
The role of surgeon’s intuition for acute type A aortic dissection in an era of evidence-based medicine: a prospective cohort study
Highlight box
Key findings
• Each point increase in the Surgeon’s Score [1–10] is associated with a 32% increase in the risk of mortality.
What is known and what is new?
• We all rely on intuition to some extent in our clinical practice.
• Surgeon’s instinct can predict the surgical mortality of acute type A aortic dissection.
What is the implication, and what should change now?
• Intuition has a place alongside evidence-based medicine. The duet of intuition and statistics-based scoring systems allows us to make more accurate predictions.
Introduction
Human beings often want to predict the future to gain peace of mind or to aid in decision-making. This is especially true when the future is highly uncertain or potentially dangerous, as in the case of acute type A aortic dissection (ATAAD). Once ATAAD develops, the mortality rate is estimated to be as high as 1% per hour, and about 50% of patients die in two days if they do not undergo surgery (1). Even with surgery, the operative mortality rate is as high as above 10% in most centers (2). The increasingly powerful statistics-based predictive models have gained a growing amount of attention and popularity. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) II is a cardiac risk model for predicting mortality after cardiac surgery (including thoracic aortic surgery) (3). The German Registry for Acute Aortic Dissection Type A (GERAADA) score has been proposed recently to predict mortality in patients who undergo surgery for ATAAD specifically (4). Both prediction systems are used worldwide for risk assessment and as benchmarks for assessing the quality of cardiovascular surgery services.
In contrast to statistics- or evidence-based prediction models, the value of the operating surgeons’ intuition is difficult to quantify. Intuition is not a well-appreciated asset in clinical practice and may not even be recognized by some as a legitimate part of the physician’s decision-making process because it is not clearly rooted in scientific evidence. However, Jerome Groopman wrote in his popular book How Doctors Think that “Clinical intuition is a complex sense that becomes refined over years and years of practice, of listening to literally thousands of patients’ stories, examining thousands of people, and most important, remembering when you were wrong” (5). Whether we admit it or not, we all rely on intuition to some extent in our clinical practice.
This study was designed to evaluate whether surgeons’ intuition plays a role in predicting the operative mortality of ATAAD. It also contributes to our rethinking and understanding of empirical and evidence-based medicine in a broader sense, which is important but yet to be well explored. We present this article in accordance with the STROCSS reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-630/rc) (6).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The prospective cohort study was approved by the Institutional Review Board of Guangdong Provincial People’s Hospital on July 6th, 2021 (ID No. 2019-842H-1), with informed consent not required due to its observational nature. The project has been registered at the Chinese Clinical Trial Registry (ChiCTR) (http://www.chictr.org.cn/), with the approval number ChiCTR2200056715. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data collection
All consecutive ATAAD patients admitted to the Guangdong Provincial People’s Hospital (Guangdong, China) from January 2021 to December 2021 were enrolled. Anthropometric, radiologic, laboratory, and operative data were manually accrued from individual electronic medical records and hospital charts. EuroSCORE II (https://www.euroscore.org) and GERAADA score (https://www.dgthg.de/de/GERAADA_Score) were calculated using the officially issued websites. After admission (before surgery), attending surgeons were asked to rate the mortality possibility on a scale of 1 to 10, with 1 to 3 representing unlikely, 4–6 possible, and 7–10 very likely.
Variables definition
The primary endpoints were operative mortality, which included in-hospital mortality, and 30-day postoperative mortality. Secondary endpoints were stroke, paraplegia, and continuous renal replacement therapy (CRRT). Malperfusion syndrome was defined using the Penn classification (7). Penn Class Aa indicated no ischemia, Penn Class Ab indicated localized ischemia, Penn Class Ac indicated generalized ischemia/circulatory collapse, and Penn Class Abc indicated combined ischemia. Circulatory collapse included any of the following signs: newly reduced left ventricular ejection fraction of 50%; new right ventricular dysfunction; pericardial tamponade; acute coronary ischemia; myocardial infarction; the need for an intra-aortic balloon pump (IABP), or need for veno-arterial extracorporeal membrane oxygenation (ECMO).
Surgical techniques
The surgical strategies have been reported in detail previously (8). Briefly, the standard procedure in our center for ATAAD involving aortic arch included total arch replacement with a four-branched graft and frozen elephant trunk. The preferred cardiopulmonary bypass (CPB) protocol involved right axillary artery cannulation, selective antegrade cerebral perfusion, and moderate hypothermic circulatory arrest.
Statistical analysis
Continuous variables were tested for normality distribution with the Kolmogorov-Smirnov test and were expressed as a mean with standard deviation (SD) or median with an interquartile range (IQR). Independent t-tests were performed for normally distributed variables, or Mann-Whitney U tests otherwise. Categorical variables were presented as frequencies with percentages, and analyzed by Chi-square test or Fisher’s exact test, as appropriate. Least absolute shrinkage and selection operator (LASSO) regression was performed to pick the significant predictors for early mortality, with lambda min as 0.02162034. Logistic regression was performed to calculate the odds ratio (OR) and 95% confidence interval (CI). The area under the curve (AUC) of receiver operating characteristic (ROC) analysis was first performed to assess the accuracy of prediction models. Then, Integrated Discrimination Improvement (IDI) was calculated to explore whether the inclusion of the surgeon’s intuition would improve the performance of the GERAADA score. The larger the IDI, the better the prediction ability of the new model.
R software (version 3.5.1) was used for data analysis. A two-tailed P<0.05 indicated statistical significance.
Results
Our single-center data included 161 ATAAD patients, of which 145 were alive (90.1%) and 16 died (9.9%) postoperatively within 30 days. Baseline characteristics are shown in Table 1. Males accounted for 126 (78.3%), and the median age was 53.6±11.6 years. Compared to the survival group, the mortality group was older (52.8±11.4 vs. 60.6±11.4 years, P=0.010), and had a higher percentage of malperfusion [Penn Class Aa: 83 (57.2%) vs. 2 (12.5%); Penn Class Ab: 33 (22.8%) vs. 4 (25.0%); Penn Class Ac: 15 (10.3%) vs. 2 (12.5%); Penn Class Abc: 14 (9.7%) vs. 8 (50.0%), P<0.001]. The mortality group was more likely to develop aortic regurgitation [44 (30.3%) vs. 10 (62.5%), P=0.022], had a lower platelet count [187.0×109/L (146.0, 235.0)×109/L vs. 139.0×109/L (127.0, 186.5)×109/L, P=0.008], and a decreased creatinine clearance rate [87.5 (63.4, 107.7) vs. 59.4 (49.6, 77.4) mL/min, P=0.005]. All other baseline variables were comparable between the two groups.
Table 1
| Variables | Overall (n=161) | Survival (n=145) | Mortality (n=16) | P value |
|---|---|---|---|---|
| Male, n (%) | 126 (78.3) | 113 (77.9) | 13 (81.3) | 1.000 |
| Age (year), mean (SD) | 53.6 (11.6) | 52.8 (11.4) | 60.6 (11.4) | 0.010 |
| BMI (kg/m2), median [IQR] | 24.5 [22.5, 26.8] | 24.4 [22.5, 27.0] | 25.4 [21.5, 26.2] | 0.865 |
| Penn classification, n (%) | <0.001 | |||
| Penn Class Aa | 85 (52.8) | 83 (57.2) | 2 (12.5) | |
| Penn Class Ab | 37 (23.0) | 33 (22.8) | 4 (25.0) | |
| Penn Class Ac | 17 (10.6) | 15 (10.3) | 2 (12.5) | |
| Penn Class Abc | 22 (13.7) | 14 (9.7) | 8 (50.0) | |
| Aortic regurgitation, n (%) | 54 (33.5) | 44 (30.3) | 10 (62.5) | 0.022 |
| Smoking, n (%) | 29 (18.0) | 27 (18.6) | 2 (12.5) | 0.739 |
| Hypertension, n (%) | 115 (71.4) | 104 (71.7) | 11 (68.8) | 0.777 |
| Coronary artery disease, n (%) | 48 (29.8) | 41 (28.3) | 7 (43.8) | 0.250 |
| Marfan syndrome, n (%) | 4 (2.5) | 4 (2.8) | 0 | 1.000 |
| Hx of cardiovascular surgery, n (%) | 8 (5.0) | 8 (5.5) | 0 | 1.000 |
| Diabetes, n (%) | 4 (2.5) | 4 (2.8) | 0 | 1.000 |
| COPD, n (%) | 23 (14.3) | 22 (15.2) | 1 (6.3) | 0.472 |
| Bicuspid aortic valve, n (%) | 2 (1.2) | 2 (1.4) | 0 | 1.000 |
| White blood cell count (×109/L), median [IQR] | 12.0 [9.8, 14.5] | 12.1 [9.8, 14.7] | 12.0 [10.7, 13.6] | 0.959 |
| Hemoglobin (g/L), mean (SD) | 123.8 (17.6) | 124.1 (18.0) | 120.9 (14.1) | 0.487 |
| Platelet count (×109/L), median [IQR] | 176.0 [143.0, 228.0] | 187.0 [146.0, 235.0] | 139.0 [127.0, 186.5] | 0.008 |
| Creatinine clearance (mL/min), median [IQR] | 82.3 [61.3, 104.7] | 87.5 [63.4, 107.7] | 59.4 [49.6, 77.4] | 0.005 |
| D-dimer (ng/mL), median [IQR] | 8,280.0 [2,550.0, 20,000.0] |
6,530.0 [2,400.0, 20,000.0] |
16,350.0 [6,682.5, 20,000.0] |
0.053 |
| Ejection fraction, median [IQR] | 65.0 [62.0, 67.0] | 65.0 [62.0, 67.0] | 64.5 [62.8, 69.2] | 0.476 |
| Root aneurysm, n (%) | 41 (25.5) | 37 (25.5) | 4 (25.0) | 1.000 |
SD, standard deviation; BMI, body mass index; IQR, interquartile range; Hx, history; COPD, chronic obstructive pulmonary disease.
The surgical management of ATAAD is shown in Table 2. A significantly increased percentage of coronary artery bypass graft (CABG) was observed in the mortality group [8 (5.5%) vs. 7 (43.8%), P<0.001]. Patients in the mortality group underwent longer duration of CPB time [236.0 (200.0, 270.0) vs. 296.5 (223.5, 404.2) min, P=0.011] and aortic cross-clamp (ACC) time [113.0 (93.0, 144.0) vs. 142.0 (119.0, 238.0) min, P=0.003].
Table 2
| Variables | Overall (n=161) | Survival (n=145) | Mortality (n=16) | P value |
|---|---|---|---|---|
| Bentall, n (%) | 43 (26.7) | 36 (24.8) | 7 (43.8) | 0.135 |
| Total arch replacement, n (%) | 151 (93.8) | 135 (93.1) | 16 (100.0) | 0.600 |
| CABG, n (%) | 15 (9.3) | 8 (5.5) | 7 (43.8) | <0.001 |
| CPB time (min), median [IQR] | 241.0 [200.0, 275.0] | 236.0 [200.0, 270.0] | 296.5 [223.5, 404.2] | 0.011 |
| ACC time (min), median [IQR] | 116.0 [94.0, 149.0] | 113.0 [93.0, 144.0] | 142.0 [119.0, 238.0] | 0.003 |
| MHCA, n (%) | 149 (92.5) | 135 (93.1) | 14 (87.5) | 0.339 |
| MHCA time (min), median [IQR] | 19.0 [14.0, 21.0] | 18.0 [14.0, 21.0] | 19.0 [17.0, 21.0] | 0.665 |
CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; IQR, interquartile range; ACC, aortic cross-clamp; MHCA, moderate hyperthermic cardiac arrest.
Surgical outcomes are shown in Table 3. The overall mortality rate was 9.9% (16/161). Stroke rate was 7.5% (n=12) [survival vs. mortality group: 3.4% (n=5) vs. 43.8% (n=7), P<0.001]. Paraplegia rate was 4.3% (n=7) [survival vs. mortality group: 2.8% (n=4) vs. 18.8% (n=3), P=0.022]. CRRT rate was 22.4% (n=36) [survival vs. mortality group: 17.2% (n=25) vs. 68.8% (n=11), P<0.001].
Table 3
| Variables | Overall (n=161) | Survival (n=145) | Mortality (n=16) | P value |
|---|---|---|---|---|
| Early mortality, n (%) | 16 (9.9) | 0 | 16 (100.0) | <0.001 |
| Stroke, n (%) | 12 (7.5) | 5 (3.4) | 7 (43.8) | <0.001 |
| Paraplegia, n (%) | 7 (4.3) | 4 (2.8) | 3 (18.8) | 0.022 |
| CRRT, n (%) | 36 (22.4) | 25 (17.2) | 11 (68.8) | <0.001 |
CRRT, continuous renal replacement therapy.
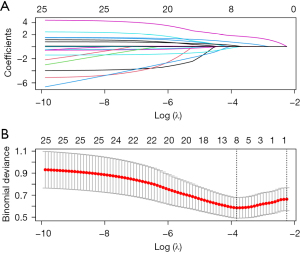
Of interest, there were no significant differences between the survival and mortality groups regarding EuroSCORE II [0.2 (0.1, 0.3) vs. 0.2 (0.2, 0.3), P=0.092]. A significantly higher GERAADA score [0.1 (0.1, 0.2) vs. 0.2 (0.2, 0.3), P=0.002] and Surgeon’s Score [5.0 (2.0, 8.0) vs. 8.0 (7.0, 10.0), P=0.003] were observed in the mortality group (Figure 1), compared to the survival group. The OR for Surgeon’s Score was 1.32 (95% CI: 1.09–1.66, P=0.009), i.e., each point increase in the Surgeon’s Score is associated with a 32% increase in the risk of mortality. Figure 2 shows the feature selection with LASSO regression, which picked the following variables as significant predictors for early mortality of ATAAD: Surgeon’s Score, Penn classification, age, aortic regurgitation, coronary artery disease, chronic obstructive pulmonary disease, platelet count, and ejection fraction. If Penn classification was not included in the LASSO regression, then the following variables would be the significant predictors for early mortality of ATAAD: GERAADA score, Surgeon’s Score, age, aortic regurgitation, coronary artery disease, diabetes mellitus, chronic obstructive pulmonary disease, platelet count, creatinine clearance, and ejection fraction. In both cases, the Surgeon’s Score remains a valuable predictor. As was demonstrated in Figure 3, the AUC for GERAADA score and Surgeon’s Score were 0.740 (95% CI: 0.625–0.854), and 0.710 (95% CI: 0.586–0.833), respectively. We then integrated GERAADA score and Surgeon’s Score using logistic regression. The combined model yielded an AUC of up to 0.761 (95% CI: 0.638–0.884). The IDI was also shown to be improved (4.39%, P=0.005) with the inclusion of the Surgeon’s Score into the GERAADA score.

Next, as demonstrated in Table 4, the cohort was divided into two groups according to the Surgeon’s Score with 7 as the cutoff: the Low-middle group (Surgeon’s Score <7, n=87) and the High group (Surgeon’s Score ≥7, n=74). Compared to the Low-middle group, the High group was significantly older (49.8±11.8 vs. 58.0±9.7 years, P<0.001), more likely to develop malperfusion (Penn classification, P<0.001], less likely to have a past open cardiac surgery [8 (9.2%) vs. 0 (0.0%), P=0.008], had a higher percentage of aortic regurgitation [21 (24.1%) vs. 33 (44.6%), P=0.007], lower platelet count [199.0×109/L (152.0, 247.0)×109/L vs. 162.0×109/L (135.2, 205.8)×109/L, P=0.003], lower creatinine clearance [101.9 (87.8, 121.6) vs. 63.0 (50.6, 74.5) mL/min, P<0.001], and higher level of D-dimer [3,660.0 (1,915.0, 10,125.0) vs. 20,000.0 (7,852.5, 20,000.0) ng/mL, P<0.001]. The mortality rate [3 (3.4%) vs. 13 (17.6%), P=0.003) and CRRT rate [13 (14.9%) vs. 23 (31.1%), P=0.022] were significantly higher in the High Surgeon’s Score group, compared with Low-middle group. Other clinical characteristics did not differ significantly between the two groups.
Table 4
| Variables | Overall (n=161) | Low-middle Surgeon’s Score (n=87) | High Surgeon’s Score (n=74) | P value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Male, n (%) | 126 (78.3) | 71 (81.6) | 55 (74.3) | 0.338 |
| Age (year), mean (SD) | 53.6 (11.6) | 49.8 (11.8) | 58.0 (9.7) | <0.001 |
| BMI (kg/m2), median [IQR] | 24.5 [22.5, 26.8] | 24.6 [22.8, 27.5] | 24.2 [22.2, 26.3] | 0.197 |
| Penn classification, n (%) | <0.001 | |||
| Penn Class Aa | 85 (52.8) | 61 (70.1) | 24 (32.4) | |
| Penn Class Ab | 37 (23.0) | 10 (11.5) | 27 (36.5) | |
| Penn Class Ac | 17 (10.6) | 11 (12.6) | 6 (8.1) | |
| Penn Class Abc | 22 (13.7) | 5 (5.7) | 17 (23.0) | |
| Aortic regurgitation, n (%) | 54 (33.5) | 21 (24.1) | 33 (44.6) | 0.007 |
| Smoking, n (%) | 29 (18.0) | 17 (19.5) | 12 (16.2) | 0.682 |
| Hypertension, n (%) | 115 (71.4) | 60 (69.0) | 55 (74.3) | 0.488 |
| Coronary artery disease, n (%) | 48 (29.8) | 26 (29.9) | 22 (29.7) | 1.000 |
| Marfan syndrome, n (%) | 4 (2.5) | 4 (4.6) | 0 | 0.125 |
| Hx of cardiovascular surgery, n (%) | 8 (5.0) | 8 (9.2) | 0 | 0.008 |
| Diabetes, n (%) | 4 (2.5) | 2 (2.3) | 2 (2.7) | 1.000 |
| COPD, n (%) | 23 (14.3) | 11 (12.6) | 12 (16.2) | 0.652 |
| Bicuspid aortic valve, n (%) | 2 (1.2) | 2 (2.3) | 0 | 0.500 |
| White blood cell count (×109/L), median [IQR] | 12.0 [9.8, 14.5] | 12.1 [9.6, 14.2] | 12.0 [10.1, 14.7] | 0.590 |
| Hemoglobin (g/L), mean (SD) | 123.8 (17.6) | 125.9 (18.9) | 121.4 (15.9) | 0.105 |
| Platelet count (×109/L), median [IQR] | 176.0 [143.0, 228.0] | 199.0 [152.0, 247.0] | 162.0 [135.2, 205.8] | 0.003 |
| Creatinine clearance (mL/min), median [IQR] | 82.3 [61.3, 104.7] | 101.9 [87.8, 121.6] | 63.0 [50.6, 74.5] | <0.001 |
| D-dimer (ng/mL), median [IQR] | 8,280.0 [2,550.0, 20,000.0] |
3,660.0 [1,915.0, 10,125.0] |
20,000.0 [7,852.5, 20,000.0] |
<0.001 |
| Ejection fraction, median [IQR] | 65.0 [62.0, 67.0] | 65.0 [62.0, 66.5] | 64.0 [62.2, 67.8] | 0.993 |
| Root aneurysm, n (%) | 41 (25.5) | 25 (28.7) | 16 (21.6) | 0.365 |
| Operative data | ||||
| Bentall, n (%) | 43 (26.7) | 25 (28.7) | 18 (24.3) | 0.594 |
| Total arch replacement, n (%) | 151 (93.8) | 79 (90.8) | 72 (97.3) | 0.110 |
| CABG, n (%) | 15 (9.3) | 8 (9.2) | 7 (9.5) | 1.000 |
| CPB time (min), median [IQR] | 241.0 [200.0, 275.0] | 236.0 [199.0, 270.5] | 245.5 [207.2, 282.2] | 0.194 |
| ACC time (min), median [IQR] | 116.0 [94.0, 149.0] | 120.0 [90.0, 151.0] | 112.0 [97.2, 143.2] | 0.845 |
| MHCA, n (%) | 149 (92.5) | 79 (90.8) | 70 (94.6) | 0.549 |
| MHCA time (min), median [IQR] | 19.0 [14.0, 21.0] | 18.0 [13.5, 21.0] | 19.0 [16.0, 22.0] | 0.542 |
| Outcomes, n (%) | ||||
| Early mortality | 16 (9.9) | 3 (3.4) | 13 (17.6) | 0.003 |
| Stroke | 12 (7.5) | 3 (3.4) | 9 (12.2) | 0.067 |
| Paraplegia | 7 (4.3) | 2 (2.3) | 5 (6.8) | 0.249 |
| CRRT | 36 (22.4) | 13 (14.9) | 23 (31.1) | 0.022 |
SD, standard deviation; BMI, body mass index; IQR, interquartile range; Hx, history; COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; MHCA, moderate hyperthermic cardiac arrest; CRRT, continuous renal replacement therapy.
Discussion
Clinical decision-making is a core competency of physicians. It involves two different types of mental processes, which exist along a continuum ranging from purely intuitive to purely analytical. In practice, depending on the complexity of the situation and the surgeon’s experience/expertise, individual decisions are usually made by a combination of the two. The proportions may not always be consistent, but intuition is certainly involved. Socrates once wrote that ‘I decided that it was not wisdom that enabled poets to write their poetry, but a kind of intuition’ (9). Instead of evading it, we would better face it head-on and even study how to take advantage of it. Surgery is a challenging and dynamic practice, and this is especially true in the management of ATAAD. Preoperatively, we routinely quote the probability of death for an ATAAD repair to patients and their families. Our group was interested in knowing how accurate such estimates were, which prompted the present study. Strikingly, we found that the surgeon’s intuition is a good predictor for operative mortality of ATAAD. In fact, the surgeon’s intuition enhances the accuracy of the traditional scoring system.
The word “intuition” comes from the Latin word “intuitiō” a contemplation, “intuērī” to gaze upon, and from “tuērī” to look at, and involves feelings you have “that something is true even when you have no evidence or proof of it” (Collins Dictionary 2015). Intuition can be described as “knowing without knowing how” or “Thinking Without Thinking”. Gut feeling, “sixth sense”, “hunch”, etc., are all related to intuition. These abstract terms contribute to the notion that intuition is associated with something mysterious. Therefore, intuition is often not a valued method in clinical practice. However, according to the Dual Process Theory (10), analysis and intuition should not be seen as two separate types of cognition but rather, as the endpoints on a sliding scale where cognition includes both analysis and intuition. The modern neuroscientist Jean-Pierre Changeux claimed that our intuition is constantly being refined and curated. The cerebral cortex is home to millions of uncommitted neurons that fuse as new memories are formed. However, the degree of fusion is not permanent but depends on subsequent utilization and the resulting reinforcement. So, intuition is then not based on innate patterns of behavior, but on previous experiences that have been reinforced to the point where assumptions can be made even in the absence of apparent logic (11).
A study in 2004 tested the clinical intuition of physicians in children’s intensive care units in Boston and Zurich (12). The trial asked clinicians to predict the likelihood of a bacterial infection. Predictions were updated daily until blood culture results were available or antibiotic therapy was started. Retrospective data on the actual presence of infection were collected after antibiotic therapy was discontinued. The study showed that clinicians have an overall discriminatory ability of 0.88, with 1 being a perfect prediction. A priori predictions made by clinicians based on experience and instinctive sensations were shown to be very accurate.
There are several reasons surgeons’ intuition can accurately predict a patient’s death in ATAAD. First, as the surgeons review the patient’s information, they immediately identify features that warrant vigilance consistent with previous tragic lessons, such as coronary involvement, pericardial tamponade, carotid artery occlusion, etc. With pattern recognition, surgeons quickly determine the patient’s prognosis with accepted accuracy. The surgeon’s intuition is also a kind of data interpretation, except that this interpretation becomes so rapid with the accumulation of experience that it seems the thinking process is not involved. Second, there is a limit to the number of variables that can be incorporated into any computational model, whereas a surgeon can assess a patient in a more comprehensive manner. In addition to the disease per se, many external factors, such as frailty, financial status, education, family support, compliance, etc., can all affect a patient’s outcome (13,14). These, however, are not included in most prognostic models. Third, surgeons know their patients well, and know themselves better, theoretically. The surgeon’s psychological and physical state can also affect the outcome of the procedure. An interesting study has even found an increase in patient mortality on the day of a surgeon’s birthday (15). Likely, a surgeon will also factor in the capability of the operation team to modify his/her predictions, as ATAAD is highly demanding of team cooperation. Fourth, the surgical techniques and outcomes for ATAAD vary from center to center. For example, the mortality rate in the current cohort is 9.9%, while some other centers report rates from 20–30% (16). When EuroSCORE II and GERAADA score are the same for a specific patient, physicians can make reasonable corrections based on the level of care at their center. Fifth, it is crucial to realize that the surgeon’s intuition and scoring system complement each other. Many of the variables on which mathematical predictive models are built are derived from physicians’ intuition to begin with. Surgeons can also help improve their prediction accuracy by learning the established model adequately. Combining the two enables more reasonable predictions. Sacks et al. have compared surgeons’ intuition and decisions with and without the use of a risk calculator. Exposure to the risk calculator made surgeons’ judgments about surgical risk less variable and more accurate (17).
Limitations
Our analysis should, however, be interpreted in the context of several limitations. First, as a large tertiary care hospital, we are all very familiar with these classic predictive models such as EuroSCORE II and GERAADA score. Although it may be unrealistic, asking those surgeons to make predictions as if they were completely unaware of the existing predictive models may enhance the independent role that intuition plays in clinical decision-making. Second, this study did not set up repeated measures for an individual patient, i.e., comparing the stability of risk assessment for the same patient by different physicians, such as senior doctors versus junior doctors. This may reveal more in-depth information.
Conclusions
Intuition certainly has a place alongside evidence-based medicine. The duet of intuition and statistics-based scoring systems allows us to make more accurate predictions, potentially resulting in more rational clinical decisions.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROCSS reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-630/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-630/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-630/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-630/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The prospective cohort study was approved by the Institutional Review Board of Guangdong Provincial People’s Hospital on July 6th, 2021 (ID No. 2019-842H-1), with informed consent not required due to its observational nature. The project has been registered at the Chinese Clinical Trial Registry (ChiCTR) (http://www.chictr.org.cn/), with the approval number ChiCTR2200056715.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Braverman AC. Acute aortic dissection: clinician update. Circulation 2010;122:184-8. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734-44; discussion 744-5. [Crossref] [PubMed]
- Czerny M, Siepe M, Beyersdorf F, et al. Prediction of mortality rate in acute type A dissection: the German Registry for Acute Type A Aortic Dissection score. Eur J Cardiothorac Surg 2020;58:700-6. [Crossref] [PubMed]
- Groopman J. How Doctors Think. Boston: Houghton Mifflin Company; 2007.
- Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [Crossref] [PubMed]
- Augoustides JG, Szeto WY, Desai ND, et al. Classification of acute type A dissection: focus on clinical presentation and extent. Eur J Cardiothorac Surg 2011;39:519-22. [Crossref] [PubMed]
- Ma WG, Zhu JM, Zheng J, et al. Sun's procedure for complex aortic arch repair: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:642-8. [PubMed]
- "Socrates Quotes." Quotes.net. STANDS4 LLC, 2023. Web. 26 Oct. 2023. Available online: https://www.quotes.net/quote/950
- Bronstein MV, Pennycook G, Joormann J, et al. Dual-process theory, conflict processing, and delusional belief. Clin Psychol Rev 2019;72:101748. [Crossref] [PubMed]
- Patel SB. Is the role of instinct important in medicine? J R Soc Med 2014;107:464-5. [Crossref] [PubMed]
- Fischer JE, Harbarth S, Agthe AG, et al. Quantifying uncertainty: physicians' estimates of infection in critically ill neonates and children. Clin Infect Dis 2004;38:1383-90. [Crossref] [PubMed]
- Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. Lancet 2019;394:1376-86. [Crossref] [PubMed]
- Bucholz EM, Sleeper LA, Goldberg CS, et al. Socioeconomic Status and Long-term Outcomes in Single Ventricle Heart Disease. Pediatrics 2020;146:e20201240. [Crossref] [PubMed]
- Kato H, Jena AB, Tsugawa Y. Patient mortality after surgery on the surgeon's birthday: observational study. BMJ 2020;371:m4381. [Crossref] [PubMed]
- McClure RS, Ouzounian M, Boodhwani M, et al. Cause of Death Following Surgery for Acute Type A Dissection: Evidence from the Canadian Thoracic Aortic Collaborative. Aorta (Stamford) 2017;5:33-41. [Crossref] [PubMed]
- Sacks GD, Dawes AJ, Ettner SL, et al. Impact of a Risk Calculator on Risk Perception and Surgical Decision Making: A Randomized Trial. Ann Surg 2016;264:889-95. [Crossref] [PubMed]



