
Neoadjuvant immunotherapy combined with chemotherapy in the treatment of stage III lung squamous cell carcinoma: a retrospective cohort study
Highlight box
Key findings
• Neoadjuvant chemotherapy plus immunotherapy in the treatment of potentially resectable stage III lung squamous cell carcinoma (LSCC) is feasible and safe.
What is known and what is new?
• Neoadjuvant chemotherapy is the standard modality of neoadjuvant therapy for locally advanced non-small cell lung cancer (NSCLC), but this treatment has high complications and low compliance. Studies have shown the efficacy of immunotherapy and chemotherapy in the treatment of advanced NSCLC.
• In the present study, we explored the therapeutic and surgical outcome for patient with stage III LSCC.
What is the implication, and what should change now?
• Neoadjuvant immunochemotherapy is safe and feasible for patients with locally advanced resectable LSCC. Our findings need to be confirmed in larger sample size randomized clinical trials in the future.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide. Non-small cell lung cancer (NSCLC) is the predominant type of lung cancer, comprising 85% of all cases of lung cancer (1). The general prognosis for patients with advanced-stage NSCLC is still poor, despite significant improvements in the treatment landscape for the disease (2). Surgical resection is the only potentially curative treatment for NSCLC, but only about 20–25% of NSCLCs are suitable for resection because the majority of patients are in the mid-to-late stage of the disease at the time of diagnosis (3,4).
NSCLC is histologically divided into adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma (5). Lung squamous cell carcinomas (LSCCs) are often of the central type, and has invaded the heart, mediastinum, and large blood vessels at the time of diagnosis, making surgical resection challenging. Neoadjuvant therapy aims to improve disease-specific survival (DSS) and overall survival (OS) in locally advanced tumors by increasing resection rates and lowering local and systemic recurrence (6-8). However, the 5-year absolute survival improvement is only 5% (9). Effective systemic treatments are still needed for nonmetastatic disease in perioperative settings.
Immune checkpoint inhibitor (ICI) therapy aimed at programmed death receptor 1 (PD-1) or its ligand (PD-L1) has drastically altered the treatment of a substantial number of patients with advanced lung cancer (10). The combination of pembrolizumab with carboplatin plus paclitaxel or nab-paclitaxel has demonstrated substantial improvements in OS and progression-free survival (PFS) compared to chemotherapy alone in patients diagnosed with previously untreated metastatic LSCC (11). According to the NADIM study, patients with stage IIIA NSCLC treated with a combination of nivolumab and chemotherapy had a major pathological response (MPR) rate of 85% following neoadjuvant therapy, as well as an OS rate of 91% and 87% at 36 and 42 months, respectively (12,13). In patients with resectable IB–IIIA NSCLCs, neoadjuvant nivolumab plus chemotherapy produced significantly longer event-free survival and a greater proportion of patients with a pathological complete response (pCR) than chemotherapy alone (14). Consequently, a neoadjuvant immunochemotherapy strategy is desirable in this population.
According to the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) staging manuals (8th edition), some late-stage tumors may also be resectable. For LSCC, due to its absence of actionable driver mutations, it is rarely suitable for targeted therapy, and the downstaging effect of neoadjuvant chemotherapy is not obvious (9). Neoadjuvant chemotherapy combined with immunotherapy shows good clinical application prospects in this part of patients (15,16). Herein, we conducted a retrospective analysis of patients with potentially resectable stage III (stage IIIA and IIIB) LSCC who underwent tumor resection after neoadjuvant immunochemotherapy to evaluate the feasibility, safety, and perioperative outcomes for these patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1175/rc) (17).
Methods
Patient selection and study design
This was a retrospective cohort study conducted between March 2020 and June 2022 at The First Affiliated Hospital of Ningbo University. The inclusion criteria for patients were as follows: (I) patients age 18 years or older, have stage IIIA or IIIB LSCC according to the 8th edition TNM staging of lung cancer of the American Joint Committee on Cancer (18) confirmed by histology or cytology, and have radiographic evidence of measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; (II) patients were required to be surgically resectable and medically operable by a multidisciplinary team (MDT); (III) no radiation or chemotherapy had been administered to the patients previously; (IV) a Karnofsky performance status above 80; (V) normal organ function and lung function can tolerate lung resection surgery. The exclusion criteria were as follows: (I) patients receiving steroids or other immunosuppressants; (II) patients with autoimmune disease; (III) patients with a prior history of malignant tumors; (IV) patients with a history of thoracic surgery or interstitial lung disease with symptoms; and (V) patients with N3 disease.
Active and passive methods were used to collect follow-up data. The active method means that patients go to the outpatient clinic for follow-up regularly, and the passive method means that we follow up patients by telephone, email, etc. To maintain accurate surveillance information, data fields relating to patient vital status, date of last contact, treatment, and recurrence were updated. Patients were followed until the date of death or the last date of follow-up (December 31, 2022). Disease-free survival (DFS) was defined as the amount of time from the start of surgery until disease progression or death. OS was defined as the length of time between the beginning of surgery and death (from any cause). Treatment-related adverse events (TRAE) were evaluated using (CTCAE) (19). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of The First Affiliated Hospital of Ningbo University (No. 2020-R229) and individual consent for this retrospective analysis was waived.
Therapy protocol
On the first day of each 21-day cycle, 200 mg of pembrolizumab, camrelizumab, or sintilimab was administered intravenously as part of immunotherapy. In addition, chemotherapeutic regimens included nab-paclitaxel 260 mg/m2 plus carboplatin area under the curve (AUC) of 5–6 mg·min/mL intravenously on day 1 every 3 weeks or a docetaxel plus cisplatin regimen, in which docetaxel 75 mg/m2 was infused over 1–2 h followed by an intravenous infusion of cisplatin 75 mg/m2 over at least 2 h on day 1.
Surgical methods
lung resections include wedge resection, lobectomy, bilobectomy, or pneumonectomy and mediastinal lymph node dissection (2R, 4R, 7, 8, and 9 for right-sided cancers; 4L, 5, 6, 7, 8, and 9 for left-sided cancers). In the thoracotomy group, surgical resection was performed with an incision placed at the fifth intercostal space (ICS). A posterolateral incision was often used. In the minimally invasive group, surgical resection was performed using bi-portal video-assisted thoracoscopic surgery (VATS) using a 10-mm, 30-degree thoracoscope. A 12-mm observation port was positioned at the seventh or eighth ICS at the midaxillary line and the operating port was located at the fourth or fifth ICS between anterior axillary line and midaxillary line. At the end of surgery, a single or double chest drain (24 or 28 Fr chest tube) was placed at the edge of the incision.
Study evaluation
All participants were staged with a chest computed tomography (CT) or positron emission tomography (PET)/CT scan, brain imaging with magnetic resonance imaging (MRI), and endobronchial ultrasound for invasive mediastinal nodal staging (20). Based on the RECIST version 1.1, the tumor’s radiological response was checked every 2 cycles (6 weeks) of the neoadjuvant regimen and before surgery. Based on tumor size and lymph node status, pathological staging was determined. Using a cryo-microtome, formalin-fixed paraffin-embedded (FFPE) tumor specimens were sliced to a thickness of 5 mm and placed on slides. For each case, the proportion of viable tumor cells was reevaluated and calculated. The definition of MPR is ≤10% of viable tumor with no viable tumor required for complete pathologic response (CPR) (21). The term “pathological complete response” (pCR) refers to the absence of any viable tumor cells upon examination of H&E slides following a comprehensive assessment of a surgically removed lung cancer specimen, which includes the evaluation of all sampled regional lymph nodes (21). The concept of pathological partial response (pPR) was established to describe a condition when the proportion of live tumor cells within the treated tumor bed is equal to or less than 50% (22). The term “pathological non-response” (pNR) was operationally defined as the presence of live tumor cells occupying more than 50% of the tumor bed (22).
Statistical analysis
Patients were characterized by demographic and clinical variables. Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were expressed as numbers (percentages). DFS and OS were determined using the Kaplan-Meier method and the statistical difference was determined using the log-rank test. R (version 4.0.5; https://www.r-project.org/) was used for statistical analysis. A two-sided P<0.05 was considered statistically significant.
Results
Patient characteristics
The study identified a total of 17 consecutive cases. A flowchart of patient selection is presented in Figure 1. The patient demographics are summarized in Table 1. All participants were male and diagnosed with stage III LSCC. The average age was 64.8±7.7 years, and the majority (70.6%) had a smoking history. Overall, 10 (58.8%) of the patients had stage IIIA disease, and 7 (41.2%) had stage IIIB disease. Of these 17 cases, 3 cases (17.6%) were stage N0 patients, 4 cases (23.5%) were stage N1 patients, and 10 cases (58.8%) were stage N2 patients. Pembrolizumab was administered to 13 (76.5%) patients, sintilimab to 3 (17.6%), and camrelizumab to 1 (5.9%). There was 1 patient (5.9%) who received 1 cycle of neoadjuvant treatment, 10 patients (58.8%) received 2 cycles, 5 patients (29.4%) received 3 cycles, and 1 patient (5.9%) received 4 cycles. The mean tumor diameter prior to immunotherapy was 51.41±22.32 mm. The average time between the last neoadjuvant treatment and surgery was 37.06±20.29 days.
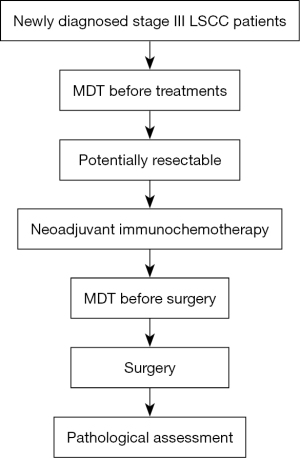
Table 1
| Characteristics | Value |
|---|---|
| Age, years, mean ± SD | 64.8±7.7 |
| Gender, n (%) | |
| Male | 17 (100.0) |
| Female | 0 |
| Smoking status, n (%) | |
| Current or ever | 12 (70.6) |
| Never | 5 (29.4) |
| Clinical stage prior to immunotherapy, n (%) | |
| IIIA | 10 (58.8) |
| IIIB | 7 (41.2) |
| Clinical nodal stage prior to immunotherapy, n (%) | |
| N0 | 3 (17.6) |
| N1 | 4 (23.5) |
| N2 | 10 (58.8) |
| Single-station | 3 (17.6) |
| Multi-station | 7 (41.2) |
| Immunotherapy regiments, n (%) | |
| Pembrolizumab | 13 (76.5) |
| Sintilimab | 3 (17.6) |
| Camrelizumab | 1 (5.9) |
| Treatment cycles, n (%) | |
| One | 1 (5.9) |
| Two | 10 (58.8) |
| Three | 5 (29.4) |
| Four | 1 (5.9) |
| Tumor diameter prior to immunotherapy, mm, mean ± SD | 51.41±22.32 |
| FEV1% predicted, mean ± SD | 79.50±10.26 |
| Days from end of neoadjuvant therapy to surgical resection, mean ± SD | 37.06±20.29 |
SD, standard deviation; FEV1%, forced expiratory volume in the first second.
Outcomes of neoadjuvant therapy
The objective response rate (ORR) was 76.5% among the 17 patients evaluated for response using RECIST 1.1, with complete response (CR) in 2 patients (11.8%), partial response (PR) in 11 patients (64.7%), and stable disease in 4 patients (23.5%). MPR was found in 12 (70.6%) of the patients, with 6 having a pCR. After neoadjuvant chemotherapy, 8 patients (47.1%) had their lymph nodes down-staged (from N2 to N1 or N0) (Figures 2,3 and Table 2).

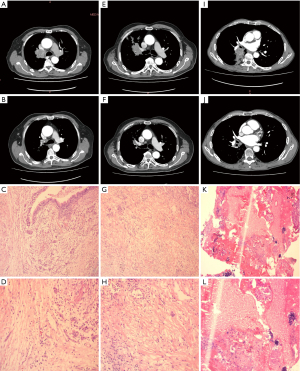
Table 2
| Variables | N (%) |
|---|---|
| Clinical response | |
| Complete response | 2 (11.8) |
| Partial response | 11 (64.7) |
| Stable disease | 4 (23.5) |
| Pathological response | |
| Non-response | 1 (5.9) |
| Partial response | 4 (23.5) |
| Major pathologic response | 12 (70.6) |
| Pathological complete response | 6 (35.3) |
| Pathological T stage | |
| T0 | 7 (41.2) |
| T1a | 3 (17.6) |
| T1b | 2 (11.8) |
| T2b | 3 (17.6) |
| T3 | 1 (5.9) |
| T4 | 1 (5.9) |
| Pathological N stage | |
| N0 | 10 (58.8) |
| N1 | 4 (23.5) |
| N2 | 2 (11.8) |
| Nx | 1 (5.9) |
| Downstaging of nodal status in patients with N2 at baseline | |
| N2 to N0 | 6 (35.3) |
| N2 to N1 | 2 (11.8) |
| N2 | 2 (11.8) |
| Adverse events (any grade) | |
| Nausea | 10 (58.8) |
| Decreased appetite | 13 (76.5) |
| Hyperbilirubinemia | 2 (11.8) |
| Leukopenia | 2 (11.8) |
| Thrombocytopenia | 3 (17.6) |
There was no grade 3 or higher TRAEs observed in any of the 17 patients following neoadjuvant treatment. A total of 13 (76.5%) patients experienced at least 1 TRAE of mild degree, including nausea, decreased appetite, hyperbilirubinemia, leukopenia, and thrombocytopenia (Table 2).
Perioperative outcome
Pre-treatment clinical stage of all 17 patients and the type of surgery each received are summarized in Table S1. Perioperative outcomes are described in Table 3. VATS was conducted in 12 cases (70.6%) and thoracotomy was performed in 5 cases (29.4%). R0 resections were achieved in 16 (94.1%) patients, with standard lobectomy in 10 (58.8%), sleeve lobectomy in 1 (5.9%), bilobectomy in 1 (5.9%), and pneumonectomy in 3 (17.6%) cases. One patient (5.9%) underwent wedge resection because of poor pulmonary function. One patient (5.9%) underwent surgical exploration and was found to have an unresectable tumor.
Table 3
| Variables | Value |
|---|---|
| Extent of resection, n (%) | |
| Lobectomy | 10 (58.8) |
| Sleeve lobectomy | 1 (5.9) |
| Bilobectomy | 1 (5.9) |
| Pneumonectomy | 3 (17.6) |
| Wedge resection | 1 (5.9) |
| Unresectable | 1 (5.9) |
| Surgical approach, n (%) | |
| VATS | 12 (70.6) |
| Thoracotomy | 5 (29.4) |
| Operative duration, min, mean ± SD | 149.24±65.43 |
| Blood loss, mL, mean ± SD | 98.82±63.63 |
| Chest tube duration, days, mean ± SD | 8.65±5.53 |
| Hospital stay after surgery, days, mean ± SD | 9.71±5.51 |
| Overall complications, n (%) | |
| Chylothorax | 1 (5.9) |
| Air leak lasting >3 days | 5 (29.4) |
| Atelectasis | 1 (5.9) |
VATS, video-assisted thoracoscopic surgery; SD, standard deviation.
Mean surgical time was 149.24±65.43 min. Mean estimated blood loss was 98.82±63.63 mL. No patient required intraoperative blood transfusion. The mean length of postoperative chest tube duration was 8.65±5.53 days and the mean duration of hospital stay after surgery was 9.71±5.51 days. A total of 7 out of the 17 patients (41.2%) developed a postoperative complication. Air leak was the most common morbidity [5 (29.4%)], 1 patient (5.9%) developed chylothorax, and 1 patient (5.9%) experienced atelectasis.
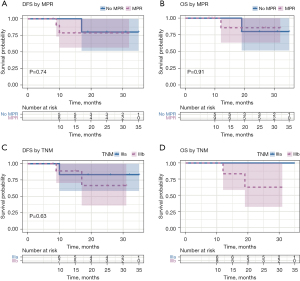
At a median follow-up of 26.0 months [interquartile range (IQR), 10.0–30.0 months] from the first day of surgery, 14 (82.4%) of the 17 patients who underwent surgical resection were still alive and had no evidence of disease, whereas 3 (17.6%) of the 17 patients had disease recurrence. Of the 3 patients who had disease recurrence, 1 patient had local recurrence (a recurrence at the bronchial stump), 1 patient had distant recurrence in the brain, and 1 patient had distant recurrence in the bone. The 2 patients with distant recurrences died. The 2-year DFS and OS rates were 76.6% and 82.5%, respectively (Figure 4). The 2-year DFS and OS rates with response to neoadjuvant therapy were both 80.0% in the no-MPR group, and 78.7% and 85.7% in the MPR group, respectively (Figure 5A,5B). The 2-year DFS and OS rates by stage were 83% and 100% for stage IIIA, respectively, and 66.7% and 62.5% for stage IIIB, respectively (Figure 5C,5D). Neither tumor-node-metastasis (TNM) stage nor response to neoadjuvant therapy was found to be associated with DFS or OS (Figure 5).

Discussion
In this retrospective study, we presented 17 cases of stage III LSCC lung resection after immunochemotherapy. 58.8% of which had stage IIIA disease, and 41.2% had stage IIIB disease. Overall, 70.6% of patients were treated via a minimally invasive approach, 5 patients underwent thoracotomy due to anticipated complexity, no conversion to thoracotomy during operation. Increased surgical difficulty after neoadjuvant therapy has always been a concern for surgeons. In the present study, the average operative time was 149.24±65.43 min, and the average blood loss was 149.24±65.43 mL. Some indicators, such as the rate of minimally invasive approaches, operating time, blood loss, and perioperative complications, can reflect operative difficulties (23). We found that after neoadjuvant therapy, the operative time was slightly longer, and the average blood loss did not increase significantly compared with conventional surgery in the same period. According to the previous study, the surgical procedure becomes more challenging following neoadjuvant immunotherapy due to the heightened presence of adhesions, hemorrhage, vascular invasion, fibrotic tissues and lymph node enlargement are difficult to separate (24). However, in another analysis on the duration of surgery, amount of blood loss, and rate of conversion to thoracotomy in a cohort of 31 patients who underwent surgical procedures subsequent to neoadjuvant immunotherapy, the authors concluded that the utilization of neoadjuvant immunotherapy did not provide a statistically significant increase in the surgical complexity (25). In this study, the overall complication rate was 41.2%, with postoperative air leak accounting for the highest proportion (29.4%). There were no mortalities within either 30 or 90 days. Even though all patients in our study were late stage, the rate of minimally invasive approach was significantly higher, and the duration of surgery was shorter than in previous studies (14,26,27). There was no postoperative mortality and most postoperative morbidity was minor in our study. Our findings show that even though the cases are more complex, that complications remain limited, and surgery is feasible and safe, although more difficult in patients with advanced LSCC.
In the era of neoadjuvant chemotherapy, the MPR rate of NSCLC is around 20%, whereas the pCR rate is less than 4% (28). The total OS of preoperative chemotherapy for NSCLC increased by only 5% (9). Ford et al. reported an MPR rate of 45% and a pCR rate of 10% in resectable NSCLC patients treated with neoadjuvant nivolumab monotherapy (29). The NEOSTAR trial, which used dual immunotherapy with nivolumab and ipilimumab for operable NSCLC, showed MPR and pCR rates of 38.1% and 28.6, respectively (30). The MPR and pCR rates in neoadjuvant immunochemotherapy have been significantly improved. In the NADIM-trial, a phase II open-label, multicenter, single-arm clinical trial, 3 cycles of nivolumab plus paclitaxel plus carboplatin preoperatively for stage IIIa NSCLC resulted in an MPR rate of 82.9% and a pCR rate of 63.4% (13). The CheckMate-816 trial was the first reported phase III neoadjuvant immunochemotherapy clinical trial for resectable NSCLC, with 3 cycles of nivolumab plus paclitaxel plus carboplatin preoperatively showing an MPR rate of 36.9% and a pCR rate of 24%, respectively (14). Our study reported an MPR rate of 70.6% and a pCR rate of 35.3%. Furthermore, 53% of patients experienced nodal downstaging following treatment, which was consistent with previous findings (31). It is critical to identify patients who can benefit from neoadjuvant therapy. Some studies have pointed out that certain biomarkers, such as the expression level of PD-L1, tumor mutation burden (TMB), and homologous recombination deficiency (HRD), can screen out the potential patients who may benefit from immunotherapy (32,33). However, which biomarkers have the highest potential for indicative capability in predicting outcomes remains unknown.
The median DFS and OS in our series were not reached. The 2-year DFS and OS rates were 76.6% and 82.5%, respectively. Previous studies used MPR as a surrogate for clinical benefit from neoadjuvant therapies and found that patients who achieved MPR after neoadjuvant chemotherapy had significantly longer DFS and OS than those who did not (3,34). Due to the small sample size and short follow-up time, we were unable to detect a significant difference in OS and DFS in our study.
There is currently no established guideline on how to process and evaluate resected lung cancer specimens, and there is a lack of precise definitions on the degree of pathological response after neoadjuvant therapy in clinical trials and clinical practice (21). In our study, 2 patients with pCR relapsed, 1 with local recurrence and 1 with distant metastasis. Traditional imaging evaluation after neoadjuvant immunotherapy can be difficult and misleading in assessing therapeutic effect on tumor cells because it does not always reflect the actual therapeutic effect (35). The International Association for the Study of Lung Cancer (IASLC) currently recommends a standardized approach to assess the percentages of (I) viable tumor, (II) necrosis, and (III) stroma (including inflammation and fibrosis), with a total of 100% (21).
Our study had the following limitations. First, this was a retrospective study with a small sample size and lack of PD-L1 scores. Second, all patients were men, and the treatment cycles and intervals could not be well controlled. Third, because the follow-up period was relatively short, additional analyses with long-term follow-up are warranted. As a result, the findings must be validated in a large-scale, multicenter prospective clinical trial.
Conclusions
Our study demonstrated that neoadjuvant immunochemotherapy was safe and feasible for patients with late-stage resectable LSCC. In the current population, the perioperative mortality and morbidity rates were comparable. Our findings need to be confirmed in larger sample size with randomized clinical trials in the future.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1175/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1175/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1175/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1175/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of The First Affiliated Hospital of Ningbo University (No. 2020-R229) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest 2001;120:1584-91. [Crossref] [PubMed]
- Jasper K, Stiles B, McDonald F, et al. Practical Management of Oligometastatic Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:635-41. [Crossref] [PubMed]
- Kim N, Kim HK, Lee K, et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat Commun 2020;11:2285. [Crossref] [PubMed]
- Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [Crossref] [PubMed]
- Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol 2002;20:247-53. [Crossref] [PubMed]
- Nagai K, Tsuchiya R, Mori T, et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 2003;125:254-60. [Crossref] [PubMed]
- Group NM-aC. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Azad NS, Gray RJ, Overman MJ, et al. Nivolumab Is Effective in Mismatch Repair-Deficient Noncolorectal Cancers: Results From Arm Z1D-A Subprotocol of the NCI-MATCH (EAY131) Study. J Clin Oncol 2020;38:214-22. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Provencio M, Serna-Blasco R, Nadal E, et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol 2022;40:2924-33. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Wang J, Lu S, Yu X, et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021;7:709-17. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr 2021;112:90-2. (Engl Ed). [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non–Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2022;20:497-530. [Crossref] [PubMed]
- Travis WD, Dacic S, Wistuba I, et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 2020;15:709-40. [Crossref] [PubMed]
- Varga Z, Christiansen A, Lukamowicz-Rajska M, et al. Next Generation Sequencing of Reactive Stroma and Residual Breast Cancer Cells in Tumor Bed after Neoadjuvant Chemotherapy. Cancers (Basel) 2022;14:5609. [Crossref] [PubMed]
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. [Crossref] [PubMed]
- Shi Y, Li J, Chen M, et al. Sarcoidosis-like reaction after neoadjuvant pembrolizumab combined with chemotherapy mimicking disease progression of NSCLC induced encouraging discovery of pathological complete response. Thorac Cancer 2021;12:3433-6. [Crossref] [PubMed]
- Jiang L, Huang J, Jiang S, et al. The surgical perspective in neoadjuvant immunotherapy for resectable non-small cell lung cancer. Cancer Immunol Immunother 2021;70:2313-21. [Crossref] [PubMed]
- Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. [Crossref] [PubMed]
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. [Crossref] [PubMed]
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504-14. [Crossref] [PubMed]
- Wu J, Hou L, E H, et al. Real-world clinical outcomes of neoadjuvant immunotherapy combined with chemotherapy in resectable non-small cell lung cancer. Lung Cancer 2022;165:115-23. [Crossref] [PubMed]
- Yarchoan M, Albacker LA, Hopkins AC, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019;4:e126908. [Crossref] [PubMed]
- Zhou Z, Ding Z, Yuan J, et al. Homologous recombination deficiency (HRD) can predict the therapeutic outcomes of immuno-neoadjuvant therapy in NSCLC patients. J Hematol Oncol 2022;15:62. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Ling Y, Li N, Li L, et al. Different pathologic responses to neoadjuvant anti-PD-1 in primary squamous lung cancer and regional lymph nodes. NPJ Precis Oncol 2020;4:32. [Crossref] [PubMed]


