
Significance of histologic subtype size as a prognostic indicator in stage IA lung adenocarcinoma
Highlight box
Key findings
• Each histologic subtype size has a powerful prognostic role in stage IA lung adenocarcinoma.
What is known and what is new?
• Since a new histologic classification has been proposed, there is some controversy in terms of predicting prognosis in non-mucinous adenocarcinoma.
• We identified that each subtype size has a prognostic role in recurrence. More increasing acinar, micropapillary, and solid subtype sizes and decreasing lepidic subtype sizes affect the prognosis.
What is the implication and what should change now?
• Unique characteristics are pathological heterogeneity in adenocarcinoma. each subtype size is more important than the subtype proportion although it is predominant or non-predominant. We speculated that each subtype size without lepidic lesion may represent invasiveness.
Introduction
Oncology has evolved with medical science’s development. In particular, various studies have been conducted for lung adenocarcinoma in terms of prognosis in thoracic oncology. A new histologic classification was proposed by the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) in 2011 (1). This classification demonstrated six histologic subtypes (acinar, papillary, lepidic, micropapillary, solid, and variant) and used the term “predominant” for all categories.
Lung adenocarcinoma shows heterogeneous patterns and variety in terms of histologic subtypes and their proportion. Since then, numerous studies have been conducted on the clinical relevance of the prognosis following the predominant histologic subtype, and this classification would be categorized into three groups following the prognosis. Lepidic predominant subtype shows favorable (low grade), acinar and papillary show intermediate, and micropapillary and solid show poor results (high grade) (2). Many studies demonstrated more clinical relevance in the histologic subtype for the prognosis rather than the entire tumor size in the early stage of lung adenocarcinoma (3,4).
We wondered about the association between tumor size and prognosis and whether histologic subtype proportion is more valuable than size. We hypothesized that histologic subtype size rather than proportion has a more prognostic impact theoretically. This study investigated the prognostic value following each histologic subtype amount or size in stage IA lung adenocarcinoma. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-913/rc).
Methods
Study population
A total of 1,133 patients with lung adenocarcinoma underwent resection with curative aim at Seoul St. Mary's Hospital and Bucheon St. Mary's Hospital from January 2010 to April 2017. Using electronic medical records (EMRs), patients with pathological tumor sizes of ≤3 cm without lymph node (LN) metastasis were reviewed following the 8th edition of the tumor-node-metastasis (TNM) classification. Patients with neoadjuvant treatment, visceral pleural invasion, and wedge resection were excluded, as well as pathologically, minimally invasive adenocarcinoma, adenocarcinoma in situ, mucinous adenocarcinoma, and incomplete resection. Patients with pathologic reports that did not include the histologic subtypes were excluded. Finally, we reviewed the EMRs and imaging studies. Patients with multifocal ground glass opacity (GGO), synchronous, or metachronous lung cancer were excluded (Figure 1).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of the Catholic University of Korea (HC22WADI0065) approved the study, and written informed consent from the patients was waived because of the retrospective design of the study.
Data collection
A total of 550 patients with pathological stage IA lung adenocarcinoma were reviewed. Preoperative studies included blood sampling, including carcinoembryonic antigen (CEA), pulmonary function test (PFT), chest computed tomography (CT), positron emission tomography-CT (PET-CT), brain magnetic resonance imaging, bone scanning, and bronchoscopy. Every tumor was classified by the IASLC/ATS/ERS classification, and the predominant subtype which occupies the greatest proportion was demonstrated. Each histologic subtype size was estimated by multiplying tumor size by the proportion of the histologic subtype. T-staging was reclassified using the estimated size of the histologic subtype following the 8th edition of the TNM classification.
Follow-up (F/U) was conducted every 3 months for 1 year postoperatively, every 4 months in the second year, and every 6 months thereafter. Chest CT evaluation was conducted on every visit. All patients were followed until recurrence and death or loss of F/U. Recurrence was defined as local or extrathoracic metastasis based on clinical and pathologic evidence.
Statistical analysis
All statistical analyzes were conducted using SPSS version 18 (SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test was used to compare continuous variables and the chi-square test and Fisher’s exact test for categorical variables. Survival curves were generated by the Kaplan-Meier method and log rank using each histologic subtype size. The Cox proportional hazards model was used to determine the association between prognostic factors and recurrence after checking the proportionality assumption. Variables with P values of <0.05 in the univariate analysis were included in the multivariate analysis using forward selection
Results
Table 1 shows the patient characteristics. The median age was 63 (range, 25–82) years, and 227 (41.3%) patients were male. The median maximum standardized uptake value (SUVmax) was 2 (range, 0–17.6). GGO was identified in 363 (66.0%) patients, including pure or mixed nodules. Video-assisted thoracic surgery (VATS) was conducted in 478 (86.9%) patients for the surgical procedure. Lobectomy was conducted in 480 (87.3%) patients.
Table 1
| Characteristic | Total (n=550) |
|---|---|
| Age (years) | 63 [25–82] |
| Male | 227 (41.3) |
| Smoking | 137 (24.9) |
| SUVmax | 2 [0–17.6] |
| GGO | 363 (66.0) |
| Lobectomy | 480 (87.3) |
| VATS | 478 (86.9) |
Data are presented as median [range] or n (%). SUVmax, maximum standardized uptake value; GGO, ground glass opacity; VATS, video-assisted thoracic surgery.
The median tumor size was 1.8 cm (range, 0.3–3 cm) for the pathologic review (Table 2). Poor differentiation was rare (7.3%) because of the early stage. The median number of dissected LNs was 11 (range, 0–53). Segmentectomy or lobectomy without mediastinal LN evaluation was performed following the surgeon’s decision based on the preoperative imaging study if the tumor was a pure GGO or a GGO-dominant lesion. Lymphovascular invasion (LVI) was identified in 121 (22.0%) patients. Following the current 8th TNM staging system, 69 patients were stage IA1 (12.5%), 301 were stage IA2 (54.7%), and 180 were stage IA3 (32.7%).
Table 2
| Characteristic | Total (n=550) |
|---|---|
| Tumor size (cm) | 1.8 [0.3–3] |
| Differentiation | |
| Well | 299 (54.4) |
| Moderate | 211 (38.4) |
| Poor | 40 (7.3) |
| Predominant subtype | |
| Acinar | 231 (42.0) |
| Papillary | 43 (7.8) |
| Lepidic | 244 (44.4) |
| MP | 5 (0.9) |
| Solid | 21 (3.8) |
| Variant | 6 (1.1) |
| Histologic subtype size (cm) | |
| Acinar | 0.66 [0–3] |
| Papillary | 0 [0–2.8] |
| Lepidic | 0.62 [0–2.5] |
| MP | 0 [0–1.89] |
| Solid | 0 [0–2.4] |
| Grade 3 (>20% of high-grade patterns) | 59 (10.7) |
| Margin (cm) | 3.5 [0.2–10] |
| Lymphovascular invasion | 121 (22.0) |
| Number of dissected LN | 11 [0–53] |
| Pathologic stage | |
| IA1 | 69 (12.5) |
| IA2 | 301 (54.7) |
| IA3 | 180 (32.7) |
Data are presented as median [range] or n (%). MP, micropapillary; LN, lymph node.
Acinar (42.0%) and lepidic (44.4%) predominant subtypes were the most common among the histologic subtypes. Otherwise, the micropapillary predominant subtype was rare (0.9%). According to grading system of lung adenocarcinoma (2,4), grade 3 (20% or more of high-grade patterns) was identified in 59 (10.7%) patients. Each histologic subtype size was estimated. The median acinar subtype size was 0.66 cm (range, 0–3 cm) and that of the lepidic subtype was 0.62 cm (range, 0–2.5 cm).
TNM staging was re-conducted using acinar and lepidic histologic size (Figure 2). A significant difference was found in DFI following the acinar histologic subtype size. The acinar histologic subtype size (>2 and ≤3 cm) showed a worse prognosis compared with the acinar subtype negative group. The estimated 5-year disease-free interval (DFI) rate was 96% in the acinar subtype negative group, 92% in acinar tumor size of ≤1 cm, 81.3% in acinar tumor size of >1 to 2 cm, and 71% in acinar tumor size of >2 to 3 cm (P=0.001). A significant difference was also found following the lepidic histologic subtype size. The only thing different from acinar histologic subtype size is that less lepidic tumor size increases the possibility of postoperative recurrence. The estimated 5-year DFI rate was 72.6% in the lepidic subtype negative group, 92.5% in lepidic tumor sizes of ≤1 cm, 96.3% in lepidic tumor sizes of >1 to 2 cm, and 100% in acinar tumor sizes of >2 to 3 cm (P<0.001).
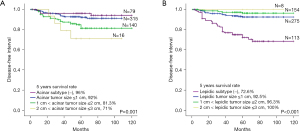
In a median of 60 months of F/U, survival analysis for DFI and overall survival (OS) was conducted to predict prognosis. Male (P=0.026), higher SUVmax (P<0.001), non-GGO pattern on preoperative CT (P<0.001), tumor size (P<0.001), acinar predominant (P=0.005), non-lepidic predominant (P<0.001), micropapillary predominant (P<0.001), solid predominant (P<0.001), poor differentiation (P<0.001), LVI (P<0.001), acinar subtype size (P<0.001), smaller lepidic subtype size (P<0.001), micropapillary subtype size (P<0.001), grade 3 (P<0.001), and solid subtype size (P<0.001) were significant variables in univariate analysis for the DFI (Table 3). Multivariate analysis revealed that non-GGO pattern (P=0.004), LVI (P=0.012), acinar subtype size (P=0.006), smaller lepidic subtype size (P=0.041), micropapillary subtype size (P<0.001), and solid subtype size (P<0.001) were significant prognostic factors for DFI (Table 3). The predominant subtype did not reach significance. Age (P=0.039), male (P=0.029), SUVmax (P=0.045), and non-lepidic predominant subtype (P=0.018) showed significance in multivariate analysis for the OS (Table 4).
Table 3
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Male | 1.826 | 1.074–3.106 | 0.026 | ||||
| SUVmax | 1.225 | 1.159–1.294 | <0.001 | ||||
| Non-GGO pattern | 7.792 | 4.108–14.782 | <0.001 | 2.866 | 1.410–5.827 | 0.004 | |
| Tumor size | 2.293 | 1.449–3.628 | <0.001 | ||||
| Acinar predominant subtype | 2.171 | 1.265–3.725 | 0.005 | ||||
| Non-lepidic predominant subtype | 11.364 | 4.105–31.459 | <0.001 | ||||
| Micropapillary predominant subtype | 11.622 | 4.191–32.227 | <0.001 | ||||
| Solid predominant subtype | 7.988 | 4.018–15.880 | <0.001 | ||||
| Poor differentiation | 3.714 | 1.914–7.207 | <0.001 | ||||
| Lymphovascular invasion | 3.412 | 2.003–5.812 | <0.001 | 2.012 | 1.165–3.475 | 0.012 | |
| Acinar subtype size | 2.247 | 1.585–3.188 | <0.001 | 1.853 | 1.194–2.874 | 0.006 | |
| Lepidic subtype size | 0.117 | 0.054–0.253 | <0.001 | 0.420 | 0.182–0.965 | 0.041 | |
| Micropapillary subtype size | 11.695 | 5.910–23.143 | <0.001 | 7.667 | 3.302–17.804 | <0.001 | |
| Solid subtype size | 3.860 | 2.555–5.829 | <0.001 | 3.529 | 2.061–6.043 | <0.001 | |
| Grade 3 (>20% of high-grade patterns) | 6.554 | 3.794–11.322 | <0.001 | ||||
DFI, disease-free interval; HR, hazard ratio; CI, confidence interval; SUVmax, maximum standardized uptake value; GGO, ground glass opacity.
Table 4
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | 1.062 | 1.016–1.110 | 0.008 | 1.047 | 1.002–1.095 | 0.039 | |
| Male | 3.040 | 1.374–6.726 | 0.006 | 2.446 | 1.096–5.458 | 0.029 | |
| SUVmax | 1.194 | 1.097–1.300 | <0.001 | 1.109 | 1.002–1.226 | 0.045 | |
| Non-GGO pattern | 2.125 | 1.008–4.481 | 0.048 | ||||
| Poor differentiation | 3.587 | 1.351–9.524 | 0.010 | ||||
| Acinar predominant subtype | 2.912 | 1.316–6.444 | 0.008 | ||||
| Non-lepidic predominant subtype | 5.301 | 1.837–15.294 | 0.002 | 3.812 | 1.252–11.604 | 0.018 | |
| Solid predominant subtype | 4.055 | 1.218–13.501 | 0.023 | ||||
| Acinar subtype size | 1.754 | 1.073–2.867 | 0.025 | ||||
| Lepidic subtype size | 0.247 | 0.106–0.572 | 0.001 | ||||
| Solid subtype size | 2.873 | 1.378–5.993 | 0.005 | ||||
| Lymphovascular invasion | 3.127 | 1.475–6.629 | 0.003 | ||||
OS, overall survival; HR, hazard ratio; CI, confidence interval; SUVmax, maximum standardized uptake value; GGO, ground glass opacity.
Discussion
Tumor burden and characteristics are believed as the most important factors for predicting prognosis. Tumor size is represented as a tumor burden, and pathology is represented as tumor characteristics. TNM classification has been continuously updated by the IASLC. The current staging system (8th edition) revised the T descriptor after survival analyzed by 1 cm increments in tumor size and pathologic IA included IA1 (tumor size of ≤1 cm), IA2 (>1 to 2 cm), and IA3 (>2 to 3 cm). Survival was statistically significant for all tumor sizes of 1 cm cut-points. The 5-year survival rate was 91% for IA1, 86% for IA2, and 81% for IA3 (5). However, predicting the prognosis for adenocarcinoma is more complex. The most common feature of early-stage adenocarcinoma is a part-solid nodule. Part-solid nodule included two components in chest CT: GGO and solid portion. Many previous studies have indicated that consolidation to tumor ratio (CTR) is a valuable factor for predicting high-risk groups for recurrence (6,7). CTR is used for surgical procedures for the early stage of lung adenocarcinoma because it presented invasiveness (8). The part-solid nodule with CTR of <0.5 is a good indication for sublobar resection following the Japanese Clinical Oncology Group Trial (9). GGO and solid portion tend to correspond to lepidic patterns and invasive lesions although this relationship is not absolute. The IASLC group proposed T-staging coding for non-mucinous lung adenocarcinoma following the invasive size, not the entire tumor size because a lot of previous data from radiologic and pathologic reports indicated that invasive size is a more valuable predictor than entire tumor size in terms of prognosis (10,11). However, controversy remains. Some studies demonstrated that invasive size was a risk factor for recurrence in stage IA lung adenocarcinoma, while the entire tumor size did not reach significance (12,13). Tsutani et al. reported similar results that invasive size was the risk factor and associated with more malignant behavior (14). Conversely, Itami et al. demonstrated that lung cancer grade, lymphatic and vascular invasion are more significant prognostic factors than tumor invasive size in stage I lung adenocarcinoma (15).
Other studies were also conducted using tumor volume regarding the prognostic impact of tumor burden because of the controversy that whether tumor diameter reflects the true tumor burden and whether tumor volume was more associated with survival (16,17). Takenaka et al. evaluated the solid part volume and revealed the solid part volume as the only prognostic factor (18).
Unique characteristics are pathological heterogeneity in adenocarcinoma. Since 2011, lung adenocarcinoma is classified into five main histologic subtypes (lepidic, acinar, papillary, micropapillary, and solid), and the predominant subtype was used because of a wide variety of distribution and proportion of the histologic subtype. Most of the studies have been conducted for the prognostic role of each histologic subtype and with no controversy about the high malignant potential of micropapillary and solid predominant subtypes. Tumor size did not reach significance in multivariate analysis in previous studies for predicting prognosis following the histologic subtype (19). However, other important prognostic factors should not be overlooked. For example, tumor size is one of the most valuable factors and one of the descriptors in TNM staging and acinar predominant subtype also has a prognostic impact according to previous our study (20). We thought that the acinar predominant subtype has an important prognostic role in stage IA lung adenocarcinoma because micropapillary and solid predominant subtypes are rare in stage IA lung adenocarcinoma. Acinar and lepidic predominant subtypes are very common in the early stage of lung adenocarcinoma. Our study revealed that each predominant subtype and grade 3 did not reach the significance for DFI in multivariate analysis. The most interesting point is that each subtype size represents risk factors for DFI. More increasing acinar, micropapillary, and solid subtype sizes and decreasing lepidic subtype sizes affect the prognosis in multivariate analysis. This indicates that each subtype size is more important than the subtype proportion although it is predominant or non-predominant. Invasive tumor size is more important than entire tumor size in non-mucinous lung adenocarcinoma. Invasive tumor size was calculated by multiplying the overall tumor size by the percentage of invasive patterns (21). This formula was similar to ours. The difference is that this formula excluded the noninvasive lepidic portion, which we included.
We speculated that each subtype size without lepidic lesion may represent invasiveness.
Survival analysis was conducted following each histologic subtype size. A significant difference was found when the analysis was conducted by 1 cm increments in acinar and lepidic tumor sizes like a current T descriptor. We found that survival decreases by increasing 1 cm in acinar tumor sizes and increases by increasing 1 cm in lepidic tumor size. This indicates that all kinds of histologic subtypes have a prognostic role in stage IA lung adenocarcinoma thus predicting prognosis depends on multifactorial causes. Most previous studies tend to focus on micropapillary and solid predominant subtypes because of the high malignant potential (22), and some studies demonstrated that non-predominant micropapillary and solid subtypes are associated with worse prognosis (23,24). However, predicting prognosis may be very difficult due to multifactorial causes according to our study. Other prognostic factors for DFI were solid mass in CT and LVI which are well-known prognostic variables from several studies (19). We also analyzed OS, SUVmax, and non-lepidic predominant subtypes. SUVmax reflects cancer aggressiveness (25). Micropapillary and solid predominant subtypes are associated with higher SUVmax. Higher SUVmax influences more LN metastasis in small-sized lung cancer. Lepidic predominant invasive lung adenocarcinoma has favorable outcomes as a low-grade malignancy. Otherwise, the non-predominant lepidic subtype is associated with a worse prognosis.
This study has several limitations. First, this study was nonrandomized and retrospective in design. Second, there was a small sample size of histologic subtypes to predict prognosis. Especially, survival analysis could not be conducted for micropapillary and solid subtype size. However, the proportion of these subtypes has been originally low. Further study will be required to identify the survival difference following these subtype sizes in large sample sizes. Third, the value of each subtype size was not an actual value. Distribution and proportion widely vary. Measuring the actual value of each subtype size respectively may be difficult. Fourth, mediastinal LN evaluation was omitted in selective cases, including pure GGO or GGO-dominant lesions (26). Mediastinal LN dissection could be omitted in selective cases, but it may affect recurrence postoperatively. Finally, this study was not conducted from multiple centers; thus, selection bias may be inevitable.
Conclusions
Each histologic subtype size may be associated with prognosis in stage IA lung adenocarcinoma. The prognosis may be affected by multifactorial causes. Each histologic subtype size without lepidic subtype may indicate invasiveness (27). Further, each subtype size may be a more valuable parameter for predicting prognosis than proportion and may provide additional information for recurrence in stage IA lung adenocarcinoma.
Acknowledgments
This manuscript was edited by ENAGO.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-913/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-913/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-913/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-913/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of the Catholic University of Korea (HC22WADI0065) approved the study, and written informed consent from the patients was waived because of the retrospective design of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Moreira AL, Ocampo PSS, Xia Y, et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal From the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol 2020;15:1599-610. [Crossref] [PubMed]
- Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013;258:1079-86. [Crossref] [PubMed]
- Jeon HW, Kim YD, Sim SB, et al. Significant difference in recurrence according to the proportion of high grade patterns in stage IA lung adenocarcinoma. Thorac Cancer 2021;12:1952-8. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Matsunaga T, Suzuki K, Takamochi K, et al. What is the radiological definition of part-solid tumour in lung cancer? Eur J Cardiothorac Surg 2017;51:242-7. [Crossref] [PubMed]
- Zhang S, Lin D, Yu Y, et al. Which will carry more weight when CTR > 0.5, solid component size, CTR, tumor size or SUVmax? Lung Cancer 2022;164:14-22. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Handa Y, Tsutani Y, Okada M. Transition of Treatment for Ground Glass Opacity-Dominant Non-Small Cell Lung Cancer. Front Oncol 2021;11:655651. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Moon Y. Is the size of the lepidic component negligible when measuring the size of the tumor to determine the stage of lung adenocarcinoma? J Thorac Dis 2021;13:1434-44. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Mimae T, et al. The prognostic role of pathologic invasive component size, excluding lepidic growth, in stage I lung adenocarcinoma. J Thorac Cardiovasc Surg 2013;146:580-5. [Crossref] [PubMed]
- Itami H, Kawaguchi T, Yoshikawa D, et al. Preference of grade and lymphovascular invasion over invasive size measurement in stage I lung adenocarcinoma. J Clin Pathol 2023;76:486-91. [Crossref] [PubMed]
- Xie HJ, Zhang X, Mo YX, et al. Tumor Volume Is Better Than Diameter for Predicting the Prognosis of Patients with Early-Stage Non-small Cell Lung Cancer. Ann Surg Oncol 2019;26:2401-8. [Crossref] [PubMed]
- Su XD, Xie HJ, Liu QW, et al. The prognostic impact of tumor volume on stage I non-small cell lung cancer. Lung Cancer 2017;104:91-7. [Crossref] [PubMed]
- Takenaka T, Yamazaki K, Miura N, et al. The Prognostic Impact of Tumor Volume in Patients with Clinical Stage IA Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1074-80. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. The Clinical Impact of Solid and Micropapillary Patterns in Resected Lung Adenocarcinoma. J Thorac Oncol 2016;11:1976-83. [Crossref] [PubMed]
- Jeon HW, Kim YD, Sim SB, et al. Prognostic impact according to the proportion of the lepidic subtype in stage IA acinar-predominant lung adenocarcinoma. Thorac Cancer 2021;12:2072-7. [Crossref] [PubMed]
- Anderson KR, Onken A, Heidinger BH, et al. Pathologic T Descriptor of Nonmucinous Lung Adenocarcinomas Now Based on Invasive Tumor Size: How Should Pathologists Measure Invasion? Am J Clin Pathol 2018;150:499-506. [Crossref] [PubMed]
- Mäkinen JM, Laitakari K, Johnson S, et al. Histological features of malignancy correlate with growth patterns and patient outcome in lung adenocarcinoma. Histopathology 2017;71:425-36. [Crossref] [PubMed]
- Chen T, Luo J, Gu H, et al. Impact of Solid Minor Histologic Subtype in Postsurgical Prognosis of Stage I Lung Adenocarcinoma. Ann Thorac Surg 2018;105:302-8. [Crossref] [PubMed]
- Zhao Y, Wang R, Shen X, et al. Minor Components of Micropapillary and Solid Subtypes in Lung Adenocarcinoma are Predictors of Lymph Node Metastasis and Poor Prognosis. Ann Surg Oncol 2016;23:2099-105. [Crossref] [PubMed]
- Tosi D, Pieropan S, Cattoni M, et al. Prognostic Value of 18F-FDG PET/CT Metabolic Parameters in Surgically Treated Stage I Lung Adenocarcinoma Patients. Clin Nucl Med 2021;46:621-26. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Significance of Lymphadenectomy in Part-Solid Lung Adenocarcinoma: Propensity Score Matched Analysis. Ann Thorac Surg 2018;106:989-97. [Crossref] [PubMed]
- Liu C, Wang LC, Chen HS, et al. Outcomes of patients with different lepidic percentage and tumor size of stage I lung adenocarcinoma. Thorac Cancer 2022;13:2005-13. [Crossref] [PubMed]



