
Role of RAPID score and surgery in the management of pleural infection: a single center retrospective study
Highlight box
Key findings
• Patients with pleural infection classified as high-risk by the RAPID [Renal (urea level), Age, Pleural fluid purulence, source of Infection and Denutrition (albumin level)] score have a lower survival rate compared to patients with low- and medium-risk groups.
What is known and what is new?
• Pleural infection is a public health issue with an increased mortality in case of delayed management.
• The RAPID score is a prognostic score to classify patients with pleural infection according to the mortality-risk.
What is the implication, and what should change now?
• Because of the increased mortality in high-risk patients, early management should be more aggressive and favor surgery.
Introduction
Background
Pleural infection is a public health problem whose incidence is increasing both in France and worldwide (1-5). The mortality rate is 17.1% in the general population and can increase up to 29.5% in patients with co-morbidities such as lung cancer (5). Pathophysiology of pleural infection is a continuum of three stages: exudative, fibrinopurulent and organised (6). The exudative phase is marked by a significant increase in pleural permeability leading to an influx of fluid, neutrophils and bacteria into the pleural cavity. The fibrinopurulent phase corresponds to the accumulation of fluid in the pleural cavity, resulting in a pleural envelope that encloses the lung in the organized phase (7-10).
Rationale and knowledge gap
Indication and optimal timing for surgery in pleural infection is still not well defined as guidelines differ between various thoracic surgery societies around the world (6,11,12). The American Association for Thoracic Surgery (AATS) recommends decortication by video-assisted thoracoscopic surgery (VATS) as first line of treatment in all operable stage II patients (12). According to the British Thoracic Society (BTS), surgical management should be the last resort in cases of persistent sepsis and collection despite optimal antibiotic and chest tube management (6). Finally, the European Association for Cardio-Thoracic Surgery (EACTS) recommends medical management by percutaneous drainage for stage I patients (11). For stage II and III patients, VATS surgery is recommended for patients that are operable (11). The RAPID [Renal (urea level), Age, Pleural fluid purulence, source of Infection and Denutrition (albumin level)] score has been developed to classify patients with pleural infection into three groups depending on mortality-risk at 3 months (13). It was elaborated from the prognostic factors of two prospective studies in which patients with pleural infection didn’t have surgery (14,15).
Objective
We hypothesized that the RAPID score would help better define patient’s care pathways in surgical department. This study aims to assess the applicability of this score at 6 and 12 months for patients managed in a surgical department and determine the impact of surgery on outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1599/rc).
Methods
Study design
This is a single-center, retrospective study, carried out at the Tenon University Hospital in Paris, from January 1st 2013 to June 30th 2019.
Inclusion criteria are: (I) patients older than 18 years old; (II) patients hospitalized for pleural infection; (III) community or hospital acquired infection.
Exclusion criteria are patients who developed pleural infection in the post-operative period following surgery or an invasive procedure (pleural puncture or drainage).
Diagnostic criteria for pleural infection are based on clinical and biological arguments: (I) fever; (II) biological inflammatory syndrome with hyperleukocytosis and increased C-reactive protein; (III) macroscopically purulent pleural fluid (defined as a thick sample that cannot be seen through); (IV) pleural fluid positive for bacterial infection (6-8); (V) imaging [computed tomography (CT) scan or chest X-ray] showing a pleural collection (8).
Presence of at least three of the previously described characteristics are necessary to establish the diagnostic.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the French Society of Thoracic and Cardiovascular Surgery (Registration No. IRB00012919). Due to the retrospective nature of this study, patients’ data were anonymised and consent was not sought after. This database is registered at Sorbonne University in order to comply with the General Data Protection Regulation (GDPR) and has been given the registration number 20220603144535.
Patient’s management
Patients’ management depended on the delay between onset of symptoms and hospital admission. Surgical management is favoured in patients hospitalized during the first 15 days after onset of symptoms: this usually corresponds to the exsudative stage of pleural infection or sometimes the fibrinopurulent stage in our experience. The preferred surgical approach was VATS. In case of dense adhesions and bleeding, a conversion for open thoracotomy was done. In both approaches, during decortication, various samples were sent for bacteriological analysis and intra-pleural saline lavage was performed before leaving one or multiple chest tubes.
Exclusive medical management was preferred in patients who were hospitalized 15 days after onset of symptoms. A thoracic drain was placed percutaneously at bedside or by an interventional radiologist. Fibrinolytic agents (urokinase—100,000 U in 10 mL saline serum) were administered through the drain three times a day after verifying potential contra-indications (external bleeding through the drain or air leakage in the drain), for a maximal of 5 days. A follow-up CT scan was performed 72 hours after surgery or drain placement to check for residual collection. In case of a persistent collection or new collection, was discussed placement of a new percutaneous drain.
Antibiotic therapy was introduced upon admission for a period of 4 to 6 weeks and adapted toward the germs identified (12).
Removal of chest tubes was based on:
- Absence of fever;
- Absence of residual collection on the CT scan;
- Three consecutive negative microbiological samples from drains.
Patients were discharged 24 hours after removal of chest tube. Follow-up included a chest X-ray and a blood sample 4 weeks later.
Data collection
Data collected included:
- Patient’s past medical history;
- Date of onset of symptoms;
- Vital parameters upon arrival in the department;
- Blood sample on arrival in the department (blood count, blood ionogram, urea, C-reactive protein, albumin);
- Bacteriology (blood culture and pleural fluid);
- Chest tubes (side, number, duration);
- Antibiotic therapy;
- Surgery;
- Length of stay;
- Last known status (alive, death).
Endpoints
The primary endpoint is the probabilities of survival at 6 and 12 months depending on the RAPID score. The RAPID score is calculated by the following parameters:
- R for renal (urea level);
- A for age;
- P for purulence in fluid (defined as a thick sample that cannot be seen through) or not;
- I for infection’s source;
- D for denutrition.
The secondary endpoint is the probabilities of survival at 6 and 12 months in patients who had surgery (surgical management group) and in patients who did not have surgery (medical management group).
Statistical analysis
The qualitative variables are reported in numbers and percentages. For quantitative variables, the results are expressed as median and interquartile range (1st and 3rd quartile). Subjects are compared according to variables of interest (RAPID score, surgery). Categorical variables are compared using the Chi-2 test. Quantitative variables are compared using the Mann-Whitney Wilcoxon test when two groups are considered. The Mood test is used to compare quantitative variables when more than two groups are considered.
To account for missing data during calculation of RAPID score in some patients, a maximum bias sensitivity analysis was used.
Survival is analyzed by using the Kaplan-Meier method and a comparison according to the risk group is made with the log-rank test. The significance level of P value was set at 5%. The R software version 4.0.3 was used (R Core Team, Auckland, USA).
Results
Patient characteristics
During the study period, 243 patients are identified. The flow chart is shown in Figure 1.
One hundred and sixty-nine patients with pleural infection after surgery or an invasive procedure were excluded. The final analysis included 74 patients. Table 1 shows their demographic characteristics.
Table 1
| Variables | Values |
|---|---|
| Age, years, median (IQR) | 54.5 (43.25, 71.75) |
| Male sex, n (%) | 55 (74.3) |
| Smoking, n (%) | |
| Non-smoker | 19 (25.7) |
| Former smokers | 19 (25.7) |
| Active | 29 (39.2) |
| Not known | 7 (9.5) |
| Alcohol, n (%) | |
| No | 26 (35.1) |
| Weaned | 5 (6.8) |
| Active | 27 (36.5) |
| Not known | 16 (21.6) |
| Source of infection, n (%) | |
| Community acquired | 58 (78.4) |
| Hospital acquired | 16 (21.6) |
| Antibiotics before diagnosis | 27 (36.5) |
| Anti-inflammatory before diagnosis | 10 (13.5) |
| Clinical symptoms, n (%) | |
| Dyspnoea | 48 (64.9) |
| Pleural pain | 42 (56.8) |
| Cough | 30 (40.5) |
| Chills | 24 (32.4) |
| Appearance of fluid | |
| Purulent | 24 (32.4) |
| Medical history, n (%) | |
| Previous pleural infection | 2 (2.7) |
| Respiratory diseases | 40 (54.1) |
| Atrial fibrillation | 4 (5.4) |
| Cardiac pathologies | 10 (13.5) |
| Hypertension | 15 (20.3) |
| Diabetes | 7 (9.5) |
| Depression | 9 (12.2) |
| Cancer | 16 (21.6) |
| Management, n (%) | |
| Medical treatment | 48 (64.9) |
| Surgical treatment | 26 (35.1) |
| Approach | |
| VATS | 20 (76.9) |
| Thoracotomy | 6 (23.1) |
IQR, interquartile range; VATS, video-assisted thoracoscopic surgery.
Median age was 54.5 years [interquartile range (IQR): 43.25–71.75 years] and 74.3% of the patients were men (n=55). Concerning tobacco intoxication, 39.2% of patients (n=29) were active smokers while 25.7% (n=19) were former smokers and 25.7% (n=19) were non-smokers. Alcohol consumption was active in 36.5% of patients (n=27), weaned in 6.8% (n=5) and absent in 35.1% (n=26).
A proportion of 64.9% of patients (n=48) were in the medical treatment group while 35.1% of patients (n=26) had surgery. In the surgical management group, 76.9% of patients (n=20) had exclusively VATS and 23.1% of patients (n=6) of patients had a thoracotomy.
Pleural samples identified a germ in 32 patients (88.9%) through pleural fluid. In 4 patients (11.1%), the germ was identified in blood cultures. Table 2 details all the germs implicated in the infections. Streptococcus (48.8%) was the most frequent germ (Table 2).
Table 2
| Variables | Values (%) |
|---|---|
| Sterile | 38 (51.4) |
| Germ(s) identified | 36 (48.6) |
| Blood cultures | 4 (11.1) |
| Pleural specimen | 32 (88.9) |
| Types of germs (%) | |
| Streptococcus spp | 21 (48.8) |
| Constellatus | 10 |
| Pneumoniae | 5 |
| Intermedius | 4 |
| Anginosus | 2 |
| Staphylococcus | 2 (4.7) |
| Aureus | 1 |
| Epidermidis | 1 |
| Enterococcus faecalis | 1 (2.2) |
| Echerichia coli | 5 (11.6) |
| Pseudomonas æruginosa | 3 (7.0) |
| Enterobacter | 3 (7.0) |
| Parvimonas micra | 2 (4.7) |
| Fusobacterium spp | 4 (9.3) |
| Necrophorum | 2 |
| Nucleatum | 2 |
| Prevotella heparinolytica | 2 (4.7) |
spp, species plurimae.
Primary endpoint
According to the RAPID score, 30 patients (40%) were classified in the low-risk group, 30 patients (40%) in the intermediate-risk group and 14 patients (20%) in the high-risk group (Table 3).
Table 3
| Variables | Low-risk (n=30) | Medium-risk (n=30) | High-risk (n=14) | P value |
|---|---|---|---|---|
| Probability of survival (CI95) | ||||
| 6 months | 0.967 (0.905–1) | 0.967 (0.905–1) | 0.571 (0.363–0.899) | |
| 12 months | 0.967 (0.905–1) | 0.967 (0.905–1) | 0.357 (0.177–0.721) | |
| Length of stay, days, median (IQR) | 12 (9.25, 18.25) | 15 (13, 22.75) | 17.5 (13.25, 26.5) | 0.075 |
| Surgical intervention, n (%) | 12 (40.0) | 12 (40.0) | 2 (14.3) | 0.193 |
| Fibrinolysis, n (%) | 16 (57.1) | 14 (51.9) | 11 (84.6) | 0.127 |
| Length of drainage, days, median [IQR] | 10 [7, 16] | 12 [9.25, 16.75] | 10 [7.25, 16.75] | 0.471 |
RAPID, Renal (urea level), Age, Pleural fluid purulence, source of Infection and Denutrition (albumin level); CI95, 95% confidence index; IQR, interquartile range.
The probability of survival at 6 and 12 months for the low- and medium-risk groups were both 0.967 [95% confidence index (CI95): 0.905–1]. For the high-risk group, the probability of survival was 0.571 (CI95: 0.363–0.899) at 6 months and 0.357 (CI95: 0.177–0.721) at 12 months. This was significantly different from the low- and medium-risk groups (P<0.0001) (Table 3, Figure 2).
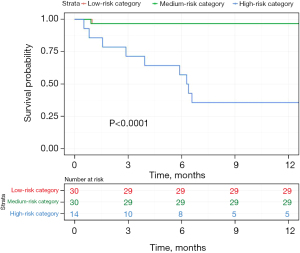
A post-hoc analysis of mortality between the different groups found a statistically significant difference between the low- and high-risk groups with a P value inferior to 0.0001. Similarly, the difference in mortality was statistically significant between the medium- and high-risk group (P=0.01). However, there was no difference between the low- and medium-risk group (P=0.19).
The length of stay did not differ significantly between the different RAPID score groups (P=0.075):
- The low-risk group had a median length of stay of 12 days (IQR: 9.25–18.25 days);
- The medium-risk group had a median length of stay of 15 days (IQR: 13–22.75 days);
- The high-risk group had a median length of stay of 17.5 days (IQR: 13.25–26.5 days).
Chest tube duration was not statistically different between the three groups (P=0.471). In the low-risk group, it was 10 days (IQR: 7–16 days). In the medium-risk group, it was 12 days (IQR: 9.25–16.75 days) and in the high-risk group, it was 10 days (IQR: 7.25–16.75 days).
Secondary endpoint
Twenty-six patients (35.1%) had surgery and 48 patients (64.9%) were in the medical treatment group. The general characteristics between those two groups were not significantly different in term of gender (P=0.512), age (P=0.113), RAPID score distribution (P=0.193) and length of stay (P=0.646) (Table 4).
Table 4
| Variables | Medical treatment (n=48) | Surgical treatment (n=26) |
|---|---|---|
| Men, n (%) | 34 (70.8) | 21 (80.8) |
| Age, years, median (IQR) | 56 (45.25, 73.25) | 52 (41.75, 61.75) |
| RAPID score, n (%) | ||
| Low-risk | 18 (37.5) | 12 (46.2) |
| Medium-risk | 18 (37.5) | 12 (46.2) |
| High-risk | 12 (25.0) | 2 (8.7) |
| Length of stay, days, median (IQR) | 15 (11.75, 23.25) | 15.5 (11.25, 21.25) |
| Probability of survival (CI95) | ||
| 6 months | 0.875 (0.786–0.974) | 0.923 (0.826–1) |
| 12 months | 0.812 (0.704–0.931) | 0.923 (0.826–1) |
IQR, interquartile range; CI95, 95% confidence index.
Probabilities of survival at 6 and 12 months tend to be higher in the surgical treatment group compared to the exclusive medical group, but the result was not significant (P=0.26). Indeed, in the medical treatment group, the probability of survival at 6 and 12 months were 0.875 (CI95: 0.786–0.974) and 0.812 (CI95: 0.704–0.931) respectively whereas in the surgical treatment group, the probabilities of survival at 6 and 12 months were both 0.923 (CI95: 0.826–1) (Table 4, Figure 3).
Discussion
Key findings
In a thoracic surgery department, the RAPID score shows a significant difference in the probabilities of survival at 6 and 12 months between the low- and medium-risk group compared to the high-risk group. But no difference was found when comparing the low-risk group with the medium-risk group.
Comparison with similar researches
These results partially confirm those of Touray et al.: in a retrospective single center study of 98 patients with stage II and III pleural infections, the 90-day mortality of low, medium- and high-risk patients were 5.3%, 8.3% and 22.6% respectively (16). In the pilot study, mortality at 3 and 12 months increased with severity of the RAPID score: patients received primary medical management and surgery would be discussed in case of failure. The 3-month mortality rates for low-, medium- and high-risk patients were 2.3%, 9.2% and 29.3% respectively (17). At 12-month, mortality rates in low-, medium- and high-risk patients were 6.1% (CI95: 3.5–10.2%), 18% (CI95: 13.6–23.3%) and 49.9% (CI95: 39.8–60%), respectively (17). We could not find any difference between the low- and medium-risk groups in our study. However, the RAPID score remains an interesting tool to assess the prognosis of patients managed for pleural infection in both medical and surgical settings. The increased risk of mortality according to the group would allow to adapt the patients’ management, initial referral to an intensive care unit and choice of an aggressive surgical treatment.
Surgery’s role in management of pleural infection remains controversial. Guidelines from various international thoracic surgery societies differ concerning indication for surgical management of pleural infection (6,11,12). We favour surgery within the first 15 days after onset of symptoms, assuming that the pleural infection’s stage is not yet organised. The probability of survival in the medically managed group was lower than in the surgically managed group. These results are similar to the conclusions by Farjah et al. (18): risk of death at 30 days was reduced by 58% in patients who had undergone surgery (after adjustment for age, sex and comorbidities) compared to patients treated medically (18).
Explanations of findings
These results are favored by a good patients’ selection before surgery: these patients are usually younger with a low- or medium-risk RAPID score. The high-risk group, in which patients were rarely operated, had a higher mortality rate. Maybe by being more aggressive in high-risk patients with surgical management would decrease mortality rate at 6 and 12 months? Bilgin et al. investigated the benefits of an early aggressive treatment which decreased length of hospital stay but didn’t change mortality rate (19).
Minimally invasive approaches by VATS are favored over thoracotomy. Their benefits have been approved by randomized studies in major lung resection for non-small cell lung cancer: indeed, Bendixen et al. have shown a decrease in post-operative pain and an improvement in quality of life in patients who VATS compared to anterolateral thoracotomy (20). In their randomized multi-centered study, Lim et al. confirmed a significant improvement in the quality of life after VATS compared to thoracotomy, on a self-assessment questionnaire (21). Length of hospital stay was reduced by one day when VATS was used compared to conventional thoracotomy (21). Similarly, Farjah et al. found fewer post-operative complications with VATS (18). VATS is not always simple to carry on in pleural infection, as it is highlighted by our high-rate of thoracotomy following conversion. The inflammatory process complicate exposure and visibility throughout the procedure.
Limits
Main limits in this study are represented by:
- The retrospective aspect which can present difficulties in data collection. Moreover, we applied the RAPID score to a population of subject after they had medical or surgical management in our department. Our selection didn’t rely on the RAPID score then. But an interesting finding by studying those cases found that we favored without knowing it surgery in patients who belong most likely to low- and medium-risk group. Therefore, this might be one of the reasons why the probabilities of survival at 6 and 12 months is higher in the surgical group;
- The monocentric design which limits the number of patients included.
However, many patients could not be included in our study because of previous thoracic surgery for major resection of non-small cell lung cancer. Indeed, the RAPID score was developed from studies excluding these patients (22,23).
Implications and actions needed
The RAPID score is intended to classify patients’ mortality. It wasn’t initially intended to be use in surgery. As this is a retrospective study, we sought to clarify our management of pleural infection and subsequently identify if the RAPID score could determine which patients had a worst prognosis. We found no difference between the low- and medium-risk groups. But we found a worst survival in the high-risk group. RAPID score seems to validate the surgeon’s decision making and is partially in line with the scoring system as there was no difference between the low- and medium-risk groups. By better identifying high-risk patients, maybe being more aggressive with surgery could improve their outcome. Prospective controlled studies are still needed to confirm the benefits of surgery compared to strictly medical management according to the RAPID score.
Conclusions
RAPID score is useful to classify mortality risk during mid- and long-term follow up in patients with pleural infection in a thoracic surgery department. In our cohort, the high-risk group had a significantly lower survival at 6 and 12 months compared to the low- and medium-risk groups. Early surgical management in patients hospitalized with pleural infection reduces mortality at 6 and 12 months compared with exclusive medical management. This result remains to be confirmed by prospective randomized trials which would also help determine the role of the RAPID score in selecting patients eligible for surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1599/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1599/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1599/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the French Society of Thoracic and Cardiovascular Surgery (Registration No. IRB00012919). Due to the retrospective nature of this study, patients’ data were anonymised and consent was not sought after.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Finley C, Clifton J, Fitzgerald JM, et al. Empyema: an increasing concern in Canada. Can Respir J 2008;15:85-9. [Crossref] [PubMed]
- Grijalva CG, Zhu Y, Nuorti JP, et al. Emergence of parapneumonic empyema in the USA. Thorax 2011;66:663-8. [Crossref] [PubMed]
- Lisboa T, Waterer GW, Lee YC. Pleural infection: changing bacteriology and its implications. Respirology 2011;16:598-603. [Crossref] [PubMed]
- Burgos J, Falcó V, Pahissa A. The increasing incidence of empyema. Curr Opin Pulm Med 2013;19:350-6. [Crossref] [PubMed]
- Bobbio A, Bouam S, Frenkiel J, et al. Epidemiology and prognostic factors of pleural empyema. Thorax 2021;76:1117-23. [Crossref] [PubMed]
- Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii41-53. [Crossref] [PubMed]
- Kroegel C, Antony VB. Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy. Eur Respir J 1997;10:2411-8. [Crossref] [PubMed]
- Alemán C, Alegre J, Monasterio J, et al. Association between inflammatory mediators and the fibrinolysis system in infectious pleural effusions. Clin Sci (Lond) 2003;105:601-7. [Crossref] [PubMed]
- Brims FJ, Lansley SM, Waterer GW, et al. Empyema thoracis: new insights into an old disease. Eur Respir Rev 2010;19:220-8. [Crossref] [PubMed]
- Reichert M, Hecker M, Witte B, et al. Stage-directed therapy of pleural empyema. Langenbecks Arch Surg 2017;402:15-26. [Crossref] [PubMed]
- Scarci M, Abah U, Solli P, et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur J Cardiothorac Surg 2015;48:642-53. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Rahman NM, Kahan BC, Miller RF, et al. A clinical score (RAPID) to identify those at risk for poor outcome at presentation in patients with pleural infection. Chest 2014;145:848-55. [Crossref] [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Touray S, Sood RN, Lindstrom D, et al. Risk Stratification in Patients with Complicated Parapneumonic Effusions and Empyema Using the RAPID Score. Lung 2018;196:623-9. [Crossref] [PubMed]
- Corcoran JP, Psallidas I, Gerry S, et al. Prospective validation of the RAPID clinical risk prediction score in adult patients with pleural infection: the PILOT study. Eur Respir J 2020;56:2000130. [Crossref] [PubMed]
- Farjah F, Symons RG, Krishnadasan B, et al. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg 2007;133:346-51. [Crossref] [PubMed]
- Bilgin M, Akcali Y, Oguzkaya F. Benefits of early aggressive management of empyema thoracis. ANZ J Surg 2006;76:120-2. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Lim E, Batchelor TJP, Dunning J, et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid 2022;1:EVIDoa2100016.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000;118:1158-71. Erratum in: Chest 2001;119:319. [Crossref] [PubMed]
- Anstadt MP, Guill CK, Ferguson ER, et al. Surgical versus nonsurgical treatment of empyema thoracis: an outcomes analysis. Am J Med Sci 2003;326:9-14. [Crossref] [PubMed]




