A potential role for VEGF in the diagnostic approach of pleural effusions
Introduction
During the last decades, advances have been made in the diagnostic approach of pleural effusions (1-3). The application of rigorous diagnostic algorithms that include invasive diagnostic modalities such as video-assisted thoracic surgery (VATS) or medical thoracoscopy may establish diagnosis in most cases (4,5). However, despite diagnostic advances, a significant percentage of pleural effusions, approximately 20% overall (5), remains without specific diagnosis even after the application of invasive diagnostic procedures (6-8). Furthermore, invasive diagnostic techniques, such as image-guided percutaneous fine needle biopsy (FNB), medical or surgical thoracoscopy, are not universally available or well tolerated by many patients (9).
In this respect, the use of non-invasive tests, such as the evaluation of pleural fluid biological markers, might be helpful in diagnostic approach of pleural effusions. In the last few years, several studies have evaluated the role of biomarkers to increase the diagnostic capacity of pleural fluid, but their diagnostic utility remains undetermined and their clinical use controversial (10-16).
Among them, vascular endothelial growth factor (VEGF) is a growth factor that may play a significant role in exudative pleural effusions that are due to increased permeability of the pleural membrane (17-19). The molecular mechanisms that lead in increased fluid accumulation in lymphocytic exudative effusions are complex (19-22). These mechanisms may involve the intrapleural production of cytokines, which are known to increase vascular permeability, such as tumor necrosis factor α (TNF-α) and transforming growth factor (TGF) (23,24). VEGF is an endothelial-cell specific growth factor, which may also contribute in this process, as it increases the permeability of human pleural mesothelial cells or microvessels (25). Previous studies have shown that several histological types of lung cancer express VEGF (26) whereas it is also produced by several inflammatory cells such as eosinophils, lymphocytes, macrophages and neutrophils (27-29). In this respect, VEGF may play a role in the pathogenesis of exudative pleural effusions secondary to malignancy and has been found increased in the pleural fluid (29,30).
In the present prospective study, we aimed to investigate whether VEGF levels in pleural fluid and serum, could be used as an adjunct for further diagnostic assessment in patients who present with lymphocytic exudative pleural effusion and initially negative diagnostic work up.
Methods
Seventy-one patients were recruited by consecutive sampling from the Respiratory Department of University Hospital of Larissa during 1-year period (January-December 2012). Patients were included in the study if they presented lymphocytic pleural effusion that remained without diagnosis after thorough clinical, radiological assessment and pleural fluid biochemical analysis, culture and cytological examination of the pleural fluid. Based on the VEGF levels in the pleural fluid of patients with non-malignant exudates on a previous study (median =469; IQR, 330–653) (27), we calculated that for a 25% increase in VEGF levels in patients with malignant pleural fluid at least 27 patients in each group would be required (P=0.05, power =80%). The study was approved by the University of Thessaly and the University Hospital of Larissa ethics committees and informed consent was obtained by the participants.
Clinical and laboratory assessment
Patients underwent baseline assessment which included medical history, complete physical examination, blood analysis, chest radiography and tuberculin test. Glucose, total protein, adenosine deaminase (ADA) and lactate dehydrogenase (LDH), bacteriologic study with the Ziehl-Neelsen stain, culture in the Lowenstein-Jensen medium and cytology were analyzed from the pleural fluid (3 samples) obtained using thoracocentesis. Having non-specific diagnosis by completing this first diagnostic approach additional procedures were performed at the discretion of the attending physician in order to gain diagnosis (immunological testing, fiberoptic bronchoscopy, image-guided aspiration etc.). In cases a specific diagnosis was not established thoracoscopy was suggested. Patients were followed up as indicated up to 2 years. Particularly, they were evaluated monthly for the first three months and at 6-month intervals thereafter or more frequently if there was clinical indication. Clinical status was ascertained and physical examination and chest radiographies were performed at each visit.
Cases were considered as malignant when diagnostic procedures that were performed during the follow up period resulted to the diagnosis of malignancy. Cases were considered as benign when a diagnosis other than malignancy, was obtained. Cases were considered of unknown origin when no specific diagnosis could be obtained during the study or when pleural effusion was resolved.
Laboratory analysis
Pleural fluid was collected and stored at the time of first tapping. The fluid samples obtained at baseline by thoracentesis were immediately analyzed for pH (Instrumentation Laboratory, USA) following the anaerobically procedure for obtaining samples. Total protein (g/L), glucose (mg/dL) and LDH (U/L) were measured in pleural fluid and serum. Pleural fluid samples were centrifuged at 1,500 g for 15 min and the supernatant from each sample was stored at –80 °C. Five hundred cells in coded May-Giemsa-Grunwald cytospins were counted in a blinded fashion by two independent investigators and averaged. Differential cell counts were manually measured under microscope using Wright-stained smears and expressed as percentage and as absolute number of cells of pleural fluid. It must be mentioned that measurement of VEGF and follow-up of patients were independent and comparison of these two components was performed only after gathering all clinical data.
Vascular endothelial growth factor (VEGF) assay
VEGF levels (pg/mL) were measured in serum and pleural fluid by using an immunoenzymometric assay (Biosource Inc; Europe S.A.). The reproducibility of these assays was confirmed by performing repeated measurements on successive days. The mean difference with limits of agreement (±2SD) for pleural effusion VEGF was 7.84 (−66.17 to 81.85) and the mean difference with limits of agreement (±2SD) for serum VEGF was 1.80 (−45.18 to 48.78).
Statistical analysis
Descriptive statistics were used to summarize baseline characteristics and the results were expressed as median (range) or stated otherwise. Normal distribution was assessed using the Shapiro-Wilk test. Receiver operating characteristics (ROC) analysis was used to evaluate the diagnostic performance of VEGF in diagnosing pleural effusions associated with adverse outcome. A P value of less than 0.05 was considered as statistically significant. MedCalc statistical software (MedCalc Software, Mariakerle, Belgium) was used for the entire analysis.
Results
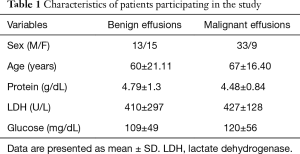
Finally 71 patients—47 males and 24 women—were enrolled in the study. The median age was 64.57 years (range, 20–88 years). Baseline characteristics of participants are shown in Table 1.
Full table
Outcomes
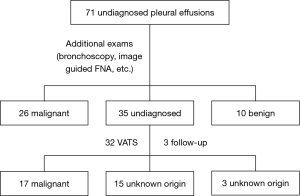
After initial assessment all patients underwent additional exams in order to gain diagnosis and indeed 26 patients were diagnosed with malignancy with other procedures rather than thoracoscopy, while 10 had diagnosis other than malignancy. Particularly, malignancy was obtained by CT-guided pleural biopsy (n=2), liver biopsy (n=1), fiberoptic bronchoscopy (n=13), gastroscopy (n=2), prostate biopsy (n=1), hysterectomy (n=1), while in 6 patients malignancy was obtained based on clinical and radiologic grounds. In benign PE group, the etiological diagnosis was attributed due to: TBC (n=2), rheumatoid arthritis (n=5), SLE (n=2), while one patient (in whom thoracoscopy had been suggested and refused), finally recalled having had previous contact with asbestos. However, 35 patients still remained without definitive diagnosis and thoracoscopy was suggested. Thirty two of them underwent thoracoscopy while 3 patients refused. Overall, malignancy was diagnosed in 17 out of these 32 patients, while no diagnosis could be gained even after thoracoscopy in 15 of them. None of them developed malignancy during the follow up and these cases were considered of unknown origin. The three remaining patients who refused to undergo thoracoscopy were also followed; no malignancy was developed and these cases were also considered of unknown origin (Figure 1).
As far as the sub-types of cancer is concerned lung cancer was diagnosed in 17 patients (7 adenocarcinoma, 6 squamous cell carcinoma, 3 SCLC and 1 NSCLC–NOS), while 8 patients had mesothelioma, 3 non-Hodgkin lymphoma, 1 melanoma, 1 leiomyosarcoma and 7 metastatic adenocarcinoma (1 prostate, 2 gastric, 1 pancreas, 2 ovaries, 1 unknown origin).
VEGF levels in pleural fluid and serum
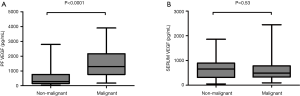
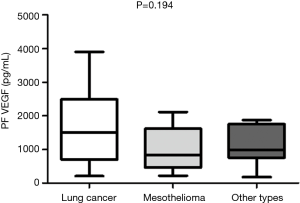
The median VEGF levels in pleural fluid and serum were 1,077 µg/dL (range, 33.52–3,902.32 µg/dL) and 699.57 µg/dL (range, 57–2,449.49 µg/dL) respectively. VEGF levels in pleural fluid of patients with malignancy was significantly higher compared to patients who had non-malignant diagnosis [1,506 (range, 177–3,902) vs. 609 (range, 33.52–2,788) pg/dL, P<0.0001] while no significant difference was found between serum VEGF levels in patients with malignancy and in patients with a non-malignant outcome [654 (range, 48.62–1,859) vs. 491.7 (range, 93.06–2,449) (P=0.53) (Figure 2A,B)]. We found no correlations between serum and pleural VEGF levels both in malignancy and controls (P=0.22 and P=0.14) respectively. Moreover, no significant difference was found in VEGF levels between effusions due to lung cancer and other malignant disease (mesothelioma, other types of cancer) (Figure 3).
VEGF and other biochemical parameters in PE
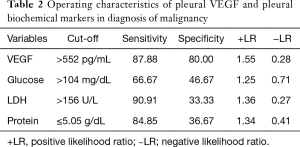
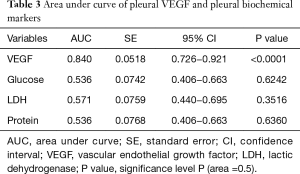
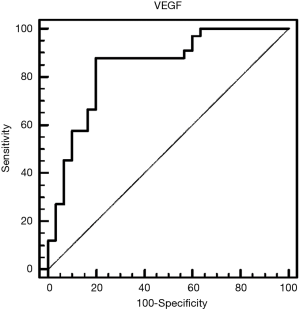
Table 2 depicts the threshold values of VEGF in distinguishing patients that presented malignancy at the end of follow-up period. Among other biochemical parameters (total protein, glucose, LDH) pleural fluid VEGF levels (cut-off 552 µg/dL) demonstrated noteworthy specificity of 80% and sensitivity of 87.88%. Table 3 shows pleural VEGF levels ROC curve where the area under the curve was 0.84 (P=0.0001). ROC curve analysis showed that VEGF had a significant diagnostic performance in the diagnosis of malignancy (Figure 4).
Full table
Full table
Discussion
Our results indicate that patients with lymphocytic pleural effusions with initial negative cytological and diagnostic workup who were finally diagnosed with malignancy had an elevated baseline VEGF level in the pleural effusion. On the contrary, patients with a non-malignant course had significantly lower VEGF levels in their pleural effusion at the time of the first thoracentesis. At this point, VEGF essay is not widely available. Considering that this is an easily performed, cheap, quick method it might be potentially applied to clinical practice as a guide for further diagnostic assessment, in combination with currently conventional diagnostic tools.
No statistically significant difference was found in serum VEGF levels among patients with malignancy and patients with a non-malignant outcome. Increased levels of circulating VEGF have been measured in a variety of physiological conditions (i.e., pregnancy) as well as autoimmune and infectious inflammatory disorders, including rheumatoid arthritis, COPD, chronic venous disease, diabetes (31,32). Therefore, the evaluation of elevated levels of serum VEGF in patients with malignancy and comorbidities could be precarious (33).
Previous studies have reported higher levels of VEGF in pleural effusions of malignant origin (34,35). Thickett et al. in a prospective, long-term follow-up study of 78 patients with pleural effusions have shown that VEGF levels were increased in malignancy, empyemas and parapneumonic pleural effusions (29). VEGF level was above 1,000 pg/mL in most malignant effusions and empyemas. Notably, if we consider only those patients with a diagnosis of malignancy, all VEGF measurements were above 1,000 pg/mL. Those results are in accordance with our findings, which suggest that VEGF levels may be elevated in malignant pleural effusions (MPEs). Moreover, in our study, VEGF had a higher specificity in differentiating non-malignant from MPEs, against other biochemical parameters that had been also evaluated.
ADA is a well-known biomarker for diagnosing TB with sensitivity and specificity in 90s. In our cases, though, ADA levels in pleural fluid were low and rather ineffective in the initial diagnostic assessment of our patients. On the other hand, in a recently published study the cancer ratio (serum LDH/pleural ADA ratio) (36) has been evaluated in diagnosing MPE, with sensitivity and specificity also in 90s. Nevertheless, further studies are warranted.
In a published study from our center, we investigated the diagnostic role of multiple biomarkers in pleural fluid in discriminating different pleural effusion groups (malignant, parapneumonic and tuberculous pleural effusion) (20). In that study we demonstrated that VEGF levels were increased in MPEs. However, VEGF was not found to be a significant parameter for the discrimination between malignant and parapneumonic pleural effusion. Furthermore, in the present study, parapneumonic pleural effusions were excluded and we investigated the role of VEGF in the diagnosis of lymphocytic pleural effusion. Shen et al. published a meta-analysis also suggesting that pleural VEGF levels may, to a certain extent, play a role in the diagnosis of MPEs, while its diagnostic value is not satisfactory (37). In this respect, VEGF may be useful as a biomarker of malignancy in exudative pleural effusions.
On our study, the ROC curve analysis with a cut off of 552 µg/dL revealed sensitivity 87.88% and specificity 80% for the diagnosis of malignancy. In the literature, the data are variable and this can be explained by different cut-off points considered. To the best of our knowledge this is the first study to focus exclusively on VEGF in lymphocytic exudative pleural effusions still remaining undiagnosed after extensive examination. This is important information since malignancy still remains diagnostic challenge in such cases.
Of course, it is widely accepted that the gold standard method to establish a neoplastic origin of a pleural effusion is thoracoscopy, which has a sensitivity of 95% (5,38-40). Thoracoscopy has the advantage of providing the opportunity of undertaking at the same time both diagnostic and therapeutic (such as talk poudrage) options. However, this diagnostic modality has also the disadvantages of being more invasive, costly, not worldwide available and hazardous especially in elderly patients.
Our study presents several limitations. Particularly, the sample size of the study is rather small and a larger study is needed to confirm our findings. Moreover, not all the malignant effusions were confirmed by cytology or biopsy, as 11 of them were finally attributed to the underneath malignancy, which is not unusual in everyday clinical practice. Future studies are warranted in order to evaluate further the clinical significance of VEGF levels as a biomarker of malignancy and their contribution in diagnostic assessment of patients with pleural effusions.
Conclusions
The present study indicates that high levels of VEGF may be helpful in discriminating patients with potential malignancy among those with lymphocytic exudative pleural effusions with negative diagnostic work up at initial assessment. These patients could benefit from more rigorous methods in order to obtain diagnosis. On the other hand, pleural effusion with low levels of VEGF at initial assessment could be addressed in a conservative manner, as the expected nature of the disease is benign.
In this respect, VEGF could be used as an adjunct in the decision of performing more invasive tests when managing patients with lymphocytic pleural effusion without the obvious diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the University of Thessaly and the University Hospital of Larissa ethics committees and informed consent was obtained by the participants.
References
- McGrath EE, Anderson PB. Diagnosis of pleural effusion: a systematic approach. Am J Crit Care 2011;20:119-27. [Crossref] [PubMed]
- Light RW. Diagnostic principles in pleural disease. Eur Respir J 1997;10:476-81. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii-4. [Crossref] [PubMed]
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii61-76. [Crossref] [PubMed]
- Chakrabarti B, Ryland I, Sheard J, et al. The role of Abrams percutaneous pleural biopsy in the investigation of exudative pleural effusions. Chest 2006;129:1549-55. [Crossref] [PubMed]
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010;138:1242-6. [Crossref] [PubMed]
- Harris RJ, Kavuru MS, Rice TW, et al. The diagnostic and therapeutic utility of thoracoscopy: a review. Chest 1995;108:828-41. [Crossref] [PubMed]
- Lee P, Colt HG. Pleuroscopy in 2013. Clin Chest Med 2013;34:81-91. [Crossref] [PubMed]
- Bennett R, Maskell N. Management of malignant pleural effusions. Curr Opin Pulm Med 2005;11:296-300. [PubMed]
- Lombardi G, Zustovich F, Nicoletto MO, et al. Diagnosis and treatment of malignant pleural effusion: a systematic literature review and new approaches. Am J Clin Oncol 2010;33:420-3. [Crossref] [PubMed]
- Shi HZ, Liang QL, Jiang J, et al. Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: A meta-analysis. Respirology 2008;13:518-27. [Crossref] [PubMed]
- Yilmaz Turay U, Yildirim Z, Türköz Y, et al. Use of pleural fluid C-reactive protein in diagnosis of pleural effusions. Respir Med 2000;94:432-5. [Crossref] [PubMed]
- Light RW. Tumor markers in undiagnosed pleural effusions. Chest 2004;126:1721-2. [Crossref] [PubMed]
- Niklinski J, Furman M. Clinical tumour markers in lung cancer. Eur J Cancer Prev 1995;4:129-38. [Crossref] [PubMed]
- Liang QL, Shi HZ, Qin XJ, et al. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax 2008;63:35-41. [Crossref] [PubMed]
- Sculier JP, Body JJ, Jacobowitz D, et al. Value of CEA determination in biological fluids and tissues. Eur J Cancer Clin Oncol 1987;23:1091-3. [Crossref] [PubMed]
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002;20:4368-80. [Crossref] [PubMed]
- Grove CS, Lee YC. Vascular endothelial growth factor: the key mediator in pleural effusion formation. Curr Opin Pulm Med 2002;8:294-301. [Crossref] [PubMed]
- Zebrowski BK, Yano S, Liu W, et al. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res 1999;5:3364-8. [PubMed]
- Daniil ZD, Zintzaras E, Kiropoulos T, et al. Discrimination of exudative pleural effusions based on multiple biological parameters. Eur Respir J 2007;30:957-64. [Crossref] [PubMed]
- Hamed EA, El-Noweihi AM, Mohamed AZ, et al. Vasoactive mediators (VEGF and TNF-α) in patients with malignant and tuberculous pleural effusions. Respirology 2004;9:81-6. [Crossref] [PubMed]
- Economidou F, Margaritopoulos G, Antoniou KM, et al. The angiogenetic pathway in malignant pleural effusions: Pathogenetic and therapeutic implications Exp Ther Med 2010;1:3-7. (Review). [PubMed]
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011-27. [Crossref] [PubMed]
- Yano S. Formation of pleural effusion by human lung adenocarcinoma directly correlates with expression of VEGF/VPF. Proc Am Assoc Cancer Res 1999;40:227a.
- Yanagawa H, Takeuchi E, Suzuki Y, et al. Vascular endothelial growth factor in malignant pleural effusion associated with lung cancer. Cancer Immunol Immunother 1999;48:396-400. [Crossref] [PubMed]
- Ishimoto O, Saijo Y, Narumi K, et al. High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology 2002;63:70-5. [Crossref] [PubMed]
- Fiorelli A, Vicidomini G, Di Domenico M, et al. Vascular endothelial growth factor in pleural fluid for differential diagnosis of benign and malignant origin and its clinical applications. Interact CardioVasc Thorac Surg 2011;12:420-4. [Crossref] [PubMed]
- Kaya A, Poyraz B, Celik G, et al. Vascular endothelial growth factor in benign and malignant pleural effusions. Arch Bronconeumol 2005;41:376-9. [PubMed]
- Thickett DR, Armstrong L, Millar AB. Vascular endothelial growth factor (VEGF) in inflammatory and malignant pleural effusions. Thorax 1999;54:707-10. [Crossref] [PubMed]
- Cheng D, Rodriguez RM, Perkett EA, et al. Vascular endothelial growth factor in pleural fluid. Chest 1999;116:760-5. [Crossref] [PubMed]
- Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem 2001;47:617-23. [PubMed]
- Larsson A, Sköldenberg E, Ericson H. Serum and plasma levels of FGF-2 and VEGF in healthy blood donors. Angiogenesis 2002;5:107-10. [Crossref] [PubMed]
- Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 1999;85:178-87. [Crossref] [PubMed]
- Sack U, Hoffmann M, Zhao XJ, et al. Vascular endothelial growth factor in pleural effusions of different origin. Eur Respir J 2005;25:600-4. [Crossref] [PubMed]
- Shu J, Sun G, Liu H, et al. Clinical utility of vascular endothelial growth factor in diagnosing malignant pleural effusions. Acta Oncol 2007;46:1004-11. [Crossref] [PubMed]
- Verma A, Abisheganaden J, Light RW. Identifying Malignant Pleural Effusion by A Cancer Ratio (Serum LDH: Pleural Fluid ADA Ratio). Lung 2016;194:147-53. [Crossref] [PubMed]
- Shen YC, Liu MQ, Wan C, et al. Diagnostic accuracy of vascular endothelial growth factor for malignant pleural effusion: a meta-analysis. Exp Ther Med 2012;3:1072-6. [PubMed]
- Alemán C, Sanchez L, Alegre J, et al. Differentiating between malignant and idiopathic pleural effusions: the value of diagnostic procedures. QJM 2007;100:351-9. [Crossref] [PubMed]
- Ferrer JS, Munoz XG, Orriols RM, et al. Evolution of idiopathic pleural effusion: a prospective, long-term follow-up study. Chest 1996;109:1508-13. [Crossref] [PubMed]
- Venekamp LN, Velkeniers B, Noppen M. Does ‘idiopathic pleuritis’ exist? Natural history of non-specific pleuritis diagnosed after thoracoscopy. Respiration 2005;72:74-8. [Crossref] [PubMed]