
Outcomes of robotic lobectomy for non-small cell lung cancer in a National Cancer Institute-Comprehensive Cancer Center vs. National Cancer Database
Highlight box
Key findings
• Compared to national benchmarks in robotic lobectomy, patients who underwent robotic lobectomy in a National Cancer Institute-designated Comprehensive Cancer Center (NCI-CCC) had higher nodal yield, higher rate of mediastinal lymphadenectomy and lower conversion rates.
What is known and what is new?
• Video-assisted thoracoscopic lobectomy (VL) had shorter median length of stay, better physical function, lower in-hospital complications compared to open lobectomy (OL). So far there have been no randomized trials comparing robotic lobectomies to VL or OL
• The manuscript adds that robotic lobectomy (RL), when done in an NCI-CCC, is associated with im-proved perioperative outcomes in terms of higher nodal yield, higher performance of mediastinal lymphadenectomy, and lower conversion rate but has comparable long-term outcomes with OL, VL, and RL benchmarks.
What is the implication, and what should change now?
• Robotic lobectomy demonstrated improved outcomes when performed in an NCI-CCC and should be considered safe in resectable primary non-small cell lung cancer.
Introduction
Minimally invasive techniques for all surgical procedures have grown remarkably, and the same holds for pulmonary resections for lung cancer. With the recent expansion of criteria for low-dose lung cancer screening from the US Preventive Services Task Force (1) and the continued growth of the aging population (2), it is expected that the incidence of resectable non-small cell lung cancers (NSCLC) will continue to increase, requiring minimally invasive surgical techniques. In a previous analysis of the National Cancer Database (NCDB) from 2010 to 2012, there was a significant increase in proportion of robotic lobectomy (RL) performed from 3.0% to 9.1% (3). According to Society of Thoracic Surgeons (STS) data, there was also an increase in using robotic technology for treating NSCLC from <1% in 2009 to 18.1% in 2016, and this is associated with a decrease in the use of open lobectomy (OL) from 53.3% to 28.0% without much change in the proportion of video-assisted thoracoscopic surgery (VATS) lobectomies (VLs) (4). The advantages of minimally invasive approaches have been proven in a multicenter randomized trial comparing VLs and OL (5). There were significantly fewer in-hospital complications, a shorter median length of stay (LOS), and better median physical function as measured by the European Organization for Research and Treatment of Cancer (EORTC) core health-related quality of life questionnaire (QLC-C30) at 5 weeks in the VL cohort than the OL cohort without compromising early oncologic outcomes. Progression-free survival, and overall survival (OS) were similar between cohorts at 52 weeks after the procedure. However, long-term outcomes are pending for this study, which is the largest randomized study comparing VL with OL so far. A large retrospective multicenter experience reported that the OS and stage-specific survival rates for RLs are consistent with those of VLs (6). In this study, which included 325 RLs, the 5-year OS for pathologic stage IA was 91%; stage IB was 88%; and stage II was 49%. So far, there have been no head-to-head comparisons for perioperative and oncologic outcomes between OLs, VLs, and RLs.
The goal of this retrospective study is to compare the perioperative and long-term oncologic outcomes of RL for NSCLC in a comprehensive cancer center (CCC) to OL, VL and RL as reported in the NCDB. More than 1500 accredited centers across the US territories submit data to this program, and it is thought to capture around 70% of all newly diagnosed lung cancer cases in the US annually (7). The authors aim to provide perspectives on how outcomes in a National Cancer Institute (NCI)-CCC compares to national benchmarks as presented in the NCDB. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1340/rc).
Methods
The Moffitt Cancer Center (MCC) database for RLs between 2010 and 2020 was used for the analysis. This database has been retrospectively collected and prospectively maintained under an Institutional Review Board (IRB)-approved protocol in the Thoracic Oncology Program, with 63 demographic, clinical, and perioperative datapoints collected for each patient. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval to report this study was obtained from Moffitt Cancer Center’s Scientific Review Committee (MCC #16728, #18761, and #19304) and by our University of South Florida’s Institutional Review Boards (USF IRB #Pro00022263 and Chesapeake IRB #Pro00017745 and #00000790). Individual consent for this retrospective analysis was waived.
Only patients diagnosed with primary lung malignancy were included. Patients with unknown pathologies, benign pathologies, small cell lung cancer, and secondary metastases to the lung were not analyzed. The demographic and clinical variables collected included age (years), sex, race/ethnicity, histologic type, tumor grade, laterality, lobe resected, Charlson comorbidity score, smoking history, steroid use, presence/absence of hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), end-stage renal disease (ESRD), and receipt of neoadjuvant therapy. The perioperative datapoints collected included the T stage, N stage, conversion to thoracotomy, additional pulmonary resections performed, estimated blood loss (EBL, in mL), and operative duration (in minutes), number of examined nodes, postoperative morbidity, chest tube duration (in days), discharge to home with tube, LOS and postoperative mortality.
To compare outcomes, we reviewed the NCDB to identify patients with NSCLC who underwent lobectomy for primary lung malignancy with a curative intent between 2010 and 2017. This group was used as a control group against our MCC RL database during the same timeframe to reflect chronological cohorts. We excluded patients who were reported to have metastatic disease or had secondary lung malignancies. Only patients who underwent a pulmonary lobectomy with reported data on the surgical approach were included. Patients who had non-anatomical wedge resections, segmentectomy, pneumonectomy, or unclear surgical resection approaches were not considered for the analysis.
Operative technique
All RLs were performed using the Intuitive daVinci robotic surgical system. From 2010 to December 2011, the S model was used. The Si model was then used from January 2012 to December 2016. From 2017 onwards, the Xi system was henceforth used. There are three surgeons in the NCI-CCC who perform RLs. Two of these surgeons use four-arm technique, and one use a three-arm technique. The port placement and technique of the surgeons did not vary with the laterality, or the lobe being resected. The first 8-mm trocar is placed, and the remaining trocars are placed under thoracoscopic guidance. Temporary insufflation with warm humidified CO2 is used to a maximum pressure of 8–10 mmHg to improve visualization of the surgical field. Robotic stapling is performed through either the anterior or posterior 12-mm trocars. The dissection is standardized, regardless of the laterality or the lobe. The inferior pulmonary ligament is divided, and this is followed by a posterior hilar dissection which includes a thorough lymphadenectomy of stations 7, 8, and 9. We then follow with an anterior hilar dissection, after which division of the vein, artery, and bronchus is performed. The sequence of division of these structures as well as the fissure differ depending on the lobe being removed. A complete mediastinal and hilar lymphadenectomy then follows—stations 2 and 4 on the right and stations 5 and 6 on the left. Stations 10 and 11 are dissected out as well. A single 28 French chest tube is inserted, and postoperative care follow the ERAS protocol.
Statistical analysis
After patient selection in each database, we aimed to compare the outcomes of RLs at MCC to OLs, VLs, and RLs as reported in the NCDB. We used the conditional logistic regression for categorical variables and mixed-effect modeling for continuous variables to compare the clinical and demographic characteristics between groups. For each comparative analysis, we matched patients from the MCC RL database to their chronological peers from the NCDB OL, NCDB VL, and NCDB RL databases. The match was performed using a propensity score that was calculated from a logistic regression including all available perioperative variables: age, sex, race/ethnicity, Charlson score, histology, tumor grade, laterality, TNM stage, and the receipt of any type of neoadjuvant therapy. Patients were matched on a ratio of 1:3 using the nearest neighbor method with a caliper width of 0.1 standard deviations (SDs), and the matched groups were checked for adequate balance. Missing data were excluded from analysis.
Surgical outcomes were defined as the number of examined nodes, performance of mediastinal lymphadenectomy, 30-day mortality, and LOS in the hospital postoperatively. These outcomes were compared based on the intention to treat using similar methods as described above (conditional logistic regression and mixed-effect modeling). The Kaplan-Meier method was used to study long-term OS outcomes. The log-rank test was used to compare OS between the study groups for each comparison. IBM SPSS Statistics v25 (Armonk, NY, USA) with the Essentials for R Plug-In for propensity score matching was used for the statistical analysis. Statistical significance was set at <0.05 throughout the study.
Results
The MCC database for RLs contained 1,021 patients diagnosed with primary lung malignancies between 2010 and 2020. Follow up varied and was reflected as the last date of contact with the patients, ranging from 2 weeks to 10 years. The mean age was 69.17±20.93 years (median, 70 years), and 596 (58.4%) patients were female. The most common tumor histology was adenocarcinoma [704 (69.0%)] followed by squamous cell carcinoma [186 (18.2%)]. Most the tumors were right-sided [661 (64.7%)] and located in the upper lobe [614 (60.1%)]. Half of the patients were healthy, as indicated by a Charlson comorbidity index of 0 [518 (50.7%)]. At the time of operation, 233 patients (22.8%) were active smokers and 21 (2.1%) were receiving systemic steroids. The mean tumor size was 2.96±1.73 cm (median, 2.5 cm), and most tumors were clinical stage I [609 (59.6%)] based on the American Joint Committee on Cancer, eighth edition of TNM staging for NSCLC. From the operative standpoint, 36 patients (3.5%) experienced conversion to OL; 28 patients (2.7%) had an additional anatomic lobectomy; and 382 (37.4%) had an additional resection (i.e., wedge resection or mediastinal mass resection). The mean EBL was 170±353 mL (median, 100 mL) and the mean operative time was 173±54 minutes (median, 163 minutes). Table 1 summarizes the clinical, demographic, and intraoperative characteristics of the MCC RL cohort.
Table 1
| Variables | Value (N=1,021) |
|---|---|
| Age (years) | 69.17±20.93, 70 |
| Sex | |
| Males | 425 (41.6) |
| Females | 596 (58.4) |
| Race/ethnicity | |
| White | 947 (92.8) |
| Black | 21 (2.1) |
| Hispanic | 25 (2.4) |
| Asian | 19 (1.9) |
| Other | 9 (0.9) |
| Histology type | |
| Adenocarcinoma | 704 (69.0) |
| Squamous cell carcinoma | 186 (18.2) |
| Neuroendocrine | 105 (10.3) |
| Other | 26 (2.5) |
| Grade | |
| Well differentiated | 289 (28.3) |
| Moderately differentiated | 452 (44.3) |
| Poorly differentiated | 250 (24.5) |
| Not reported | 30 (2.9) |
| Side | |
| Right | 661 (64.7) |
| Left | 360 (35.3) |
| Lobe | |
| Upper | 614 (60.1) |
| Middle | 84 (8.2) |
| Lower | 295 (28.9) |
| Combined (bilobectomy) | 28 (2.7) |
| Charlson score | |
| 0 | 518 (50.7) |
| 1 | 311 (30.5) |
| 2 | 128 (12.5) |
| 3+ | 64 (6.3) |
| Comorbidities | |
| Hypertension | 499 (48.9) |
| Diabetes mellitus | 161 (15.8) |
| COPD | 253 (24.8) |
| CHF | 26 (2.5) |
| ESRD | 5 (0.5) |
| Risk factors | |
| Tobacco | 233 (22.8) |
| Steroids | 21 (2.1) |
| Tumor size (cm) | 2.96±1.73, 2.5 |
| T stage | |
| Tis | 11 (1.1) |
| T1 | 534 (52.3) |
| T2 | 353 (34.6) |
| T3 | 88 (8.6) |
| T4 | 35 (3.4) |
| N stage | |
| N0 | 759 (74.3) |
| N1 | 108 (10.6) |
| N2 | 154 (15.1) |
| TNM stage | |
| Stage I | 609 (59.6) |
| Stage II | 207 (20.3) |
| Stage III | 202 (19.8) |
| Stage IV | 3 (0.3) |
| Neoadjuvant therapy | |
| Systemic | 21 (2.1) |
| Radiation | 5 (0.5) |
| Operative details | |
| Conversion to open | 36 (3.5) |
| Additional lobectomy | 28 (2.7) |
| Additional resection (wedge, mediastinal mass) | 382 (37.4) |
| Estimated blood loss (mL) | 170±353, 100 |
| Operative time (minutes) | 173±54, 163 |
Data are shown as number (percentage) or mean ± standard deviation, median. COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; ESRD, end-stage renal disease.
Regarding the 30-day postoperative outcomes, the most commonly encountered morbidity was presence of a persistent air leak for >5 days [208 (20.4%)] followed by atrial fibrillation [96 (9.4%)] and pneumonia [43 (4.2%)]. The mean duration of chest tube placement was 4.94±5.40 days (median, 3 days), and 103 patients (10.1%) were discharged home with a chest tube in place. The median LOS in the hospital was 4 days, and 10 patients (1.0%) experienced postoperative mortality within 30 days. Table 2 demonstrates the short-term postoperative outcomes of the MCC RL cohort.
Table 2
| Perioperative outcome | Value (N=1,021) |
|---|---|
| Number of examined nodes | 15.96±7.85, 15 |
| Postoperative morbidity | |
| Pneumonia | 43 (4.2) |
| Pulmonary embolism | 5 (0.5) |
| Cerebral vascular accident | 6 (0.6) |
| Atrial fibrillation | 96 (9.4) |
| Other arrhythmias | 7 (0.7) |
| Myocardial infarction | 3 (0.3) |
| Cardiac arrest | 3 (0.3) |
| Respiratory failure (ventilation >48 hours) | 11 (1.1) |
| Hemothorax | 7 (0.7) |
| Chyle leak | 36 (3.5) |
| Empyema | 4 (0.4) |
| Pneumothorax | 9 (0.9) |
| Persistent air leak | 208 (20.4) |
| Hypoxia | 11 (1.1) |
| Mucus plug | 16 (1.6) |
| Days with chest tube | 4.94±5.40, 3 |
| Home with chest tube | |
| No | 918 (89.9) |
| Yes | 103 (10.1) |
| Home days chest tube | 0.87±3.13, 0 |
| Postoperative mortality | |
| No | 1,011 (99.0) |
| Yes | 10 (1.0) |
| Length of stay (days) | 4.85±3.94, 4 |
Data are shown as mean ± standard deviation, median or number (percentage).
We selected 122,467 patients from the NCDB who underwent OL for the first comparative analysis. There were significant differences in baseline characteristics between the MCC RL and the NCDB OL groups, most remarkably in the tumor histology (adenocarcinoma, 68.4% in MCC RLs vs. 56.1% in NCDB OLs; P<0.001) and T stage (T4, 27.4% in MCC RLs vs. 1.9% in NCDB OLs; P<0.001). After calculating the propensity score, we matched 499 MCC patients with RLs to 1,497 NCDB patients with OLs on a ratio of 1:3. The matched dataset was well-balanced as manifested by resolution of all the baseline differences and a SD <0.1 across all variables. Table S1 demonstrates the comparisons between the unmatched and 1:3 matched datasets of MCC RLs and NCDB OLs.
Upon comparison of short-term outcomes, MCC RL patients had a higher mean number of retrieved nodes (14.66±6.83 vs. 9.74±7.38 nodes; P=0.001), higher reported rates of mediastinal lymphadenectomy (99.4% vs. 75.2%; P<0.001), and a 2-day shorter median LOS (4 vs. 6 days; P<0.001) than NCDB OL patients. There were no differences in 30-day postoperative mortality between the groups (1.0% in MCC RLs vs. 1.6% in NCDB OLs; P=0.176). Table S2 shows the comparison of postoperative outcomes between the MCC RL vs. NCDB OL matched groups. Kaplan-Meier analyses showed no differences between these groups in terms of long-term median OS (78.0±5.4 months for MCC RLs vs. 81.6±3.8 months for NCDB OLs; log-rank P=0.953). Five-year OS was 62% for MCC RLs vs. 60% for NCDB OLs (P=0.774). Figure 1 shows the results of the Kaplan-Meier survival analysis comparing the MCC RL to NCDB OL groups.

We repeated a similar selection process to identify 45,193 NCDB patients who underwent VLs as a control group for the second comparison. Similar baseline differences were also noted between this patient group compared to our MCC RL group, most notably in the histology distribution and tumor T stage. We matched 492 MCC RL patients to 1,476 NCDB VL patients with adequate adjustment of all the baseline differences. Table S3 shows a summary of the unmatched and 1:3 matched datasets of MCC RL vs. NCDB VL patient groups.
When comparing short-term outcomes, MCC RL patients had a higher nodal yield (14.66±6.83 vs. 11.52±8.79; P<0.001); higher rates of reported mediastinal lymphadenectomy (99.4% vs. 83.6%; P<0.001); and a lower conversion rate (4.1% vs. 13.8%; P<0.001) than the NCDB VL patients. There were no differences in postoperative mortality (1.0% for MCC RLs vs. 1.6% for NCDB VLs; P=0.379) or median LOS (4 days for MCC RLs vs. 4 days for NCDB VLs; P=0.351). Table S4 shows the comparison of short-term outcomes between MCC RL vs. NCDB VL patients. Moreover, survival analyses showed no differences in median OS between these 2 groups (78.0±5.3 months for MCC RLs vs. 78.1±2.7 months for NCDB VLs; P=0.720). Five-year OS was not statistically significantly different between the groups (62% for MCC RLs vs. 61% for NCDB VLs; P=0.735). Figure 2 demonstrates the Kaplan-Meier survival analyses for the MCC RL vs. NCDB VL groups.

Finally, an identical methodology was followed to select 15,816 NCDB RL patients for comparison against the MCC RL patients. A comparable difference in the baseline profiles were noted between the groups, with resolution of all differences upon matching 493 MCC RL to 1,479 NCDB RL patients. Table 3 shows the comparison between these 2 groups before and after the propensity score matching.
Table 3
| Variables | Unmatched dataset | Matched dataset 1:3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MCC RL (N=667) | NCDB RL (N=15,816) | SD | P | MCC RL (N=493) | NCDB RL (N=1,479) | SD | P | ||
| Age, years | 68.62±9.22 | 67.80±9.54 | 0.017 | 0.007* | 68.42±9.23 | 68.22±9.71 | 0.004 | 0.702 | |
| Sex | 0.016 | 0.046* | 0.015 | 0.506 | |||||
| Males | 269 (40.3) | 6,999 (44.3) | 189 (38.3) | 592 (40.0) | |||||
| Females | 398 (59.7) | 8,817 (55.7) | 304 (61.7) | 887 (60.0) | |||||
| Race/ethnicity | 0.069 | <0.001* | 0.027 | 0.829 | |||||
| White | 631 (94.6) | 12,885 (81.5) | 463 (93.9) | 1,391 (94.1) | |||||
| Black | 15 (2.2) | 1,359 (8.6) | 14 (2.8) | 31 (2.1) | |||||
| Hispanic | 6 (0.9) | 879 (5.6) | 5 (1.0) | 20 (1.4) | |||||
| Asian | 10 (1.5) | 343 (2.2) | 8 (1.6) | 29 (2.0) | |||||
| Other | 5 (0.7) | 350 (2.2) | 3 (0.6) | 8 (0.5) | |||||
| Charlson score | 0.031 | <0.001* | 0.022 | 0.813 | |||||
| 0 | 348 (52.2) | 8,278 (52.3) | 255 (51.7) | 775 (52.4) | |||||
| 1 | 188 (28.2) | 5,056 (32.0) | 143 (29.0) | 409 (27.7) | |||||
| 2 | 80 (12.0) | 1,760 (11.1) | 57 (11.6) | 190 (12.8) | |||||
| 3+ | 51 (7.6) | 722 (4.6) | 38 (7.7) | 105 (7.1) | |||||
| Histology | 0.073 | <0.001* | 0.013 | 0.958 | |||||
| Adenocarcinoma | 456 (68.4) | 9,088 (57.5) | 331 (67.1) | 1,011 (68.4) | |||||
| SCC | 124 (18.6) | 3,575 (22.6) | 92 (18.7) | 267 (18.1) | |||||
| Neuroendocrine | 76 (11.4) | 1,245 (7.9) | 59 (12.0) | 167 (11.3) | |||||
| Other | 11 (1.6) | 1,908 (12.1) | 11 (2.2) | 34 (2.3) | |||||
| Grade | 0.063 | <0.001* | 0.02 | 0.845 | |||||
| Well difference | 198 (29.7) | 3,140 (19.9) | 159 (32.3) | 490 (33.1) | |||||
| Moderately difference | 286 (42.9) | 6,838 (43.2) | 205 (41.6) | 632 (42.7) | |||||
| Poorly difference | 168 (25.2) | 4,422 (28.0) | 117 (23.7) | 324 (21.9) | |||||
| Missing | 15 (2.2) | 1,416 (9.0) | 12 (2.4) | 33 (2.2) | |||||
| Side | 0.003 | 0.891 | |||||||
| Right | 430 (64.5) | 9,706 (61.4) | 321 (65.1) | 968 (65.4) | |||||
| Left | 233 (34.9) | 6,110 (38.6) | 172 (34.9) | 511 (34.6) | |||||
| Missing | 4 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| Lobe | 0.035 | <0.001* | 0.01 | 0.907 | |||||
| Upper | 411 (61.6) | 8,914 (56.4) | 307 (62.3) | 897 (60.6) | |||||
| Middle | 58 (8.7) | 933 (5.9) | 45 (9.1) | 134 (9.1) | |||||
| Lower | 198 (29.7) | 5,969 (37.7) | 141 (28.6) | 448 (30.3) | |||||
| T stage | 0.364 | <0.001* | 0.069 | 0.1 | |||||
| Tis | 1 (0.1) | 65 (0.4) | 1 (0.2) | 3 (0.2) | |||||
| T1 | 363 (54.4) | 9,411 (59.5) | 360 (73.0) | 1,090 (73.7) | |||||
| T2 | 54 (8.1) | 3,849 (24.3) | 53 (10.8) | 204 (13.8) | |||||
| T3 | 44 (6.6) | 692 (4.4) | 44 (8.9) | 119 (8.0) | |||||
| T4 | 183 (27.4) | 142 (0.9) | 13 (2.6) | 43 (2.9) | |||||
| Tx | 22 (3.3) | 1,657 (10.5) | 22 (4.5) | 20 (1.4) | |||||
| N stage | 0.112 | <0.001* | 0.061 | 0.103 | |||||
| N0 | 512 (76.8) | 13,242 (83.7) | 411 (83.4) | 1,242 (84.0) | |||||
| N1 | 62 (9.3) | 801 (5.1) | 34 (6.9) | 110 (7.4) | |||||
| N2 | 93 (13.9) | 655 (4.1) | 48 (9.7) | 112 (7.6) | |||||
| Nx | 0 (0.0) | 1,118 (7.1) | 0 (0.0) | 15 (1.0) | |||||
| TNM stage | 0.14 | <0.001* | 0.059 | 0.195 | |||||
| Stage 0 | 0 (0.0) | 66 (0.4) | 0 (0.0) | 0 (0.0) | |||||
| Stage I | 410 (61.5) | 11,397 (72.1) | 312 (63.3) | 979 (66.2) | |||||
| Stage II | 131 (19.6) | 1,715 (10.8) | 101 (20.5) | 284 (19.2) | |||||
| Stage III | 123 (18.4) | 882 (5.6) | 78 (15.8) | 195 (13.2) | |||||
| Stage IV | 3 (0.4) | 5 (0.0) | 2 (0.4) | 0 (0.0) | |||||
| Unstageable | 0 (0.0) | 1,751 (11.1) | 0 (0.0) | 21 (1.4) | |||||
| Neoadjuvant systemic | 15 (2.2) | 476 (3.0) | 0.009 | 0.258 | 11 (2.2) | 29 (2.0) | 0.008 | 0.712 | |
| Neoadjuvant radiation | 3 (0.4) | 203 (1.3) | 0.015 | 0.058 | 3 (0.6) | 3 (0.2) | 0.032 | 0.157 | |
Data are shown as mean ± SD or number (percentage). *, statistically significant. RL, robotic lobectomy; MCC, Moffitt Cancer Center; NCDB, National Cancer Database; SD, standard difference; SCC, squamous cell carcinoma.
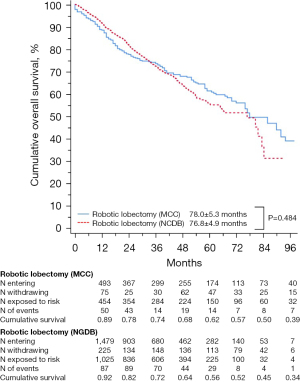
Regarding short-term outcomes, MCC RL patients had a higher nodal yield (14.68±6.81 vs. 11.53±8.25; P<0.001), higher rates of reported mediastinal lymphadenectomy (99.4% vs. 87.3%; P<0.001) and a lower conversion rate (4.1% vs. 9.5%; P<0.001) than NCDB RL patients. There were no differences in postoperative mortality (1.0% for MCC RLs vs. 1.1% for NCDB RLs; P=0.899) or in median LOS (4 days for MCC RLs vs. 4 days for NCDB RLs; P=0.252). Table 4 summarizes the postoperative outcomes of MCC RL vs. NCDB RL groups. Similarly, survival analyses showed no difference in median OS between the groups (78.0±5.3 years for MCC RLs vs. 76.8±4.9 years for NCDB RLs; P=0.484). Five-year OS was 62% for the MCC RLs group vs. 56% for the NCDB RLs group (P=0.524). Figure 3 demonstrates the survival curves of the MCC RL vs. NCDB RL groups.
Table 4
| Postoperative outcome | MCC RL (N=493) | NCDB RL (N=1,479) | HR (95% CI) | P |
|---|---|---|---|---|
| Examined nodes | 14.68±6.81 | 11.53±8.25 | 0.743 (0.621–0.833) | <0.001* |
| Mediastinal lymphadenectomy | 490 (99.4) | 1,291 (87.3) | 0.842 (0.713–0.932) | <0.001* |
| Conversion to open | 20 (4.1) | 141 (9.5) | 2.492 (1.542–4.027) | <0.001* |
| 30-day mortality | 5 (1.0) | 16 (1.1) | 1.067 (0.389–2.929) | 0.899 |
| LOS in days | 4 [3–6] | 4 [3–7] | 1.058 (0.834–1.585) | 0.252 |
Data are shown as mean ± standard deviation, median [interquartile range] or number (percentage). *, statistically significant. MCC, Moffitt Cancer Center; RL, robotic lobectomy; NCDB, National Cancer Database; HR, hazard ratio; CI, confidence interval; LOS, length of stay.
Discussion
Several studies have repeatedly shown that, between the 2 minimally invasive approaches to a lobectomy, VL is associated with higher odds of conversion to thoracotomy compared to RL, and our data support this. Each group’s criteria for conversion to thoracotomy varies considerably. In our institution, in cases of bleeding that cannot be controlled or treated with robotic surgery, then conversion to thoracotomy happens. Other criteria would be injuries to airway that cannot be repaired robotically, as well as severe pleural symphysis from prior surgeries that cause difficulty in entering the pleural space. There are no criteria regarding operative duration beyond which a conversion to open is necessary, as opposed to other institutions (8,9).
In a previous propensity score-matched study of the NCDB database, VL was associated with a 5% difference in conversion rates compared to RL, with predictors of conversion being the procedure occurring within a community hospital, tumor size of 4.5 cm or greater, and an increasing Charlson score (10). Another study analyzing predictors of conversion to thoracotomy for VL found that the hilar calcification score, assessed via CT scans, independently predicts conversion. However, this study was performed in a location with reported endemic histoplasmosis, and so the authors developed a scoring system to determine whether the extent and location of calcifications can be used to predict severity of inflammatory hilar changes. A calcification score of ≤2 had a 17% rate of conversion and was ideal for a VL, whereas patients with a calcification score >3 had a conversion rate of at least 25% (11). Notably, these conversion rates are higher than previous reports; this is likely because of the incidence of histoplasmosis in the area and the fact that the period of the surgeons’ initial learning curves when performing VLs were included within the study period.
Another study by Park et al. explored conversions and showed that 41% of the conversions to thoracotomy were due to hilar nodal anthracofibrosis and hilar adhesions (12). The group performed a retrospective review of chest CT scans, which revealed that hilar calcifications were present in 71% of patients. Our study, which analyzed matched datasets of MCC RLs and NCDB VLs, showed significantly higher conversion rates to OL with NCDB VLs (4.1% with MCC RLs vs. 13.8% with NCDB VLs; P<0.001; Table S4). When matched RL datasets were compared between MCC and NCDB, the conversion rates remained higher for the NCDB dataset (4.1% for MCC vs. 9.5% for NCDB; P<0.001; Table 4). This difference could be explained by the higher volume of cases performed at our NCI-CCC than at the community hospitals and academic institutions that reported their results to NCDB. Additionally, the lower rate of conversion at MCC may be related to the increased experience our surgeons have with this procedure. The NCDB reflects data collected from both community hospitals and academic institutions, whereas our data was sourced from only 1 CCC. However, the specific factors leading to this difference may be worth exploring in a prospective study.
Recently, Servais et al. analyzed the STS General Thoracic Surgery Database (GTSD) to evaluate predictors and consequences of conversion of VL and RL to OL (13); the group found that lower-volume centers had increased rates of conversion for both VL and RL. Conversion occurred in 11% of VLs and 6% of RLs. Independent predictors for VL conversion included clinical stage II/III, preoperative chemotherapy, forced expiratory volume in 1 second (FEV1), body mass index (BMI), and a left-sided resection. An increased BMI, including overweight up to class 3 obesity was a significant predictor for VL conversion to OL. Conversely, independent predictors for RL conversion were clinical stage III, a left-sided resection, and FEV1. Each 5% decrease in percent of predicted FEV1 was associated with conversion for both VL and RL.
The results of this paper are consistent with our data in that there were fewer conversions with RL than with VL; this may be primarily because of case volume and surgeon proficiency in our center, considering that MCC is a high-volume cancer center. Additionally, procedures at MCC are performed by thoracic surgeons, as opposed to the NCDB, which represents a diverse spectrum of surgeons and institutions. Unfortunately, there are no data in the STS GTSD on long-term outcomes, specifically OS and cancer-specific outcomes, which limits the paper’s use for impact evaluation. The inclusion of long-term outcomes made NCDB a more ideal point of comparison than the STS GTSD database for our study.
Concerns regarding adequacy of lymphadenectomy have been longstanding limitations of minimally invasive surgeries. We know that a thorough assessment of the mediastinal and hilar lymph node stations is important in ensuring accurate staging as well as detecting occult metastasis. Recently, the Commission of Cancer (CoC) of the American College of Surgeons defined one of the quality metrics of lung cancer resection as having a specific minimum number of lymph nodes separate from the tissue specimen. This quality metric is defined as the presence of lymph nodes from at least 3 mediastinal lymph node stations and at least one hilar lymph node station. It is yet unknown as to how the adherence to the CoC metric will influence long-term outcomes of lung cancer. This problem on adequacy of lymphadenectomy has been more notable for the VATS approach than the robotic or open approaches because of the rigidity of the instruments, the required experience of the surgeon performing the operation, and clinical factors. A previous analysis of the NCDB and STS GTSD showed that there is, in fact, a lower rate of nodal upstaging for VLs than OLs (14,15). However, it is important to note that the facility where the lobectomy was performed varied throughout the NCDB dataset. If a patient was treated in an academic or a research facility, there were no longer any differences in the nodal upstaging between OLs and VLs. With the advent of the high-definition camera on the robotic platform as well as the wristed instrumentation leading to better maneuverability inside the thorax, the frequency of RLs has increased remarkably. In a propensity score-weighted analysis of a large collaborative database from 2 institutions, namely the Ohio State University Wexner Medical Center (Columbus, Ohio) and the Ruhrlandklinik of the University of Duisburg-Essen (Essen, Germany), it was reported that RLs were associated with similar lymph node assessment and pathologic staging as OLs and that pathologic upstaging in VLs was significantly less frequent than with OLs (16).
Our study is the first to compare a large RL database from an NCI-CCC to a national database, and we found significant differences in the total number of lymph nodes and reported rates of mediastinal lymphadenectomy. Specifically, there was a higher number of total lymph nodes dissected and higher rates of mediastinal lymphadenectomy for the MCC RL group than chronological peers from NCDB who received a RL, VL, or OL. Although our data do not include information on pathologic upstaging after surgery, evidence from a previously published study shows that pathologic upstaging for stage I NSCLC is common and is associated with a greater number of lymph nodes sampled, among other variables (7). Another paper by Zirafa and colleagues compared nodal upstaging between their consecutive cN0 NSCLC patients who underwent OL and RL, and found that although the number of lymph nodes harvested were similar between the OL and RL cohorts, there was a statistically higher number of nodal stations in RL compared to OL, and nodal upstaging was observed in 20.8% of RL patients and in 17.9% of OL patients (17). Other papers however report a conflicting finding. For instance, in a recent article by Haruki et al., the median total LN numbers, though significantly different between RL and VL, were not associated with overall nodal upstaging (18). The article by Shindo et al. mirrors these results in cN0 patients (19). The association between lymph node assessment and pathologic upstaging is still currently controversial and needs to be further studied. Suffice it to say that these differences, if any, in lymph node assessment and upstaging between the different approaches have not been found to translate to differences in long-term oncologic outcomes. It will be interesting to see if the recently released CoC quality metric translates to differences in outcomes in the long run. This was not possible to track in our retrospective study that compares it to NCDB due to a high percentage of missing data.
Generally, our paper’s surgical outcomes were superior to those of NCDB counterparts, in terms of nodal yield, rates of reported mediastinal lymphadenectomy, and conversion rates. Median LOS, 30-day mortality and 5-year OS were similar. Although there is clearly selection bias in the paper, our group suggests that surgical outcomes were superior for several reasons: (I) our institution perform more RLs than most institutions, so there is more experience, (II) there are dedicated OR nursing teams and dedicated OR bedside assistants for robotic thoracic surgical procedures, and (III) there is a dedicated telemetry ward for our postoperative inpatients, and we follow ERAS and clinical pathways for all of our patients.
It is also important to note that the most common postoperative complication in our database is prolonged air leak, and this had an incidence of 20.4% in our cohort of RL patients. Note however that 24.8% of the patients had a diagnosis of COPD, and that 22.8% were either currently smoking or recently quit within 90 days of surgery. These are two of the main risk factors for prolonged air leak after lung resections and could potentially explain the reason behind the relatively higher incidence of prolonged air leak in our cohort, and relatively longer duration of chest tube use.
Long-term survival remains an incredibly important measure of oncologic efficacy for any surgical procedure. Multiple papers have reported excellent survival after RL for early-stage lung cancers. For example, Park reported a 3-year OS of 88% to 97% for stage I NSCLC and 72% for stage II NSCLC (12). Cerfolio et al., on the other hand, reported an estimated 5-year OS of 77% to 83% for stage I NSCLC and 68% to 70% for stage II NSCLC (20). However, there are limited data comparing RL oncologic outcomes with those of VL and OL.
In one retrospective analysis performed at a single CCC, no differences were found in 5-year OS between RL, VL, and OL. On multivariate analyses, RL showed no difference in OS and recurrence-free survival compared to VL and OL (21). Yang et al. found that, among patients with stage I NSCLC who underwent resections, 5-year OS after RL was 78%; no differences were found between VL (74%) and OL (78%) approaches (22). In our study, we demonstrated that the 5-year OS rates were similar for MCC RL patients compared to their OL-, VL-, and RL-matched counterparts from the NCDB. Once more, this confirms the feasibility and noninferiority of the RL approach for lung cancer surgery.
Limitations
One major limitation of our study was its retrospective nature, subjecting it to unmeasured confounding variables. To study lobectomies in a more critical and granular manner, we must perform a prospective randomized trial that to compare VL to RL by both center volume and by stage after adjusting for potential confounding variables and making risk adjustments; then, we must use an appropriate follow-up period so we can make appropriate conclusions regarding clinical implications and, more importantly, oncologic outcomes. There is selection bias in our study, since all three surgeons reserve OLs for large tumors (>8 cm), tumors requiring chest wall resection, and sleeve lobectomies. Thoracoscopic lobectomies (VL) were only performed before the robotic platform became available. In addition, our study only considered the total number of lymph nodes and reports of mediastinal lymphadenectomy. Pathologic upstaging was not evaluated, which would have been a clinically relevant variable in lobectomies for resected NSCLC. Recurrence rates were also not considered. As the patient population was from a database in an NCI-CCC, these may not be generalizable to the population of patients getting pulmonary resections in the community hospitals.
Conclusions
The frequency of RL for NSCLC has remarkably increased over the years, and compared to national benchmarks of OL, VL and RL as presented in the NCDB, RL performed in an NCI-CCC appears to provide improved perioperative outcomes and comparable long-term OS. Although these results need to be verified in a prospective randomized trial with a long-term follow-up to confirm oncologic outcomes, this study provides a preponderance of evidence suggesting the suitability of RL in resectable lung cancers.
Acknowledgments
Editorial assistance was provided by the Moffitt Cancer Center’s Office of Scientific Publishing by Daley Drucker and Gerard Hebert; no compensation was given beyond their regular salaries.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1340/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1340/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1340/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1340/coif). E.M.T. and J.P.F. have had financial relationships with Intuitive Surgical, Inc., in the form of honoraria received as robotic thoracic surgery observation sites and proctors. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval to report this study was obtained from Moffitt Cancer Center’s Scientific Review Committee (MCC #16728, #18761, and #19304) and by our University of South Florida’s Institutional Review Boards (USF IRB #Pro00022263 and Chesapeake IRB #Pro00017745 and #00000790). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States 2016. American Community Survey Reports, ACD-38, U.S. Census Bureau, Washington DC, 2018. (Accessed on 18 June 2022).
- Rajaram R, Mohanty S, Bentrem DJ, et al. Nationwide Assessment of Robotic Lobectomy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;103:1092-100. [Crossref] [PubMed]
- Feczko AF, Wang H, Nishimura K, et al. Proficiency of Robotic Lobectomy Based on Prior Surgical Technique in The Society of Thoracic Surgeons General Thoracic Database. Ann Thorac Surg 2019;108:1013-20. [Crossref] [PubMed]
- Lim E, Batchelor TJP, Dunning J, et al. Video-assisted thoracoscopic or open lobectomy in early-stage lung cancer. NEJM Evidence 2022;1:EVIDoa2100016.
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Bott MJ, Patel AP, Crabtree TD, et al. Pathologic Upstaging in Patients Undergoing Resection for Stage I Non-Small Cell Lung Cancer: Are There Modifiable Predictors? Ann Thorac Surg 2015;100:2048-53. [Crossref] [PubMed]
- Takase Y, Miyajima M, Chiba Y, et al. Causes and management of intraoperative complications in robot-assisted anatomical pulmonary resection for lung cancer. J Thorac Dis 2022;14:3221-33. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Hendriksen BS, Hollenbeak CS, Taylor MD, et al. Minimally Invasive Lobectomy Modality and Other Predictors of Conversion to Thoracotomy. Innovations (Phila) 2019;14:342-52. [Crossref] [PubMed]
- Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [Crossref] [PubMed]
- Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011;35:590-5. [Crossref] [PubMed]
- Servais EL, Miller DL, Thibault D, et al. Conversion to Thoracotomy During Thoracoscopic vs Robotic Lobectomy: Predictors and Outcomes. Ann Thorac Surg 2022;114:409-17. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, Mitchell JD, Onaitis M. Lymph node evaluation by open or video-assisted approached in 11,500 anatomic lung cancer resections. Ann Thorac Surg. 2012;94:347-53. [Crossref] [PubMed]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref] [PubMed]
- Kneuertz PJ, Cheufou DH, D'Souza DM, et al. Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1457-1466.e2. [Crossref] [PubMed]
- Zirafa C, Aprile V, Ricciardi S, et al. Nodal upstaging evaluation in NSCLC patients treated by robotic lobectomy. Surg Endosc 2019;33:153-8. [Crossref] [PubMed]
- Haruki T, Takagi Y, Kubouchi Y, et al. Comparison between robot-assisted thoracoscopic surgery and video-assisted thoracoscopic surgery for mediastinal and hilar lymph node dissection in lung cancer surgery. Interact Cardiovasc Thorac Surg 2021;33:409-17. [Crossref] [PubMed]
- Shindo Y, Miyajima M, Nakamura Y, et al. Number of lymph nodes dissected and upstaging rate of the N factor in robot-assisted thoracic surgery versus video-assisted thoracic surgery for patients with cN0 primary lung cancer. Surg Today 2023;53:428-34. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, Veronesi G, Spaggiari L, Park BJ. The long-term survival of robotic lobectomy for non-small cell lung cancer: a mul-ti-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Kneuertz PJ, D'Souza DM, Richardson M, et al. Long-Term Oncologic Outcomes After Robotic Lobectomy for Early-stage Non-Small-cell Lung Cancer Versus Video-assisted Thoracoscopic and Open Thoracotomy Approach. Clin Lung Cancer 2020;21:214-224.e2. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]


