Surgery and pleuro-pulmonary tuberculosis: a scientific literature review
Introduction
Tuberculosis (TB) is considered a leading cause of morbidity and mortality worldwide and, therefore, is a public health priority, especially in low-and middle-income countries, where TB incidence is estimated to be the highest. Over 9.6 million new TB cases and 1.5 million deaths are estimated to have occurred in 2014, mostly in Africa and South-East Asia (1).
People living with HIV (PLHIV) are disproportionately affected by TB; however, other medical conditions have been associated with an increased risk of TB (2-5).
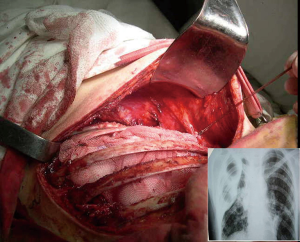
A significant decline in TB incidence has been observed in high income countries since the second half of the 20th century, following the improvement of socio-economic conditions and the introduction of the first anti-TB drugs (6). When rifampicin began to be routinely used in the 1960s, the therapeutic approach to TB was revolutionized, making chemotherapy the first therapeutic option and surgical treatment a less convenient approach (7). Current drug regimens achieve a cure rate >85%, with poorer outcomes in geographical areas where multidrug-resistant (MDR) strains are prevalent. MDR-TB, whose estimated incidence was approximately 480,000 cases in 2014, is a TB form caused by Mycobacterium tuberculosis strains resistant to at least rifampicin and isoniazid (1). However, only one third of the estimated cases are actually diagnosed and a quarter receives a second-line treatment (8). MDR-TB is a man-made phenomenon, resulting from clinical mismanagement of TB cases (e.g., inappropriate regimens and/or drug dosages, insufficient duration of treatment and poor adherence) (9). The World Health Organization (WHO) guidelines on MDR-TB management suggest a combination of five to seven second-line drugs, whose effectiveness and safety profiles are currently sub-optimal (10). Although clinical observational and experimental research on repurposed (carbapenems, linezolid, mefloquine, etc.) and new (bedaquiline and delamanid) drugs has showed interesting findings, a lot of work is still needed to assess the best options to treat MDR-TB (11-15). In this critical global scenario with few effective drug options, TB surgery could be crucial to clinically and bacteriologically address severe forms of pleuro-pulmonary MDR-TB in combination with appropriate chemotherapy (16,17). The need for surgery is estimated to have increased from 5% to 15% over the last twenty years due to the growing emergence of MDR-TB (18). Old and new surgical techniques are still in use, often in absence of a clear evidence on their effectiveness (Figure 1).
Aim of this review is to describe past and present surgical practices supporting the clinician to better manage diagnosis and treatment of complex TB cases.
Methods
We carried out a non-systematic literature review based on a PubMed search using specific key-words, including various combinations of TB, surgery, MDR-TB. References of the most important papers were retrieved to improve the search sensitivity.
Manuscripts written in English and Russian were selected.
The main indications for surgery to support diagnosis and treatment of TB patients include both old and modern approaches; they are presently summarized as follows:
- Diagnosis of complicated cases;
- Elimination of contagious persisting cavities, despite appropriate chemotherapy;
- Treatment of destroyed lung;
- Resection of tuberculomas;
- Treatment of tuberculous pleural empyema.
After describing each of these areas of intervention we will provide a summary of the evidence emerging from the literature search and concluding remarks.
Areas of intervention
Diagnosis of complicated cases
This explorative surgical approach can be recommended in patients with suspected lung cancer, in those with a pulmonary dissemination and those with mediastinal lymphadenopathy of unknown origin.
Distinguishing between TB and lung cancer can be rather challenging. The coexistence of TB and lung cancer, either within the same lesion or in different ones, has also been reported. Diagnostic delay and inappropriate treatment can be avoided through prompt identification of TB and/or lung cancer. Pulmonary cavitations should be carefully assessed before administering anti-TB drugs since a considerable proportion of elderly patients may also have an underlying malignancy (19).
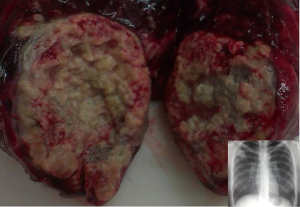
The increasing incidence of pulmonary TB in older patients in high-income countries, together with a more indolent course of lung cancer, make surgical exploration a reasonable option. It is estimated that TB is confirmed in up to 40% of surgical specimens from patients suspected of having lung cancer (Figure 2) (20). The discrepancy between presumptive and confirmed malignancy may be very high, up to 34% versus 6.25% according to some case series, and TB-associated lung cancer can be found in more than 25% of patients (21). Also, radiographic criteria for malignancy can be identified in up to 66% of patients with a confirmed tuberculoma (22).
When TB and lung cancer coexist, the neoplastic portion of the lesion is usually characterized by irregular margins and convergence of peripheral vessels on High Resolution Computed Tomography (HRCT) scans showing gradual enhancement on time-attenuation curve, while smooth margins and a cavity with peripheral contrast-enhancement are typical features of the benign portion (23). Also, homogeneous contrast-enhancement is commonly observed in carcinomas, while capsular or no enhancement are suggestive of tuberculomas or hamartomas (24,25). Some radiographic signs should be evaluated in case of undefined solitary lesions: (I) nodular conglomeration in the area of previous tuberculous involvement; (II) recently developed satellite lesions surrounding tuberculous sequelae; or (III) parenchymal cavitations in areas where only nodular lesions were previously noted.
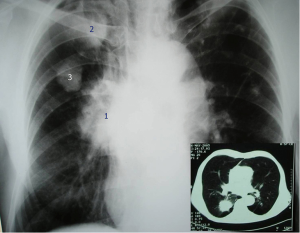
Carcinoma-related subpleural scars are usually adenocarcinomas. According to some reports, nearly 90% of patients with scar cancer show stage I disease, as a consequence of a regular radiologic TB monitoring (Figure 3) (26).
If disseminated pulmonary lesions (often associated with hilar and/or mediastinal lymphadenopathies) are detected, TB must always be suspected especially in immunocompromised individuals, although other conditions should also be considered such as cardiovascular disorders, systemic syndromes, and several occupational diseases (27-29). In such cases, surgery, based on minimally invasive techniques, can help to diagnose TB disease (30). Radiological findings are not reliable to definitely diagnose a TB case (31). For instance, hilar and mediastinal lymphadenopathy may be identified in primary and secondary TB patients. Such nodes, frequently associated with post-inflammatory adhesions in the interlobar fissures, must be carefully considered before performing Video-Assisted Thoracoscopic Surgery (VATS) or thoracotomy for lymph node biopsy in the absence of parenchymal lesions (32). Cervical mediastinoscopy plays a marginal role in the diagnosis of mediastinal TB since the proportion of patients benefitting from this procedure is considerably limited (33,34). Sayar et al. reported a series of 19 patients with no parenchymal abnormalities who underwent mediastinoscopy and TB was confirmed in 16 of them. However, a minimum of 3–5 nodal station biopsies is thought to be needed to increase the diagnostic probabilities (35).
The diagnostic value of transbronchial lung biopsy in patients with a miliary TB pattern ranges between 30% and 58% or 64% and 90% depending on inclusion criteria (36,37). Percutaneous biopsy of parenchymal lung lesions shows a poorer performance, with 20–55% of diagnostic yield (38). Surgical lung biopsy may, therefore, be required for diagnosis in a proportion of patients suspected of having TB, although operative and infectious risks should be cautiously evaluated (39).
Elimination of contagious persisting cavities despite appropriate chemotherapy
TB patients with a persistently positive sputum smear may benefit from surgical resection of bacillary pulmonary lesions. This therapeutic approach is mainly reserved to MDR-TB cases and aims to cure patients and prevent further M. tuberculosis transmission, increasing the probability of treatment success (40,41).
MDR-TB radiographic patterns are usually associated with the underlying drug-resistant form: patients with primary resistance are more likely to have non-cavitary solid lesions, pleural effusions and primary TB classical features, while those who develop secondary drug resistance often present with cavitary consolidations and a post-primary TB pattern (42,43).
Patients with one or more of the following clinical features can be considered as potential candidates to surgery: (I) persistently positive sputum smear and/or culture despite appropriate chemotherapy; (II) relapse; (III) high risk of relapse based on drug resistance profile (e.g., XDR-TB cases) (44,45).
Surgical resection is recommended for infectious TB patients after at least 6–8 months of appropriate anti-TB therapy (10,46-48).
A recent meta-analysis reported a treatment success of 84% for pulmonary resection in M/XDR-TB patients, with a 6% failure rate, 3% relapse, and 5% mortality (49). Excessive surgical delay may favour disease progression and development of further drug resistances (50). Although some authors suggested that surgery can be performed after only three months of medical therapy, a period of six to eight months is now recommended to make treatment success more likely (51). Other important prognostic factors include unilateral pulmonary involvement, absence of TB bronchitis, radiologic improvement of parenchymal sequelae, complicated pulmonary TB (e.g., haemoptysis, superimposed infections, etc.).
A maximally parenchymal-sparing surgery is recommended whenever possible since disease relapse may occur despite adequate surgical intervention and appropriate chemotherapy (Figure 4). According to the scientific literature, lobectomy is performed more frequently compared to pneumonectomy (63.7% vs. 21.8%) (52).

High rate of pneumonectomy is reported in series including patients with multicavitary forms involving >1 lobe (53,54).
In patients with bilateral lung involvement (40–90% of the total cases), dominant lesions are usually unilateral; consequently, small contralateral nodules or cavities are managed only with post-operative medical therapy. However, if sputum conversion does not occur after surgery for dominant lesions, small contralateral cavitary lesions should be removed as well (48). Post-operative complications in MDR-TB patients undergoing pulmonary resection are summarized in (Table 1) (55-60).

Full table
Treatment of destroyed lung
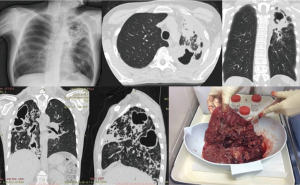
The term “destroyed lung” refers to the radiographic appearance of fibrotic cavities combined with caseous lesions, frequently accompanied by sub-lobar atelectasis or bronchiectasis. Other typical signs are the upward displacement of the lung hilus, the mediastinal shift towards the affected side and a narrow intercostal space (Figure 5). Most patients with this medical condition have a history of exposure to multiple TB therapies, with delayed recovery and/or drug resistance (17,61). The surgical rationale is to interrupt further M. tuberculosis transmission and to prevent life-threatening complications.
In high TB burden areas, TB is the leading cause of haemoptysis and recurrent episodes may reflect tubercular cavitations or destroyed lungs (62-65). Although haemoptysis represents an indication for surgery, an assessment of lesions not responsible for bleeding can be very difficult and mortality rate may be up to 40% for procedures performed in emergency situations (66). The risk of complications after embolization of bronchial arteries is usually low, with 75–94% of patients immediately clinically recovering; however, the recurrence rate ranges between 18% and 42% (67,68). If massive haemoptysis occurs and embolization cannot be performed, emergency thoracotomy is recommended (62,69).
In a single-centre cohort study published in 2007, four risk factors were associated with recurrence after successful embolization for life-threatening haemoptysis: (I) lack of complete cessation of haemoptysis within seven days after the procedure; (II) need for blood transfusion; (III) presence of aspergillomas; (V) absence of TB (70).
In patients with haemoptysis and destroyed lung, surgical delay may be life-threatening. In a series of 38 patients with destroyed lungs combined with aspergillomas and haemoptysis, undergoing pneumonectomy or pleuro-pneumonectomy, one out of seven with post-surgical empyema died (71).
Bronchial stenosis, often combined with a destroyed lung, should be distinguished from TB of the bronchus; however, it does not constitute an obstacle for pneumonectomy (72). An extrapleural approach is usually required since other interventions are not feasible (Figure 5).
A conservative treatment with thoracic drainage is suggested as initial procedure in case of destroyed lung complicated by empyema, especially if a broncho-pleural fistula is also present. In such cases, surgery may be carried out only after achieving a complete infection control except for selected patients without broncho-pleural fistulae (73).
Resection of tuberculomas
Surgery in patients with tuberculomas is recommended to reduce their infectiousness. It usually radiologically appears as a post-primary lesion and can be confused with a malignant neoplasia (74).
Tuberculomas, sized between 1 and 10 cm, are estimated to be one of the most frequent benign nodules, representing up to 25% of all resected single pulmonary nodules (75,76). They can occur during both primary and post-primary TB (31,77). In resected tuberculomas M. tuberculosis strains can be isolated in ~85% of cases. They may include a cavity or calcifications, and margins are usually smooth and sharp (78,79). Tuberculomas can be found in the upper lobes, often surrounded by satellite nodules in 80% of the cases (80,81).
Because of increased glucose metabolism caused by granulomatous inflammation, they can accumulate F-18-fluorodeoxyglucose (FDG) during positron emission tomography (PET); then, they could be misdiagnosed with pulmonary tumours (Figure 3) (82). Choline C-11 PET scans can differentiate between lung cancer and tuberculoma, being tuberculoma’s choline C-11 uptake lower if compared with lung cancer (83).
Natural history of pulmonary tuberculoma can be divided into progressive, stationary, and regressive. Stationary status accounts for 30-50% of all patients with a tuberculoma (84,85).
Reported progression rate was 5.8% in patients with tuberculomas sized <2 cm vs. 14.8% and 25.0% when tuberculoma is sized <4 and >4 cm, respectively (86).
Atypical and typical mono- and bi-segmental resections are recommended for patients with tuberculoma. Lobectomy may be necessary only for large lesions or for those located near the lung hilum.
If anti-TB therapy is not performed pre-operatively (differential diagnosis with lung cancer), it is usually administered post-operatively (87,88).
Treatment of tuberculous pleural empyema
Pleural adhesions can occur since the first inflammatory phases. In case of a delayed diagnosis, an open or VATS decortication is the only therapeutic option.
In some TB patients pleural effusion cannot be associated with a clear parenchymal disease.
In patients without any radiologic signs of TB, diagnosis of TB pleuritis can be performed histologically only after removal of thickened pleura.
If pleural empyema occurs with radiologic TB signs, conservative treatment with prolonged chest tube aspiration is strongly recommended. Surgical therapy (open approach) is suggested only for cases with major residual pleural thickenings (89).
In case of a bronchopleural fistula the tube thoracostomy is the initial treatment option. Open thoracostomy is usually recommended for severely ill patients with a high operative risk. Two-stage management with open thoracostomy and subsequent muscle plombage of the cleaned residual space is also suggested as a reasonable alternative in patients with poor general conditions and extensive calcifications precluding decortications (90).
In patients operated for tuberculous empyema, the need for the lung resection can not always be assessed with certainty before the operation. When such a need is confirmed during the operation, the remaining lung may be so destroyed that pneumonectomy has to be considered. However, several Authors do not recommend a similar therapeutic approach, arguing that ТВ and concomitant empyema are relevant risk factors for procedure-related morbidity and mortality (91-94).
After decortications, residual pleural space may be managed either with muscle plombage or with thoracoplasty. A four-rib thoracoplasty with a stable and retracted mediastinum may be carried out (95).
In patients without radiologic signs of TB, a diagnostic approach is suggested.
In patients with a bronchopleural fistula chest tube thoracostomy is a suitable initial, but not infrequently a definitive treatment option. However, two-stage procedures with open thoracostomy and subsequent residual cavity obliteration may be possible in clinically-compensated patients.
Open thoracostomy is usually recommended for severely ill patients with a high operative risk.
Indications, contraindications and risks
The indications for lung resection have been discussed above. As resection is performed with a full or muscle-sparing thoracotomy, dense adhesions can be a relative surgical contraindication, particularly with a video-thoracoscopic approach. In patients with poor lung function or advanced age VATS lobectomy is probably the most suitable option (96).
Preoperative bronchoscopy is mandatory, including biopsies from the anticipated suture line and from sites with suspected active disease. Endoscopic confirmation of tuberculous bronchitis is a contraindication for surgery, because endobronchial granulation tissue, erosion, and ulceration of the mucosa may predispose to the development of bronchopleural fistula (97).
Operative mortality after lung resection for TB is lower than 5%, an acceptable proportion if we consider that mortality following lobectomy for lung cancer is around 3%. The proportion of post-operative complications is 9–26%, the commonest being persistent air leakage (40%) (98).
Summary of the evidence
- Reliable radiographic criteria are unavailable to distinguish between malignant and TB lesions. Surgical exploration with ex tempore histological examination may be helpful and should not be delayed (22-25,28,31).
- If bronchoscopic biopsies failed to confirm diagnosis, video-assisted thoracoscopy or mediastinoscopy are valid alternatives. Diagnostic thoracotomy is rarely necessary, but may be considered in selected cases (30,33-38).
- In MDR-TB patients, surgical resection of pulmonary bacillary lesions may be useful to improve treatment outcome if chemotherapy failed, but before complete destruction of lung parenchyma and associated decline in pulmonary function (16,41,44,48,49,99).
- Maximally parenchymal-sparing surgery should be preferred in MDR-TB patients due to their high relapse risk (45,50-55,57,58).
- Surgical treatment can be performed in patients meeting appropriate criteria provided that contralateral lung lesions are stable (57,58).
- Bronchoscopic biopsy of the bronchial mucosa aimed to exclude TB is of utmost importance before proceeding to pneumonectomy for a destroyed lung (36,37).
- Tuberculoma should be surgically treated to reduce infectiousness. Appropriate imaging techniques should be adopted to discriminate lung cancer and tuberculoma (81-84,88).
- A diagnostic surgical approach is recommended when pleural effusion occurs without any radiologic signs. Open surgery is suggested only for cases with major residual pleural thickenings when TB pleuritis is immediately detected. Bronchopleural fistula should be managed with a chest tube thoracostomy (89-92).
- Post-operative chemotherapy is mandatory; after the removal of the main lung lesion as some nodules and tiny cavities may persist and can favour relapses. The following duration of anti-TB therapy is recommended (99). For culture-positive patients at the time of surgery:
- With drug-susceptible TB four to six months of therapy after culture conversion;
- With MDR-TB at least 18 months of therapy after culture conversion;
- With XDR-TB at least 24 months of therapy after culture conversion.
- For culture-negative patients at the time of surgery:
- With drug-susceptible TB at least four months of therapy after surgery;
- With M/XDR-TB six to eight months of therapy after surgery.
Concluding remarks
The rate of treatment success after surgery ranges between 75% and 98%, as shown in Tables 2 and 3. Unfortunately, the most severe cases (e.g., bilateral cavities or destroyed lung) who would benefit most from surgery cannot undergo such procedures due to the high operative and post-operative morbidity and mortality.
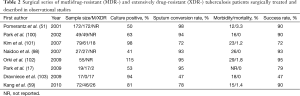
Full table

Full table
The experience of high level reference centres for MDR-TB suggest that a proper individualized synergy of chemotherapy and surgery might increase the chances of treatment success (16,44,45).
Evidence-based guidelines are necessary in order to better understand which patient categories and surgical techniques should be recommended. Interventional trials carried out in high burden settings could include adequate sample sizes to improve the statistical power of the epidemiological studies and to provide reliable findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Global tuberculosis report 2015. Geneva: World Health Organization (WHO), 2015.
- Walker NF, Meintjes G, Wilkinson RJ. HIV-1 and the immune response to TB. Future Virol 2013;8:57-80. [Crossref] [PubMed]
- Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV 2015;2:e438-44. [Crossref] [PubMed]
- Ferrara G, Murray M, Winthrop K, et al. Risk factors associated with pulmonary tuberculosis: smoking, diabetes and anti-TNFα drugs. Curr Opin Pulm Med 2012;18:233-40. [Crossref] [PubMed]
- Sotgiu G, Matteelli A, Migliori GB., et al. Diabetes and tuberculosis: what else beyond? Int J Tuberc Lung Dis 2015;19:1127-8. [Crossref] [PubMed]
- Hermans S, Horsburgh CR Jr, Wood R. A Century of Tuberculosis Epidemiology in the Northern and Southern Hemisphere: The Differential Impact of Control Interventions. PloS one 2015;10:e0135179. [Crossref] [PubMed]
- Fisher L. Rifampin--new and potent drug for TB treatment. Bull Natl Tuberc Respir Dis Assoc 1971;57:11-2. [PubMed]
- Falzon D, Mirzayev F, Wares F, et al. Multidrug-resistant tuberculosis around the world: what progress has been made? Eur Respir J 2015;45:150-60. [Crossref] [PubMed]
- Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2009;13:1320-30. [PubMed]
- Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization, 2014.
- Pontali E, Sotgiu G, D'Ambrosio L, et al. Bedaquiline and multidrug-resistant tuberculosis: a systematic and critical analysis of the evidence. Eur Respir J 2016;47:394-402. [Crossref] [PubMed]
- Chang KC, Yew WW, Sotgiu G. Clinical research in the treatment of tuberculosis: current status and future prospects. Int J Tuberc Lung Dis 2015;19:1417-27. [Crossref] [PubMed]
- Tiberi S, D'Ambrosio L, De Lorenzo S, et al. Ertapenem in the treatment of multidrug-resistant tuberculosis: first clinical experience. Eur Respir J 2016;47:333-6. [Crossref] [PubMed]
- Sotgiu G, Pontali E, Centis R, et al. D'Ambrosio L, Delamanid (OPC-67683) for treatment of multi-drug-resistant tuberculosis. Expert Rev Anti Infect Ther 2015;13:305-15. [Crossref] [PubMed]
- Tiberi S, Payen M C, Sotgiu G, et al. Effectiveness and safety of meropenem/clavulanate-containing regimens in the treatment of MDR-and XDR-TB. Eur Respir J 2016;47:1235-43. [Crossref] [PubMed]
- Fox GJ, Mitnick CD, Benedetti A, et al. Surgery as an Adjunctive Treatment for Multidrug-Resistant Tuberculosis: An Individual Patient Data Metaanalysis. Clin Infect Dis 2016;62:887-95. [Crossref] [PubMed]
- Park SK, Kim JH, Kang H, et al. Pulmonary resection combined with isoniazid- and rifampin-based drug therapy for patients with multidrug-resistant and extensively drug-resistant tuberculosis. Int J Infect Dis 2009;13:170-5. [Crossref] [PubMed]
- Moran JF. Surgical treatment of pulmonary tuberculosis. In: Sabiston DC Jr, Spencer FC. editors. Surgery of the chest. 6th ed. Philadelphia: W.B. Saunders Company, 1995:752-72.
- Tariq SM, Tariq S. Empirical treatment for tuberculosis: survey of cases treated over 2 years in a London area. J Pak Med Assoc 2004;54:88-95. [PubMed]
- Mouroux J, Maalouf J, Padovani B, et al. Surgical management of pleuropulmonary tuberculosis. J Thorac Cardiovasc Surg 1996;111:662-70. [Crossref] [PubMed]
- Rizzi A, Rocco G, Robustellini M, et al. Results of surgical management of tuberculosis: experience in 206 patients undergoing operation. Ann Thorac Surg 1995;59:896-900. [Crossref] [PubMed]
- Ishida T, Yokoyama H, Kaneko S, et al. Pulmonary tuberculoma and indications for surgery: radiographic and clinicopathological analysis. Respir Med 1992;86:431-6. [Crossref] [PubMed]
- Ashizawa K, Matsuyama N, Okimoto T, et al. Coexistence of lung cancer and tuberculoma in the same lesion: demonstration by high resolution and contrast-enhanced dynamic CT. Br J Radiol 2004;77:959-62. [Crossref] [PubMed]
- Yamashita K, Matsunobe S, Tsuda T, et al. Solitary pulmonary nodule: preliminary study of evaluation with incremental dynamic CT. Radiology 1995;194:399-405. [Crossref] [PubMed]
- Swensen SJ, Morin RL, Schueler BA, et al. Solitary pulmonary nodule: CT evaluation of enhancement with iodinated contrast material--a preliminary report. Radiology 1992;182:343-7. [Crossref] [PubMed]
- Jackson D, Greenberg SD, Howel JF. Pulmonary scar carcinoma. A case with two primaries. Cancer 1984;54:361-6. [Crossref] [PubMed]
- Kwong JS, Carignan S, Kang EY, et al. Miliary tuberculosis. Diagnostic accuracy of chest radiography. Chest 1996;110:339-42. [Crossref] [PubMed]
- Hong SH, Im JG, Lee JS, et al. High resolution CT findings of miliary tuberculosis. J Comput Assist Tomogr 1998;22:220-4. [Crossref] [PubMed]
- Im JG, Itoh H, Han MC. CT of pulmonary tuberculosis. Semin Ultrasound CT MR 1995;16:420-34. [Crossref] [PubMed]
- Ensminger SA, Prakash UB. Is bronchoscopic lung biopsy helpful in the management of patients with diffuse lung disease? Eur Respir J 2006;28:1081-4. [Crossref] [PubMed]
- Krysl J, Korzeniewska-Kosela M, Müller NL, et al. Radiologic features of pulmonary tuberculosis: an assessment of 188 cases. Can Assoc Radiol J 1994;45:101-7. [PubMed]
- Sihoe AD, Shiraishi Y, Yew WW. The current role of thoracic surgery in tuberculosis management. Respirology 2009;14:954-68. [Crossref] [PubMed]
- Otto TL, Zaslonka J, Lukiański M. Experience with mediastinoscopy. Thorax 1972;27:463-7. [Crossref] [PubMed]
- Jacob B, Parsa R, Frizzell R, et al. Mediastinal tuberculosis in Bradford, United Kingdom: the role of mediastinoscopy. Int J Tuberc Lung Dis 2011;15:240-5. i. [PubMed]
- Sayar A, Ölçmen A, Metin M, et al. Role of mediastinoscopy in intrathoracic tuberculous lymphadenitis. Asian Cardiovascular and Thoracic Annals 2000;8:253-5. [Crossref]
- Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest 2000;117:385-92. [Crossref] [PubMed]
- Charoenratanakul S, Dejsomritrutai W, Chaiprasert A. Diagnostic role of fiberoptic bronchoscopy in suspected smear negative pulmonary tuberculosis. Respir Med 1995;89:621-3. [Crossref] [PubMed]
- Lacasse Y, Wong E, Guyatt GH, et al. Transthoracic needle aspiration biopsy for the diagnosis of localised pulmonary lesions: a meta-analysis. Thorax 1999;54:884-93. [Crossref] [PubMed]
- Cooper JD, Perelman M, Todd TR, et al. Precision cautery excision of pulmonary lesions. Ann Thorac Surg 1986;41:51-3. [Crossref] [PubMed]
- Olcmen A, Gunluoglu MZ, Demir A, et al. Role and outcome of surgery for pulmonary tuberculosis. Asian Cardiovasc Thorac Ann 2006;14:363-6. [Crossref] [PubMed]
- van Leuven M, De Groot M, Shean KP, et al. Pulmonary resection as an adjunct in the treatment of multiple drug-resistant tuberculosis. Ann Thorac Surg 1997;63:1368-72; discussion 1372-3. [Crossref] [PubMed]
- Fishman JE, Sais GJ, Schwartz DS, et al. Radiographic findings and patterns in multidrug-resistant tuberculosis. J Thorac Imaging 1998;13:65-71. [Crossref] [PubMed]
- Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol 2008;191:834-44. [Crossref] [PubMed]
- Iseman MD, Madsen L, Goble M, et al. Surgical intervention in the treatment of pulmonary disease caused by drug-resistant Mycobacterium tuberculosis. Am Rev Respir Dis 1990;141:623-5. [Crossref] [PubMed]
- Pomerantz M, Madsen L, Goble M, et al. Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg 1991;52:1108-11; discussion 1112. [Crossref] [PubMed]
- Hu Y, Coates AR, Mitchison DA. Sterilizing activities of fluoroquinolones against rifampin-tolerant populations of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2003;47:653-7. [Crossref] [PubMed]
- Sulochana S, Rahman F, Paramasivan CN. In vitro activity of fluoroquinolones against Mycobacterium tuberculosis. J Chemother 2005;17:169-73. [Crossref] [PubMed]
- Shiraishi Y, Nakajima Y, Katsuragi N, et al. Resectional surgery combined with chemotherapy remains the treatment of choice for multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2004;128:523-8. [Crossref] [PubMed]
- Xu HB, Jiang RH, Li L. Pulmonary resection for patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. J Antimicrob Chemother 2011;66:1687-95. [Crossref] [PubMed]
- Marrone MT, Venkataramanan V, Goodman M, et al. Surgical interventions for drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2013;17:6-16. [Crossref] [PubMed]
- Pomerantz BJ, Cleveland JC Jr, Olson HK, et al. Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg 2001;121:448-53. [Crossref] [PubMed]
- Somocurcio JG, Sotomayor A, Shin S, et al. Surgery for patients with drug-resistant tuberculosis: report of 121 cases receiving community-based treatment in Lima, Peru. Thorax 2007;62:416-21. [Crossref] [PubMed]
- Kim HJ, Kang CH, Kim YT, et al. Prognostic factors for surgical resection in patients with multidrug-resistant tuberculosis. Eur Respir J 2006;28:576-80. [Crossref] [PubMed]
- Naidoo R, Reddi A. Lung resection for multidrug-resistant tuberculosis. Asian Cardiovasc Thorac Ann 2005;13:172-4. [Crossref] [PubMed]
- Man MA, Nicolau D. Surgical treatment to increase the success rate of multidrug-resistant tuberculosis. Eur J Cardiothorac Surg 2012;42:e9-12. [Crossref] [PubMed]
- Kir A, Inci I, Torun T, et al. Adjuvant resectional surgery improves cure rates in multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2006;131:693-6. [Crossref] [PubMed]
- Shiraishi Y, Katsuragi N, Kita H, et al. Aggressive surgical treatment of multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2009;138:1180-4. [Crossref] [PubMed]
- Mohsen T, Zeid AA, Haj-Yahia S. Lobectomy or pneumonectomy for multidrug-resistant pulmonary tuberculosis can be performed with acceptable morbidity and mortality: a seven-year review of a single institution's experience. J Thorac Cardiovasc Surg 2007;134:194-8. [Crossref] [PubMed]
- Kang MW, Kim HK, Choi YS, et al. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg 2010;89:1597-602. [Crossref] [PubMed]
- Wang H, Lin H, Jiang G. Pulmonary resection in the treatment of multidrug-resistant tuberculosis: a retrospective study of 56 cases. Ann Thorac Surg 2008;86:1640-5. [Crossref] [PubMed]
- Bai L, Hong Z, Gong C, et al. Surgical treatment efficacy in 172 cases of tuberculosis-destroyed lungs. Eur J Cardiothorac Surg 2012;41:335-40. [Crossref] [PubMed]
- Ozgül MA, Turna A, Yildiz P, et al. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006;54:243-8. [PubMed]
- Kim YG, Yoon HK, Ko GY, et al. Long-term effect of bronchial artery embolization in Korean patients with haemoptysis. Respirology 2006;11:776-81. [Crossref] [PubMed]
- Gross AM, Diacon AH, van den Heuvel MM, et al. Management of life-threatening haemoptysis in an area of high tuberculosis incidence. Int J Tuberc Lung Dis 2009;13:875-80. [PubMed]
- Dewan RK. Surgery for pulmonary tuberculosis-a 15-year experience. Eur J Cardiothorac Surg 2010;37:473-7. [PubMed]
- Fernando HC, Stein M, Benfield JR. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg 1998;133:862-6. [Crossref] [PubMed]
- Osaki S, Nakanishi Y, Wataya H, et al. Prognosis of bronchial artery embolization in the management of hemoptysis. Respiration 2000;67:412-6. [Crossref] [PubMed]
- Swanson KL, Johnson CM, Prakash UB, et al. Bronchial artery embolization: experience with 54 patients. Chest 2002;121:789-95. [Crossref] [PubMed]
- Erdogan A, Yegin A, Gürses G, et al. Surgical management of tuberculosis-related hemoptysis. Ann Thorac Surg 2005;79:299-302. [Crossref] [PubMed]
- van den Heuvel MM, Els Z, Koegelenberg CF, et al. Risk factors for recurrence of haemoptysis following bronchial artery embolisation for life-threatening haemoptysis. Int J Tuberc Lung Dis 2007;11:909-14. [PubMed]
- Souilamas R, Riquet M, Barthes FP, et al. Surgical treatment of active and sequelar forms of pulmonary tuberculosis. Ann Thorac Surg 2001;71:443-7. [Crossref] [PubMed]
- Williams DJ, York EL, Nobert EJ, et al. Endobronchial tuberculosis presenting as asthma. Chest 1988;93:836-8. [Crossref] [PubMed]
- Kim YT, Kim HK, Sung SW, et al. Long-term outcomes and risk factor analysis after pneumonectomy for active and sequela forms of pulmonary tuberculosis. Eur J Cardiothorac Surg 2003;23:833-9. [Crossref] [PubMed]
- Good CA, Wilson TW. The solitary circumscribed pulmonary nodule; study of seven hundred five cases encountered roentgenologically in a period of three and one-half years. J Am Med Assoc 1958;166:210-5. [Crossref] [PubMed]
- Andreu J, Cáceres J, Pallisa E, et al. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol 2004;51:139-49. [Crossref] [PubMed]
- Palmer PE. Pulmonary tuberculosis--usual and unusual radiographic presentations. Semin Roentgenol 1979;14:204-43. [Crossref] [PubMed]
- Woodring JH, Vandiviere HM, Fried AM, et al. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol 1986;146:497-506. [Crossref] [PubMed]
- Zwirewich CV, Vedal S, Miller RR, et al. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology 1991;179:469-76. [Crossref] [PubMed]
- Lee KS, Song KS, Lim TH, et al. Adult-onset pulmonary tuberculosis: findings on chest radiographs and CT scans. AJR Am J Roentgenol 1993;160:753-8. [Crossref] [PubMed]
- Goodwin RA, Des Prez RM. Apical localization of pulmonary tuberculosis, chronic pulmonary histoplasmosis, and progressive massive fibrosis of the lung. Chest 1983;83:801-5. [Crossref] [PubMed]
- Sochocky S. T uberculoma of the lung. Am Rev Tuberc 1958;78:403-10. [PubMed]
- Goo JM, Im JG, Do KH, et al. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology 2000;216:117-21. [Crossref] [PubMed]
- Hara T, Kosaka N, Suzuki T, et al. Uptake rates of 18F-fluorodeoxyglucose and 11C-choline in lung cancer and pulmonary tuberculosis: a positron emission tomography study. Chest 2003;124:893-901. [Crossref] [PubMed]
- Bleyer JM, Marks JH. Tuberculomas and hamartomas of the lung; comparative study of 66 proved cases. Am J Roentgenol Radium Ther Nucl Med 1957;77:1013-22. [PubMed]
- Grenville-Mathers R. The natural history of so-called tuberculomas. J Thorac Surg 1952;23:251-2. [PubMed]
- Lee HS, Oh JY, Lee JH, et al. Response of pulmonary tuberculomas to anti-tuberculous treatment. Eur Respir J 2004;23:452-5. [Crossref] [PubMed]
- Perel'man MI, Kravtsova IV. Is preoperative chemotherapy necessary in pulmonary tuberculoma? Probl Tuberk 1989.19-22. [PubMed]
- Prytz S, Hansen JL. Surgical Treatment of “Tuberculoma” A Follow-up Examination of Patients with Pulmonary Tuberculosis Resected on Suspicion of Tumour. Scand J Thorac Cardiovasc Surg 1976;10:179-82. [Crossref] [PubMed]
- Kerti CA, Miron I, Cozma GV, et al. The role of surgery in the management of pleuropulmonary tuberculosis–seven years' experience at a single institution. Interact Cardiovasc Thorac Surg 2009;8:334-7. [Crossref] [PubMed]
- García-Yuste M, Ramos G, Duque JL, et al. Open-window thoracostomy and thoracomyoplasty to manage chronic pleural empyema. Ann Thorac Surg 1998;65:818-22. [Crossref] [PubMed]
- Halezeroglu S, Keles M, Uysal A, et al. Factors affecting postoperative morbidity and mortality in destroyed lung. Ann Thorac Surg 1997;64:1635-8. [Crossref] [PubMed]
- Olgac G, Yilmaz MA, Ortakoylu MG, et al. Decision-making for lung resection in patients with empyema and collapsed lung due to tuberculosis. J Thorac Cardiovasc Surg 2005;130:131-5. [Crossref] [PubMed]
- Shiraishi Y, Nakajima Y, Koyama A, et al. Morbidity and mortality after 94 extrapleural pneumonectomies for empyema. Ann Thorac Surg 2000;70:1202-6; discussion 1206-7. [Crossref] [PubMed]
- Pomerantz M. Surgery for the management of Mycobacterium tuberculosis and nontuberculous mycobacterial infections of the lung. In: Shields TW, LeCicero J, Ponn RB. editors. General thoracic surgery. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2000:1066-75.
- Hopkins RA, Ungerleider RM, Staub EW, et al. The modern use of thoracoplasty. Ann Thorac Surg 1985;40:181-7. [Crossref] [PubMed]
- Garzon JC, Ng CS, Sihoe AD, et al. Video-assisted thoracic surgery pulmonary resection for lung cancer in patients with poor lung function. Ann Thorac Surg 2006;81:1996-2003. [Crossref] [PubMed]
- Donath J, Khan FA. Tuberculous and posttuberculous bronchopleural fistula. Ten year clinical experience. Chest 1984;86:697-703. [Crossref] [PubMed]
- Naidoo R. Active pulmonary tuberculosis: experience with resection in 106 cases. Asian Cardiovasc Thorac Ann 2007;15:134-8. [Crossref] [PubMed]
- The role of surgery in the treatment of pulmonary TB and multidrug- and extensively drug-resistant TB. Copenhagen, Denmark: The Regional Office for Europe of the World Health Organization, 2014.
- Park SK, Lee CM, Heu JP, et al. A retrospective study for the outcome of pulmonary resection in 49 patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2002;6:143-9. [PubMed]
- Kim HR, Hwang SS, Kim HJ, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis 2007;45:1290-5. [Crossref] [PubMed]
- Orki A, Kosar A, Demirhan R, et al. The value of surgical resection in patients with multidrug resistant tuberculosis. Thorac Cardiovasc Surg 2009;57:222-5. [Crossref] [PubMed]
- Dravniece G, Cain KP, Holtz TH, et al. Adjunctive resectional lung surgery for extensively drug-resistant tuberculosis. Eur Respir J 2009;34:180-3. [Crossref] [PubMed]
- Chan ED, Laurel V, Strand MJ, et al. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med 2004;169:1103-9. [Crossref] [PubMed]
- Törün T, Tahaoğlu K, Özmen I, et al. The role of surgery and fluoroquinolones in the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2007;11:979-85. [PubMed]
- Keshavjee S, Gelmanova IY, Farmer PE, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet 2008;372:1403-9. [Crossref] [PubMed]
- Kwon YS, Kim YH, Suh GY, et al. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis 2008;47:496-502. [Crossref] [PubMed]


