
Changes in T lymphocyte subsets predict the efficacy of atezolizumab in advanced non-small cell lung cancer: a retrospective study
Highlight box
Key findings
• T lymphocyte subsets were shown to be altered and to predict the efficacy for advanced NSCLC patients during atezolizumab treatment.
What is known and what is new?
• Immune checkpoint inhibitors have improved clinical outcomes for NSCLC patients, However, not all advanced NSCLC patients benefit from immunotherapy. When cellular immune function declines, the CD4+/CD8+ ratio decreases, and the patients experience a state of immunosuppression.
• The level of CD3+, CD4+, and CD8+ T lymphocytes and CD4+/CD8+ indexes between the healthy group and the advanced NSCLC group was significantly different. Atezolizumab altered the number of CD3+, CD4+, and CD8+ T lymphocytes and CD4+/CD8+ indexes in advanced NSCLC patients.
What is the implication, and what should change now?
• The results showed predictive and prognostic value of peripheral blood lymphocyte subsets for advanced NSCLC patients treated with PD-1 inhibitors. Longer follow-up and a larger sample size is needed confirm the efficacy.
Introduction
Lung cancer is one of the most common malignant tumors in the world, which can be divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) according to tissue types; NSCLC accounts for about 85% of all lung cancer cases (1). The human immune system (cellular immunity, humoral immunity, and congenital immunity) plays an important role in the occurrence and development of lung cancer (2). Cellular immunity, in which lymphocytes participate, is an important part of the immune system (3). A large number of lymphocytes infiltrating around the tumor is the core of the anti-tumor response of cellular immunity and the cell line basis of immunotherapy (4). Many studies have focused on tumor-infiltrating lymphocytes in the tumor microenvironment (5), including immune elimination, homeostasis, and escape (6,7). Cancers are closely related to cellular immunity in the body (8). These lymphocytes are not limited to the lesion, the level of lymphocyte subsets in peripheral blood can also reflect the immune state of the body (9). In lymphocytes, T cells exert major antitumor effect (10). T lymphocytes include effector T cells and suppressor T cells. The level and ratio of effector T cells and suppressor T cells affect the immune function of the body (10). Among them, CD4+ assisted/induced T cells play a core role in the process of anti-tumor immunity (11). Cytokines secreted by CD4+ T lymphocytes could not only enhance cellular immunity positively, also can promote the B cell proliferation, which is conducive to the antibody production and assist humoral immunity to play an anti-tumor role (12). CD8+ T cells can produce inhibitory cytokines while playing a cytotoxic role, and negatively inhibit the expression of CD4+ T lymphocytes, which has an inhibitory effect on humoral and cellular immunity. Increased CD8+ T cells create conditions for proliferation and metastasis in tumor cells (12). Normally, CD4+ T/CD8+ T indexes are relatively balanced. When the cellular immune function declines and CD4+ T/CD8+ T indexes are decreased, the killing effect on tumor cells is weakened, and the patient enters a state of immunosuppression (13). Therefore, the monitoring of lymphocyte subsets plays an important guiding role in the judgment of drug efficacy. What’s more, when programmed cell death ligand 1 (PD-L1) on tumor cells binds to programmed cell death protein 1 (PD-1) on activated immune cells, the proliferation, migration, and cytokine release of immune cells are inhibited, thus eliminating the function of effector immune cells to kill tumor cells (14). In the last 3 years, the development of immunotherapy has enabled great progress in the treatment of various malignant tumors, such as epithelial mesenchymal tumors, gastric cancer, pancreatic cancer, and hepatocellular carcinoma (15-18), especially for some advanced cases. For example, patients with advanced SCLC have exhibited particularly positive outcomes from immunotherapy (19). And immune checkpoint inhibitors (ICIs) have significantly improved clinical outcomes in patients with NSCLC (20,21). In particular, CD4+ T/CD8+ T was independent factor for disease-free survival of stage I–IIIA lung cancer (22). PD-1 and PD-L1 inhibitors block the binding of PD-L1 on the surface of tumor cells and PD-1 on the surface of activated lymphocytes, and enhance the killing effect of immune cells on tumor cells (23,24). However, not all patients with advanced NSCLC benefit from immunotherapy. Some patients with high expression of PD-1 and PD-L1 do not respond well to ICIs (25). Therefore, in order to improve compliance with immunotherapy in patients with advanced NSCLC, it is necessary to predict the effectiveness of drugs and find interventions to improve the efficacy of immunotherapy.
Mounting evidence is emphasizing the predictive and prognostic value of peripheral blood lymphocyte (PBL) subsets in the treatment of advanced tumor patients with PD-1 inhibitors (26). The level of PBL subsets has been shown to correlate with the efficacy of PD-1 inhibitors (27). However, very few such PBL subsets have been identified to predict the efficacy of PD-L1 inhibitors in the treatment of advanced tumors. In this study, in order to evaluate the predictive and prognostic value of PBL subsets in advanced NSCLC patients treated with PD-L1 inhibitors, we analyzed the changes in PBL subsets of advanced NSCLC patients during atezolizumab treatment. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1169/rc).
Methods
Patient information
Observation group: a total of 30 patients with NSCLC (stage IV) who received the PD-L1 ICI atezolizumab as a single drug and were admitted to The Affiliated Hospital of Qingdao University from October 2021 to October 2022 were selected. All cancer patients were pathologically diagnosed as advanced lung adenocarcinoma with positive expression of PD-1/PD-L1. All cancer patients were followed up according to chemotherapy cycle. The inclusion criteria were as follows: patients with advanced lung adenocarcinoma treated with atezolizumab; patients had not received any other antitumor therapy before atezolizumab; EFGR gene, ALK, ROS1 rearrangement negative or unknown; PD-L1 expression ≥1%; physical activity status (PS) score 0–2 and stage IVA NSCLC. The exclusion criteria were as follows: patients with recent apparent infection; patients with NSCLC without measurable lesions; patient experiencing or who had experienced an immune or blood disease. Control group: 30 healthy people who underwent a physical examination during the same period were selected as the control group. The inclusion of NSCLC cases and healthy individuals was continuous. The flow of participants is displayed in Figure 1. The baseline data of the 2 groups including sex (male/female), age (<60/≥60 years), smoking status (ever/never) are shown in Table 1.
Table 1
| Characteristics | Control group | Observation group | P value |
|---|---|---|---|
| Gender, n | 0.2974 | ||
| Male | 15 | 19 | |
| Female | 15 | 11 | |
| Age (years), n | 0.5921 | ||
| ≥60 | 10 | 12 | |
| <60 | 20 | 18 | |
| Smoking status, n | 0.0371 | ||
| Ever | 13 | 21 | |
| Never | 17 | 9 |
This study was conducted retrospectively in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of The Affiliated Hospital of Qingdao University (No. QYFYWZLL27611). Written informed consent was provided by all patients.
Monitoring methods
A total of 2 mL of venous blood was collected for ethylenediamine tetraacetic acid (EDTA)-K2 anticoagulation and PBL subsets were detected according to the prescribed time. The collection of venous blood and counting of the various types of lymphocyte subsets were performed by experienced laboratory technicians in order to ensure the accuracy of the data. All the researchers carefully examined all of the data.
Efficacy evaluation
All tumors were evaluated by imaging, and the evaluation criteria was guided by the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guide (28). The assessment criteria were defined as follows: complete response (CR): all target lesions disappeared completely; partial response (PR): the total diameter of all measurable target lesions was ≥30% lower than baseline; stable disease (SD): the sum of baseline lesion diameters decreased but did not meet the criteria for PR, or increased but did not meet the criteria for progressive disease (PD); PD: the total diameter of the target lesions increased by 20% beyond baseline or new lesions appeared.
Statistical methods
The data were analyzed by using GraphPad Prism 8.4.3 (GraphPad Software, San Diego, CA, USA). The counting data were expressed by χ2 test, the measurement data were expressed by mean ± standard deviation, and comparisons were performed by Mann-Whitney U test. The independent prognostic value of the T cells subset was determined using univariate and multivariate logistic analysis. The indeterminate results were excluded and there were no missing data. Receiver operating characteristic (ROC) curves were employed and area under the curve (AUC) were used to confirm the sensitivity and specificity of the T cells subset in predicting prognosis outcomes in NSCLC treated with atezolizumab. The calculation method of 95% CIs of AUC is Wilson/Brown. P value <0.05 was considered statistically significant, two-side.
Results
Comparison of lymphocyte subsets between the observation group and the control group before treatment
There were no significant differences in gender, age, and smoking status between the 30 patients with advanced NSCLC in the observation group and the 30 healthy adults in the control group (all P>0.05) (Table 1). We then compared the levels of lymphocyte subsets in the two groups. Before treatment, the number of CD3+ T lymphocytes (P<0.05), the number of CD4+ T lymphocytes (P<0.05), and CD4+/CD8+ indexes (P<0.05) in the observation group were significantly decreased compared to the control group, whereas the number of CD8+ T lymphocytes was significantly increased (P<0.05) (Figure 2).
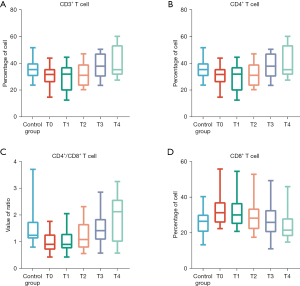
Changes of lymphocyte subsets before and after atezolizumab treatment in the observation group
In this study, no obvious adverse reactions occurred in the observation group throughout the treatment period. The 30 patients in the observation group were treated with 4 cycles of atezolizumab treatment in total. Along with atezolizumab treatment, the number of CD3+ T lymphocytes, the number of CD4+ T lymphocytes, and CD4+/CD8+ indexes gradually increased, whereas the number of CD8+ T lymphocytes gradually decreased. After the fourth cycle, the number of CD3+ T lymphocytes (P<0.05), the number of CD4+ T lymphocytes (P<0.05), and CD4+/CD8+ indexes (P<0.05) were significantly increased, and the number of CD8+ T lymphocytes was significantly decreased (P<0.05) compared with before treatment (Figure 2).
Efficacy of atezolizumab and changes of T lymphocyte subsets in the observation group
Among the 30 patients with advanced NSCLC in the observation group, 22 patients (73.3%) achieved PR/SD after 4 cycles of treatment with atezolizumab, and 8 patients (26.7%) achieved PD. There were no significant differences in gender (P=0.0571), age (P=0.5002), smoking status (P=0.5888), and PD-L1 expression (P=0.5002) between those who achieved PR/SD or PD after 4 cycles of treatment (Table 2). In addition, there was no significant difference in lymphocyte subsets between those who achieved PR/SD and those who achieved PD before treatment, including the number of CD3+ T lymphocytes (P>0.05, Figure 3A), the number of CD4+ T lymphocytes (P>0.05, Figure 3B), the number of CD4+/CD8+ T lymphocytes (P>0.05, Figure 3C), and CD8+ indexes (P>0.05, Figure 3D). However, the lymphocyte subsets of the two cohorts showed significant differences after 4 cycles of treatment, including the number of CD3+ T lymphocytes (P<0.05, Figure 3E), the number of CD4+ T lymphocytes (P<0.05, Figure 3F), the number of CD4+/CD8+ T lymphocytes (P<0.05, Figure 3G), and CD8+ indexes (P>0.05, Figure 3H).
Table 2
| Characteristics | PR/SD | PD | P value |
|---|---|---|---|
| Gender, n | 0.0571 | ||
| Male | 14 | 5 | |
| Female | 8 | 3 | |
| Age (years), n | 0.5002 | ||
| ≥60 | 8 | 4 | |
| <60 | 14 | 4 | |
| Smoking status, n | 0.5888 | ||
| Ever | 16 | 5 | |
| Never | 6 | 3 | |
| PD-L1 expression, n | 0.5002 | ||
| ≥50% | 14 | 4 | |
| <50% | 8 | 4 |
PR, partial response; SD, stable disease; PD, progressive disease; PD-L1, programmed cell death ligand 1.
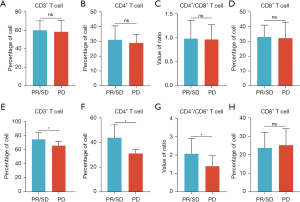
The degree of change of T lymphocyte subsets between PR/SD and PD patients after treatment with atezolizumab
In the observation group, after 4 cycles treatment, the number of CD3+ T lymphocytes (P<0.05, Figure 4A), the number of CD4+ T lymphocytes (P<0.05, Figure 4B), and CD4+/CD8+ indexes (P<0.05, Figure 4C) in 22 patients with advanced NSCLC with PR/SD were significantly increased compared with those before treatment. The number of CD8+ T lymphocytes was significantly lower than that before treatment (P<0.05, Figure 4D). However, there were no significant changes in lymphocyte subsets in 8 patients with advanced NSCLC who achieved PD efficacy (all P>0.05, Figure 4E-4H).

Lymphocyte subset level predicted the efficacy of atezolizumab in NSCLC
According to the changes of lymphocyte subsets before and after treatment and each cycle of the observation group, an ROC curve was drawn, showing CD3+ (AUC) =0.7727, Figure 5A], CD4+ (AUC =0.8352, Figure 5B), CD4+/CD8+ (AUC =0.7699, Figure 5C), and combined detection of CD3+, CD4+, CD8+ T (AUC =0.9018, Figure 5D). These results suggested that the changes in the levels and ratios of CD3+, CD4+, and CD8+ T lymphocyte subsets could predict the therapeutic effect of atezolizumab to a certain extent.

Discussion
In this study, it was found that the levels of CD3+ T, CD4+ T, and CD4+ T/CD8+ T in the observation group were significantly decreased compared with those in the control group before treatment, and the level of CD8+ T lymphocyte subsets was significantly increased. Visible, T lymphocyte subsets’s differences in values indicate that the cellular immunity may be in a suppressed state in malignant tumor. With the reduction in the number of positive immune cells, the increase in number of negative immune cells, the cellular immune function decline, and immunosuppressive factors are overexpressed. The resulting immunosuppressive microenvironment creates conditions for immune escape and malignant proliferation of tumor cells.
In this study, it was found that the values of CD3+ T, CD4+ T, and CD4+ T/CD8+ T were significantly higher after 4 cycles of atezolizumab treatment than before treatment, whereas the values of CD8+ T cells were significantly lower, which was consistent with the findings of previous research (29). It can be speculated that atezolizumab alleviates the immunosuppressive state of the body to a certain extent, plays a positive role in regulating the immune system, and enhances the anti-tumor immune activity. As suggested in a study, ICIs may restore the function of tissue-resident memory T cells in tumor immune surveillance (30). In addition, ICIs suppresses the escape of solid cancer cells from B cell-mediated cytotoxicity (31). However, the changes in the value of those lymphocyte subsets after 1 and 2 cycles of atezolizumab treatment were not statistically significant, suggesting that the efficacy of immunotherapy was slow, and multi-cycle maintenance therapy had more significant efficacy against tumor.
Some adverse drug reactions are associated with ICIs (32,33). In this study, no obvious adverse reactions occurred in all patients in the observation group. After 4 cycles of treatment with atezolizumab, 22 patients of 30 patients achieved SD/PR. We speculate that such a high response rate to immunotherapy may be associated with all patients included in this study were PD-L1 positive and more than half of the patients were PD-L1 positive >50%. The lymphocyte subsets of SD/PR patients were significantly changed compared with those before treatment. Contrastingly, the lymphocyte subsets of PD patients were not statistically significant changed compared with those before treatment. It may be suggested that in patients with a good response to atezolizumab, the positive regulatory effect of T lymphocytes is enhanced, and the negative regulatory effect is weakened, which enhances the anti-tumor immune response of the body, so as to obtain a better prognosis and longer survival. Therefore, periodic monitoring of T lymphocyte subsets in patients with immunotherapy is of certain value in predicting the therapeutic effect. It may provide a reference for determination of a treatment plan and prognosis of tumor patients.
In previous studies, the percentage of B lymphocytes before immunotherapy was an independent prognostic factor for OS in NSCLC patients receiving immunotherapy, and univariate survival analysis showed that low B cell percentage predicted poor OS in ICI patients with NSCLC. Both CD4+ T/CD8+ T ratio and B cell percentage decreased after immunotherapy (34). In this study, we found that CD4+ T, CD8+ T cells, and CD4+/CD8+ T lymphocyte ratios change with immunotherapy progression. Therefore, in the process of receiving immunotherapy, it is necessary to monitor lymphocyte subsets along with the cycle. By analyzing the periodic changes of lymphocyte subsets, it may be possible to indicate the response of patients to immunotherapy in the follow-up treatment, and provide possible auxiliary indicators for judging the prognosis. However, assessing the association between PBL subsets and PFS in immunotherapy patients with NSCLC will require the recruitment of additional NSCLC patients for monitoring in future studies.
The participants in this study were followed up until the end of the fourth cycle of immunotherapy. Whether lymphocytes can be used as an indicator to predict the efficacy of immunotherapy remains to be further verified by further follow-up and an expanded sample size.
Conclusions
Patients with advanced NSCLC are immunosuppressed compared to healthy adults. The PD-L1 inhibitor atezolizumab can alter the level of lymphocyte subsets in patients suffered advanced NSCLC after 4 cycles treatment, and the change in lymphocyte subsets may predict the efficacy of atezolizumab in patients with advanced NSCLC.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1169/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1169/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1169/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1169/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted retrospectively in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of The Affiliated Hospital of Qingdao University (No. QYFYWZLL27611). Written informed consent was provided by all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Smok-Kalwat J, Mertowska P, Mertowski S, et al. The Importance of the Immune System and Molecular Cell Signaling Pathways in the Pathogenesis and Progression of Lung Cancer. Int J Mol Sci 2023;24:1506. [Crossref] [PubMed]
- Daëron M. The immune system as a system of relations. Front Immunol 2022;13:984678. [Crossref] [PubMed]
- Bai Z, Zhou Y, Ye Z, et al. Tumor-Infiltrating Lymphocytes in Colorectal Cancer: The Fundamental Indication and Application on Immunotherapy. Front Immunol 2021;12:808964. [Crossref] [PubMed]
- Fang F, Zhang T, Li Q, et al. The tumor immune-microenvironment in gastric cancer. Tumori 2022;108:541-51. [Crossref] [PubMed]
- Lowery FJ, Krishna S, Yossef R, et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science 2022;375:877-84. [Crossref] [PubMed]
- Liu D, Heij LR, Czigany Z, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res 2022;41:127. [Crossref] [PubMed]
- Carlisle JW, Steuer CE, Owonikoko TK, et al. An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin 2020;70:505-17. [Crossref] [PubMed]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Wu Z, Li S, Zhu X. The Mechanism of Stimulating and Mobilizing the Immune System Enhancing the Anti-Tumor Immunity. Front Immunol 2021;12:682435. [Crossref] [PubMed]
- Xiao M, Xie L, Cao G, et al. CD4(+) T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy. J Immunother Cancer 2022;10:e004022. [Crossref] [PubMed]
- Mariucci S, Rovati B, Manzoni M, et al. Lymphocyte subpopulation and dendritic cell phenotyping during antineoplastic therapy in human solid tumors. Clin Exp Med 2011;11:199-210. [Crossref] [PubMed]
- Leone K, Poggiana C, Zamarchi R. The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy. Diagnostics (Basel) 2018;8:59. [Crossref] [PubMed]
- Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27:111-22. [Crossref] [PubMed]
- Huang Y, Meng Q. Research Progress of Immune Checkpoint Inhibitors in Malignant Pleural Mesothelioma. Zhongguo Fei Ai Za Zhi 2021;24:441-6. [Crossref] [PubMed]
- Huang Z, Dewanjee S, Chakraborty P, et al. CAR T cells: engineered immune cells to treat brain cancers and beyond. Mol Cancer 2023;22:22. [Crossref] [PubMed]
- Lv JB, Yin YP, Zhang P, et al. Safety and efficacy of laparoscopic surgery in locally advanced gastric cancer patients with neoadjuvant chemotherapy combined with immunotherapy. Zhonghua Wei Chang Wai Ke Za Zhi 2023;26:84-92. [Crossref] [PubMed]
- Minaei N, Ramezankhani R, Tamimi A, et al. Immunotherapeutic approaches in Hepatocellular carcinoma: Building blocks of hope in near future. Eur J Cell Biol 2023;102:151284. [Crossref] [PubMed]
- Zou Y, Ren X, Zhang H, et al. Efficacy and safety of durvalumab + chemotherapy vs. atezolizumab + chemotherapy in the treatment of small-cell lung cancer: a retrospective comparative cohort study. J Thorac Dis 2023;15:3339-49. [Crossref] [PubMed]
- Qu J, Jiang M, Wang L, et al. Mechanism and potential predictive biomarkers of immune checkpoint inhibitors in NSCLC. Biomed Pharmacother 2020;127:109996. [Crossref] [PubMed]
- Lin A, Wei T, Meng H, et al. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer 2019;18:139. [Crossref] [PubMed]
- Xu L, Luo Y, Tian J, et al. A validated nomogram integrating baseline peripheral T-lymphocyte subsets and NK cells for predicting survival in stage I-IIIA non-small cell lung cancer after resection. Ann Transl Med 2022;10:250. [Crossref] [PubMed]
- van Gulijk M, Belderbos B, Dumoulin D, et al. Combination of PD-1/PD-L1 checkpoint inhibition and dendritic cell therapy in mice models and in patients with mesothelioma. Int J Cancer 2023;152:1438-43. [Crossref] [PubMed]
- Ali LR, Garrido-Castro AC, Lenehan PJ, et al. PD-1 blockade and CDK4/6 inhibition augment nonoverlapping features of T cell activation in cancer. J Exp Med 2023;220:e20220729. [Crossref] [PubMed]
- Sade-Feldman M, Jiao YJ, Chen JH, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun 2017;8:1136. [Crossref] [PubMed]
- Yan Y, Wang X, Liu C, et al. Association of lymphocyte subsets with efficacy and prognosis of immune checkpoint inhibitor therapy in advanced non-small cell lung carcinoma: a retrospective study. BMC Pulm Med 2022;22:166. [Crossref] [PubMed]
- Barua S, Fang P, Sharma A, et al. Spatial interaction of tumor cells and regulatory T cells correlates with survival in non-small cell lung cancer. Lung Cancer 2018;117:73-9. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Li WW, Jiao J, Wang ZY, et al. Clinical efficacy of immunotherapy combined with chemotherapy in patients with advanced gastric cancer, its effect on nutritional status and Changes of peripheral blood T lymphocyte subsets. Pak J Med Sci 2021;37:1902-7. [Crossref] [PubMed]
- Mami-Chouaib F, Blanc C, Corgnac S, et al. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer 2018;6:87. [Crossref] [PubMed]
- Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol 2017;14:203-20. [Crossref] [PubMed]
- Gu T, Jiang A, Zhou C, et al. Adverse reactions associated with immune checkpoint inhibitors and bevacizumab: A pharmacovigilance analysis. Int J Cancer 2023;152:480-95. [Crossref] [PubMed]
- Pluye M, Gouraud A, Herve M, et al. Adverse drug reactions associated with immune checkpoint inhibitors: An exploratory nested case-control study in a historical cohort. Therapie 2023;78:303-11. [Crossref] [PubMed]
- Xu X, Wang D, Chen W, et al. A nomogram model based on peripheral blood lymphocyte subsets to assess the prognosis of non-small cell lung cancer patients treated with immune checkpoint inhibitors. Transl Lung Cancer Res 2021;10:4511-25. [Crossref] [PubMed]


