, and differences for non-normal continuous variables were tested by Mann-Whitney U test. A Fisher’s exact test or a Chi-square test was used to test the differences of categorical variables. In addition, the variables with P value <0.05 in the univariate analysis were further included for multivariate logistic regression analysis. A multivariate logistic regression model was constructed via a backward method analysis to evaluate the independent influencing factors of PD-L1. Finally, PD-L1 was divided into positive and negative groups based on the threshold of 1%. The detection efficiency and optimal cut-off value of STMs used to predict the expression of PD-L1 were estimated by plotting the receiver operating characteristic (ROC) curve and calculating the area under the curve (AUC). A P value <0.05 to indicate test results with statistical significance, and all statistical tests were bilateral. R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS software version 25.0 (IBM Corp., Armonk, NY, USA) were used to perform statistical analysis.
Results
Patient’s characteristics
The main clinical characteristics of all subjects at baseline are shown in Table 2. One hundred and thirty-six advanced ADC (stage III–IV) patients were enrolled in the study. According to the eighth edition of the TNM classification of lung cancer of the International Lung Cancer Research Association (39), 19.9% were stage III, and the remaining 80.1% were stage IV. The median level of STMs at baseline was 7.36 ng/mL for CEA (IQR, 3.73–35.49 ng/mL), 19.40 ng/mL for NSE (IQR, 14.36–38.39 ng/mL), and 6.20 ng/mL for CY21-1 (IQR, 2.82–14.80 ng/mL).
Table 2
| Characteristics | Values |
|---|---|
| Age (years) | 65.43±9.01 |
| Sex | |
| Female | 48 (35.3) |
| Male | 88 (64.7) |
| BMI (kg/m2) | |
| Female | 22.94±3.75 |
| Male | 22.54±3.36 |
| WBC (×109/L) | 7.25 (5.70–9.42) |
| Neutrophils (×109/L) | 4.75 (3.60–6.50) |
| Lymphocytes (×109/L) | 1.56±0.59 |
| NLR | 3.10 (2.19–4.98) |
| BUN/Cr | 88.40±23.45 |
| Alb (g/L) | 38.26±4.87 |
| LDH (U/L) | 230.60 (178.55–349.05) |
| T12 SMD (HU) | 47.71±11.53 |
| SMI | 1,095.17±284.66 |
| Clinical stage | |
| III | 27 (19.9) |
| IV | 109 (80.1) |
| PD-L1 | |
| <1% | 81 (59.6) |
| ≥1% | 55 (40.4) |
| CEA (ng/mL) | 7.36 (3.73–35.49) |
| NSE (ng/mL) | 19.40 (14.36–38.39) |
| CY21-1 (ng/mL) | 6.20 (2.82–14.80) |
| Stage | |
| T | |
| 1 | 26 (19.1) |
| 2 | 34 (25.0) |
| 3 | 23 (16.9) |
| 4 | 53 (39.0) |
| N | |
| 0 | 22 (16.2) |
| 1 | 7 (5.1) |
| 2 | 51 (37.5) |
| 3 | 56 (41.2) |
| M | |
| 0 | 27 (19.9) |
| 1 | 109 (80.1) |
Data are presented as mean ± SD, median (25–75th percentiles), or n (%). BMI, body mass index; WBC, white blood cell; NLR, neutrophil to lymphocyte ratio; BUN, blood urea nitrogen; Cr, creatinine; Alb, albumin; LDH, lactic dehydrogenase; T12 SMD, skeletal muscle density at the level of the T12; SMI, skeletal muscle index; PD-L1, programmed cell death-ligand 1; CEA, carcinoembryonic antigen; NSE, neuron specific enolase; CY21-1, cytokeratin 19 fragment; T, tumor; N, node; M, metastasis; SD, standard deviation.
Clinicopathologic correlates of PD-L1 expression
The included patients were divided into the following two categories according to the PD-L1 expression level: PD-L1 negative with a TPS <1% (n=81, 59.6%), and PD-L1 positive with a TPS ≥1% (n=55, 40.4%) (Table 3). Compared to the negative group, the proportion of male patients in the PD-L1 positive group was higher (76.4% vs. 56.8%, P=0.019), and more commonly diagnosed at earlier stages (stage IV: 67.3% vs. 88.9%, P=0.002). STMs expression levels such as CEA (4.28 vs. 15.28 ng/mL, P<0.001) and NSE (17.30 vs. 22.18 ng/mL, P=0.001) were lower in the positive group. The increase of white blood cell (WBC) was also correlated with the high expression of PD-L1 (8.40×109/L vs. 6.40×109/L, P<0.001). There was no significant difference in age, BMI, Alb, LDH, BUN, Cr, CY21-1, and neutrophil to lymphocyte ratio (NLR) between the two groups (all P>0.05). Surprisingly, although SMI was higher in the PD-L1 negative group, there was no statistical difference between the two groups (Figure 1).
Table 3
| Characteristics | PD-L1 | P value | |
|---|---|---|---|
| <1% (n=81) | ≥1% (n=55) | ||
| Age (years) | 64.73±9.39 | 66.47±8.37 | 0.269 |
| Sex | 0.019 | ||
| Female | 35 (43.2) | 13 (23.6) | |
| Male | 46 (56.8) | 42 (76.4) | |
| BMI (kg/m2) | 23.06±3.54 | 22.13±3.38 | 0.128 |
| WBC (×109/L) | 6.40 (5.40–7.95) | 8.40 (6.80–10.30) | <0.001 |
| Neutrophils (×109/L) | 4.60 (3.55–6.25) | 5.10 (3.80–6.90) | 0.199 |
| Lymphocytes (×109/L) | 1.56±0.56 | 1.57±0.65 | 0.907 |
| NLR | 2.86 (2.10–4.39) | 3.31 (2.47–6.21) | 0.058 |
| BUN/Cr | 85.41±22.43 | 92.80±24.41 | 0.071 |
| Alb (g/L) | 38.78±4.05 | 37.50±5.84 | 0.163 |
| LDH (U/L) | 217.10 (178.70–407.25) | 230.60 (174.10–312.10) | 0.338 |
| T12 SMD (HU) | 47.49±11.09 | 48.04±12.24 | 0.783 |
| SMI | 1,098.50±280.22 | 1,090.27±293.61 | 0.869 |
| Clinical stage | 0.002 | ||
| III | 9 (11.1) | 18 (32.7) | |
| IV | 72 (88.9) | 37 (67.3) | |
| CEA (ng/mL) | 15.28 (4.85–81.42) | 4.28 (2.12–9.70) | <0.001 |
| NSE (ng/mL) | 22.18 (15.72–57.45) | 17.30 (10.49–23.79) | 0.001 |
| CY21-1 (ng/mL) | 6.21 (3.26–14.96) | 6.20 (2.57–14.92) | 0.698 |
| Stage | |||
| T | 0.002 | ||
| 1 | 11 (13.6) | 15 (27.3) | |
| 2 | 16 (19.8) | 18 (32.7) | |
| 3 | 15 (18.5) | 8 (14.5) | |
| 4 | 39 (48.1) | 14 (25.5) | |
| N | 0.056 | ||
| 0 | 11 (13.6) | 11 (20.0) | |
| 1 | 2 (2.5) | 5 (9.1) | |
| 2 | 30 (37.0) | 21 (38.2) | |
| 3 | 38 (46.9) | 18 (32.7) | |
| M | 0.002 | ||
| 0 | 9 (11.1) | 18 (32.7) | |
| 1 | 72 (88.9) | 37 (67.3) | |
Data are presented as mean ± SD, median (25–75th percentiles), or n (%). PD-L1, programmed cell death-ligand 1; ADC, adenocarcinoma; BMI, body mass index; WBC, white blood cell; NLR, neutrophil to lymphocyte ratio; BUN, blood urea nitrogen; Cr, creatinine; Alb, albumin; LDH, lactic dehydrogenase; T12 SMD, skeletal muscle density at the level of the T12; SMI, skeletal muscle index; CEA, carcinoembryonic antigen; NSE, neuron specific enolase; CY21-1, cytokeratin 19 fragment; T, tumor; N, node; M, metastasis; SD, standard deviation.
Evaluation of STM model performance in predicting PD-L1 expression status
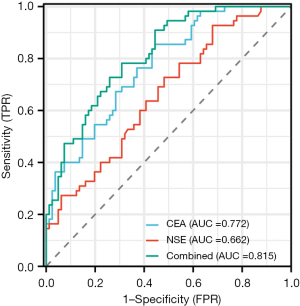
The STM values were evaluated in the PD-L1 cohort to distinguish the PD-L1 positive (PD-L1 TPS ≥1%) from the negative (PD-L1 TPS <1%) patients. Moreover, the binary task of distinguishing PD-L1 positive from the negative group, the AUCs of CEA, NSE, and their combined model were 0.772 [95% confidence interval (CI): 0.694–0.849], 0.662 (95% CI: 0.570–0.754) and 0.815 (95% CI: 0.745–0.884), respectively. The optimal cut-off values for identifying ADC patients with PD-L1 positive were CEA ≤13.38 ng/mL and NSE ≤42.35 ng/mL, with sensitivity and specificity of 85.4% and 55.6%, and 92.7% and 32.1%, respectively (Figure 2).
Multivariate analysis and correlation between PD-L1 and clinical characteristics
We selected the variables with P<0.05 in the univariate analysis and then included them in the multivariate logistic regression analysis to further explore the predictive factors of PD-L1 positive expression. The results showed CEA [odds ratio (OR) =0.954; 95% CI: 0.928–0.982; P=0.001], NSE (OR =0.993; 95% CI: 0.987–0.999; P=0.016), T stage (OR =0.559; 95% CI: 0.377–0.827; P=0.004), and WBC (OR =1.180; 95% CI: 1.013–1.374; P=0.033) were all significantly correlated with PD-L1 (Table 4).
Table 4
| Variables | B | OR | 95% CI | P value |
|---|---|---|---|---|
| Sex | 0.277 | 1.319 | 0.493–3.528 | 0.582 |
| Clinical stage | 0.510 | 1.666 | 0.869–3.194 | 0.125 |
| CEA | −0.047 | 0.954 | 0.928–0.982 | 0.001 |
| NSE | −0.007 | 0.993 | 0.987–0.999 | 0.016 |
| T | −0.582 | 0.559 | 0.377–0.827 | 0.004 |
| M | −1.359 | 0.257 | 0.054–1.217 | 0.087 |
| WBC | 0.165 | 1.180 | 1.013–1.374 | 0.033 |
PD-L1, programmed cell death-ligand 1; B, regression coefficient; OR, odds ratio; CI, confidence interval; CEA, carcinoembryonic antigen; NSE, neuron specific enolase; T, tumor; M, metastasis; WBC, white blood cell.
Discussion
In recent years, the ICIs, such as PD-1/PD-L1 inhibitors, have been widely used in the treatment of advanced cancer. PD-L1 expression has been found to predict the efficacy of immunotherapy and poor prognosis in patients with advanced NSCLC used as a critical biomarker for identifying patients more suitable for ICI treatment (40). It is essential to quantify the expression of PD-L1 accurately and rapidly in order to further guide clinical decision-making. However, the dynamic changes in the proportion of tumor cells expressing PD-L1, and the nature of invasive tissue/biopsy limits the applicability of PD-L1 detection compared with hematology-based assays. Therefore, a non-invasive, accurate and reliable method is needed to evaluate the status of PD-L1.
In this study, we evaluated the baseline demographic data, routine blood test, serum biochemical indicators, clinical stage, histological types, and the baseline level of STMs were measured routinely in clinical practice to explore their relationship with PD-L1 expression in advanced ADC patients. We confirmed that sex, clinical stage including T and M stage, WBC, CEA, and NSE were all significantly associated with PD-L1 expression in advanced ADC patients. The following multivariable logistic regression analysis observed that CEA, NSE, T stage, and WBC could be used as independent predictors of PD-L1 expression via histological analysis.
At present, many studies have reported the relationship between various clinicopathological parameters in NSCLC and the therapeutic efficacy of ICIs (41,42). However, few studies have been performed to assess the prediction of PD-L1 expression before therapy (15). In addition, there were discrepancies of the results between different studies. Therefore, the evaluation of PD-L1 expression before immunosuppressive therapy is crucial for the formulation of therapeutic regimens in NSCLC, especially for patients with advanced stage disease.
CEA, a serum glycoprotein, is the most widely used biomarker of colorectal, breast, and lung cancer (43). In our study, we found that high serum CEA levels were related to negative PD-L1 expression, which was consistent with the results of Kato et al. (26); however, they did not verify the efficiency of CEA in evaluating PD-L1 positive expression, nor did they identify its optimal cut-off value. NSE is one of the enolase involved in the glycolysis pathway and widely exists in nerve and neuroendocrine tissues. NSE has been found to exist in tumors related to the origin of neuroendocrine tissues, especially in small cell lung cancer (SCLC) where there is excessive expression of NSE, leading to a significant increase in serum levels. The correlation between NSE and PD-L1 expression has not been reported yet. In this study, we determined the AUCs produced by CEA and NSE to identify PD-L1 positive expression reach 0.772 and 0.662, respectively, and the optimal cut-off values for identifying patients with PD-L1 positive were CEA ≤13.38 ng/mL and NSE ≤42.35 ng/mL, with sensitivity and specificity of 85.4% and 55.6%, and 92.7% and 32.1%, respectively.
In addition, we proposed a model where CEA and NSE were combined to predict PD-L1 expression. Furthermore, the AUC of the combined model was higher than that of CEA alone (0.815 vs. 0.772, P=0.135). This is the first study to report the combination of serum CEA and NSE to evaluate the expression of PD-L1. In a multivariable analysis that included the clinicopathological results, it was further found that serum CEA and NSE were related to PD-L1 expression status.
Inflammation is an important part of the tumor microenvironment, which promotes tumor proliferation, angiogenesis, metastasis, and adaptive immune system destruction (44). The degree and extent of inflammation are related to the prognosis of malignant tumors. Inflammatory factors can participate in intercellular communication, thus, leading to excessive tissue remodeling and genetic and epigenetic alterations by disrupting the regulation of the immune systems. The combined effects of various inflammatory conditions increase the risk of cancer and promote distant metastasis of the tumor (45). A series of studies have been reported on the correlation between inflammatory biomarkers and the poor prognosis of NSCLC patients treated with ICIs (46,47). However, the study on the association between WBC and PD-L1 has not been reported; therefore, this is the first study regarding WBC predicting PD-L1 expression levels, suggesting that an increase in WBC indicates the positive expression of PD-L1, which is expected to be an independent predictor of PD-L1 expression. WBC are primarily involved in systemic inflammation, while PD-L1 reflects the immune response state of cancer patients. The mechanism of the relationship between the two needs further investigation.
Some studies have shown that PD-L1 is a biomarker indicating a poor prognosis and a low survival rate in patients with advanced NSCLC (48-50). When discussing the influence of clinicopathological data on PD-L1 expression, we were surprised to find that T and M staging, especially the T stage, were significantly negatively correlated with PD-L1 levels. A multivariate logistic regression analysis further confirmed that T stage could be a predictor of PD-L1 expression, which was inconsistent with the results of Liu et al. (51). In response to this phenomenon, a comprehensive analysis is needed to consolidate all available data and provide further insights on this issue.
The inconsistency between the current and previous research results may be due to the following reasons. First, the baseline demographic characteristics of the patients included in these studies are heterogeneous, and the differential expression of PD-L1 in different histological types and clinical stages may affect the analysis of clinical outcomes. Second, the threshold used to characterize PD-L1 positive expression in different studies was also different. In some studies, only patients with a percentage of PD-L1 positive tumor cells ≥50% were analyzed (52,53). Third, the techniques and protocols used in these studies may also vary. The impact of cumulative variability of laboratory techniques in each study during the outcome analysis may be difficult to estimate and elucidate.
The advantage of this study is that we have identified the STMs, especially CEA and NSE, as a convenient and effective method to detect and predict PD-L1 in ADC patients. We also determined the optimal cut-off values, and built a model for CEA combined with NSE to evaluate PD-L1 expression cooperatively, in order to judge whether ADC may show a positive expression of PD-L1. Additionally, we found that the T stage and WBC counts were significantly correlated with the expression of PD-L1 in advanced ADC patients, which could be used as independent predictors to indicate the expression of PD-L1. Currently, the detection of PD-L1 expression is based on the evaluation of clinical pathological tissues. Because of the dynamic change and individual heterogeneity of the value expression, it is difficult to evaluate the expression level of PD-L1 in all NSCLC patients in a typical treatment practice. However, for lung cancer, especially advanced ADC patients, the early prediction and evaluation of ICIs treatment effect are necessary, because anti-PD-L1 immunotherapy will affect the long-term prognosis and quality of life of patients with advanced lung cancer (54). Due to this reason, we believe that this paper has successfully identified a more convenient alternative to the standard detection method to predict the expression of PD-L1, ensuring clinicians make an earlier diagnosis leading to more rapid treatment decisions.
However, this study has several limitations. First of all, this retrospective study was conducted at a single center, which may lead to a certain degree of selection bias. Therefore, multicenter research with larger and more diverse samples are needed in the future to verify our results. Second, despite a relatively large number of patients enrolled in our cohort, considering the high prevalence of ADC, the scale of this study was still too small. Therefore, it is necessary to conduct additional research using a large-scale, prospective, and multi-center design to evaluate the primary results of this study.
In addition, liquid biopsy, as a diagnostic tool, is becoming increasingly important in the clinical management of lung cancer patients (55). It is worth noting that liquid biopsy-based testing has been proved to be very helpful in identifying operable tumor markers, especially when tissue biopsy specimens are insufficient or unavailable. In order to predict PD-L1 expression in a more non-invasive way and provide results quickly, we are willing to combine these haematological assays with liquid biopsy in future research to avoid invasive methods.
Conclusions
In conclusion, the results of this study support the concept that STMs, T staging, and WBC are related to PD-L1 measurements. A simple measurement of the above indicators can be convenient and valuable biological indicators for evaluating PD-L1 expression in advanced ADC patients. Our data also proposes a new strategy for integrating CEA and NSE and is more predictive of PD-L1 expression in patients with advanced ADC, and emphasizes their importance as potential predictive biomarkers of PD-L1 expression before treatment with ICIs. Further research is needed to evaluate the role of these markers in different clinical and pathological characteristics and even in combination with some techniques such as liquid biopsy, in larger multicenter patient populations, in order to provide a more powerful theoretical basis for the evaluation of PD-L1 expression.
Acknowledgments
The authors thank AiMi Academic Services (https://www.aimieditor.com) for English language editing and review services.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-523/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-523/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-523/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-523/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Review Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2021LSK-339). Written informed consent was waived due to retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang X, Romero-Gutierrez CW, Kothari J, et al. Prediagnosis Smoking Cessation and Overall Survival Among Patients With Non-Small Cell Lung Cancer. JAMA Netw Open 2023;6:e2311966. [Crossref] [PubMed]
- Zhang Q, Gong X, Sun L, et al. The Predictive Value of Pretreatment Lactate Dehydrogenase and Derived Neutrophil-to-Lymphocyte Ratio in Advanced Non-Small Cell Lung Cancer Patients Treated With PD-1/PD-L1 Inhibitors: A Meta-Analysis. Front Oncol 2022;12:791496. [Crossref] [PubMed]
- Cui R, Yang Z, Liu L. What does radiomics do in PD-L1 blockade therapy of NSCLC patients? Thorac Cancer 2022;13:2669-80. [Crossref] [PubMed]
- Freeman B, Mamallapalli J, Bian T, et al. Opportunities and Challenges of Kava in Lung Cancer Prevention. Int J Mol Sci 2023;24:9539. [Crossref] [PubMed]
- Liu SY, Zhang JT, Zeng KH, et al. Perioperative targeted therapy for oncogene-driven NSCLC. Lung Cancer 2022;172:160-9. [Crossref] [PubMed]
- Garassino MC, Gadgeel S, Speranza G, et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol 2023;41:1992-8. [Crossref] [PubMed]
- Brahmer JR, Lee JS, Ciuleanu TE, et al. Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J Clin Oncol 2023;41:1200-12. [Crossref] [PubMed]
- Novello S, Kowalski DM, Luft A, et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J Clin Oncol 2023;41:1999-2006. [Crossref] [PubMed]
- de Castro G Jr, Kudaba I, Wu YL, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy as First-Line Therapy in Patients With Non-Small-Cell Lung Cancer and Programmed Death Ligand-1 Tumor Proportion Score ≥ 1% in the KEYNOTE-042 Study. J Clin Oncol 2023;41:1986-91. [Crossref] [PubMed]
- Rosner S, Reuss JE, Zahurak M, et al. Five-Year Clinical Outcomes after Neoadjuvant Nivolumab in Resectable Non-Small Cell Lung Cancer. Clin Cancer Res 2023;29:705-10. [Crossref] [PubMed]
- Schoenfeld JD, Giobbie-Hurder A, Ranasinghe S, et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol 2022;23:279-91.
- Liu L, Li F, Zhao J, et al. The Real-world Therapeutic Analysis of First-line Immunotherapy in Chinese Patients with Drive Gene Positive for Advanced Non-Small Cell Lung Cancer. J Cancer 2023;14:952-65. [Crossref] [PubMed]
- Nassar AH, Adib E, Abou Alaiwi S, et al. Ancestry-driven recalibration of tumor mutational burden and disparate clinical outcomes in response to immune checkpoint inhibitors. Cancer Cell 2022;40:1161-72.e5. [Crossref] [PubMed]
- Hamada K, Tsunoda T, Yoshimura K. Emerging Immune-Monitoring System for Immune Checkpoint Inhibitors. Life (Basel) 2022;12:1229. [Crossref] [PubMed]
- Stinchcombe TE. Narrative review: blood and tumor biomarker testing in non-small cell lung cancer without an oncogenic driver. Transl Lung Cancer Res 2023;12:158-67. [Crossref] [PubMed]
- Ricciuti B, Wang X, Alessi JV, et al. Association of High Tumor Mutation Burden in Non-Small Cell Lung Cancers With Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol 2022;8:1160-8. [Crossref] [PubMed]
- Ma T, Jiao J, Huo R, et al. PD-L1 expression, tumor mutational burden, and immune cell infiltration in non-small cell lung cancer patients with epithelial growth factor receptor mutations. Front Oncol 2022;12:922899. [Crossref] [PubMed]
- Fischer A, Bankel L, Hiltbrunner S, et al. Mutational Landscape and Expression of PD-L1 in Patients with Non-Small Cell Lung Cancer Harboring Genomic Alterations of the MET gene. Target Oncol 2022;17:683-94. [Crossref] [PubMed]
- Dacic S. State of the Art of Pathologic and Molecular Testing. Hematol Oncol Clin North Am 2023;37:463-73. [Crossref] [PubMed]
- Chen Z, Liu L, Zhu F, et al. Dynamic monitoring serum tumor markers to predict molecular features of EGFR-mutated lung cancer during targeted therapy. Cancer Med 2022;11:3115-25. [Crossref] [PubMed]
- Raza A, Mohsen R, Kanbour A, et al. Serum immune mediators as novel predictors of response to anti-PD-1/PD-L1 therapy in non-small cell lung cancer patients with high tissue-PD-L1 expression. Front Immunol 2023;14:1157100. [Crossref] [PubMed]
- He X, Wang M. Application Value of Serum TK1 and PCDGF, CYFRA21-1, NSE, and CEA plus Enhanced CT Scan in the Diagnosis of Nonsmall Cell Lung Cancer and Chemotherapy Monitoring. J Oncol 2022;2022:8800787. [Crossref] [PubMed]
- Jiang C, Zhao M, Hou S, et al. The Indicative Value of Serum Tumor Markers for Metastasis and Stage of Non-Small Cell Lung Cancer. Cancers (Basel) 2022;14:5064. [Crossref] [PubMed]
- Jiang Y, Shi Y, Liu Y, et al. Efficacy and safety of alectinib in ALK-positive non-small cell lung cancer and blood markers for prognosis and efficacy: a retrospective cohort study. Transl Lung Cancer Res 2022;11:2521-38. [Crossref] [PubMed]
- Onuki Y, Matsubara H, Koizumi R, et al. Prognostic evaluation of preoperative serum tumor marker-negative cases in non-small cell lung cancer: A retrospective study. Cancer Rep (Hoboken) 2023;6:e1696. [Crossref] [PubMed]
- Kato Y, Kashima J, Watanabe K, et al. Association Between Clinicopathological Features and Programmed Death Ligand 1 Expression in Non-small Cell Lung Cancer. Anticancer Res 2018;38:1077-83. [Crossref] [PubMed]
- Sun Z, Hu S, Ge Y, et al. Radiomics study for predicting the expression of PD-L1 in non-small cell lung cancer based on CT images and clinicopathologic features. J Xray Sci Technol 2020;28:449-59. [Crossref] [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. [Crossref] [PubMed]
- Yuan S, Larsson SC. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023;144:155533. [Crossref] [PubMed]
- Jogiat U, Jimoh Z, Turner SR, et al. Sarcopenia in Lung Cancer: A Narrative Review. Nutr Cancer 2023;75:1485-98. [Crossref] [PubMed]
- Matsuguma H, Hasumi K, Wakamatsu I, et al. Preoperative diagnosis of sarcopenia and postoperative outcome in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg 2022;63:ezad001. [Crossref] [PubMed]
- Cortiula F, Hendriks LEL, van de Worp WRPH, et al. Physical exercise at the crossroad between muscle wasting and the immune system: implications for lung cancer cachexia. J Cachexia Sarcopenia Muscle 2022;13:55-67. [Crossref] [PubMed]
- Tantai X, Liu Y, Yeo YH, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;76:588-99. [Crossref] [PubMed]
- Ged Y, Sanchez A, Patil S, et al. Associations between Pretreatment Body Composition Features and Clinical Outcomes among Patients with Metastatic Clear Cell Renal Cell Carcinoma Treated with Immune Checkpoint Blockade. Clin Cancer Res 2022;28:5180-9. [Crossref] [PubMed]
- Wang J, Dong P, Qu Y, et al. Association of computed tomography-based body composition with survival in metastatic renal cancer patient received immunotherapy: a multicenter, retrospective study. Eur Radiol 2023;33:3232-42. [Crossref] [PubMed]
- Li S, Liu Z, Ren Y, et al. Sarcopenia Was a Poor Prognostic Predictor for Patients With Advanced Lung Cancer Treated With Immune Checkpoint Inhibitors. Front Nutr 2022;9:900823. [Crossref] [PubMed]
- Avilés-Salas A, Flores-Estrada D, Lara-Mejía L, et al. Modifying factors of PD-L1 expression on tumor cells in advanced non-small-cell lung cancer. Thorac Cancer 2022;13:3362-73. [Crossref] [PubMed]
- Ghiringhelli F, Bibeau F, Greillier L, et al. Immunoscore immune checkpoint using spatial quantitative analysis of CD8 and PD-L1 markers is predictive of the efficacy of anti- PD1/PD-L1 immunotherapy in non-small cell lung cancer. EBioMedicine 2023;92:104633. [Crossref] [PubMed]
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Lahiri A, Maji A, Potdar PD, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer 2023;22:40. [Crossref] [PubMed]
- Ichiki Y, Fukuyama T, Ueno M, et al. Immune profile analysis of peripheral blood and tumors of lung cancer patients treated with immune checkpoint inhibitors. Transl Lung Cancer Res 2022;11:2192-207. [Crossref] [PubMed]
- Zheng L, Xiong A, Wang S, et al. Decreased monocyte-to-lymphocyte ratio was associated with satisfied outcomes of first-line PD-1 inhibitors plus chemotherapy in stage IIIB-IV non-small cell lung cancer. Front Immunol 2023;14:1094378. [Crossref] [PubMed]
- Hashinokuchi A, Haratake N, Takenaka T, et al. Clinical significance of the combination of preoperative SUVmax and CEA in patients with clinical stage IA lung adenocarcinoma. Thorac Cancer 2022;13:2624-32. [Crossref] [PubMed]
- Afshari AR, Sanati M, Mollazadeh H, et al. Nanoparticle-based drug delivery systems in cancer: A focus on inflammatory pathways. Semin Cancer Biol 2022;86:860-72. [Crossref] [PubMed]
- Lai H, Liu Y, Wu J, et al. Targeting cancer-related inflammation with non-steroidal anti-inflammatory drugs: Perspectives in pharmacogenomics. Front Pharmacol 2022;13:1078766. [Crossref] [PubMed]
- Minichsdorfer C, Gleiss A, Aretin MB, et al. Serum parameters as prognostic biomarkers in a real world cancer patient population treated with anti PD-1/PD-L1 therapy. Ann Med 2022;54:1339-49. [Crossref] [PubMed]
- Hwang M, Canzoniero JV, Rosner S, et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J Immunother Cancer 2022;10:e004688. [Crossref] [PubMed]
- Mori M, Kanayama M, Kuwata T, et al. Prognostic impact of PD-L1 and TIGIT expression in non-small cell lung cancer following concurrent chemo-radiotherapy. Sci Rep 2023;13:3270. [Crossref] [PubMed]
- Xu X, Li J, Yang Y, et al. The correlation between PD-L1 expression and metabolic parameters of (18)FDG PET/CT and the prognostic value of PD-L1 in non-small cell lung cancer. Clin Imaging 2022;89:120-7. [Crossref] [PubMed]
- Rekulapelli A, E, Flausino L, Iyer G, et al. Effectiveness of immunological agents in non-small cell lung cancer. Cancer Rep (Hoboken) 2023;6:e1739. [Crossref] [PubMed]
- Liu LU, Xie B, Zhu W, et al. High expression of PD-L1 mainly occurs in non-small cell lung cancer patients with squamous cell carcinoma or poor differentiation. Oncol Res 2023;31:275-86. [Crossref] [PubMed]
- O'Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274-86. [Crossref] [PubMed]
- Talb J, Takam Kamga P, Mayenga M, et al. Gene expression profile of high PD-L1 non-small cell lung cancers refractory to pembrolizumab. Cancer Immunol Immunother 2022;71:2791-9. [Crossref] [PubMed]
- de Miguel-Perez D, Russo A, Arrieta O, et al. Extracellular vesicle PD-L1 dynamics predict durable response to immune-checkpoint inhibitors and survival in patients with non-small cell lung cancer. J Exp Clin Cancer Res 2022;41:186. [Crossref] [PubMed]
- Malapelle U, Pisapia P, Pepe F, et al. The evolving role of liquid biopsy in lung cancer. Lung Cancer 2022;172:53-64. [Crossref] [PubMed]


