Recognition of “aggressive” behavior in “indolent” ground glass opacity and mixed density lesions
Introduction
Ground-glass opacity (GGO) is defined as slightly increased density on high-resolution computed tomography (CT), in which the bronchial and vascular textures are still visible (1,2). Generally, ground-glass nodule (GGN) is subdivided in pure ground glass nodules and part-solid glass nodules according whether have solid part (2). According to the 2011 IASLC/ATS/ERS classification (3), pathologically such areas may be associated with atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), and minimally invasive adenocarinoma (MIA) and even invasive adenocarcinomas. Currently, due to the increased use of computed tomography and lung cancer screening trials, and advances in radiologic techniques, such as high-resolution CT (HRCT) scanning and the widespread use of low-dose helical CT screening, the detection of GGO-manifesting lung cancer is increasing, and has become a focus of attention (4-6).
Generally, GGO lesions have been unanimously considered "relatively" less aggressive biologically inert. It has already been well proven that GGO-manifesting lung cancers have excellent long-term outcomes (7,8). Cho et al. documented a 98.6% 5-year overall survival rate for pure GGO and 95.5% in a mixed GGO group and the 3-year recurrence-free survival (RFS) was also more than 95% (9). Lymph node metastasis, one of the most important issues in lung cancer biological behavior, patients with GGO-dominant tumors rarely had lymph node metastases. Suzuki et al. found no lymph node involvement in GGO-dominant lung cancer (8). However, nodal metastasis has still been seen as a phenomenon in several prior studies of GGO resections. Kodama et al. found lymph node involvement in 4 (4/52) tumors with a <50% GGO component (10). Tsutani et al. also have found lymph node involvement in 2 (2/239) tumors with a >50% GGO component (11). These data, interestingly, have posed a practical dilemma concerning recognition and removal of lymph nodes in a GGO operation. Do GGO dominant lung cancers still need systematic lymph node dissection and should sampling be done as a routine procedure in lung cancer surgery?
As such, it is truly the case that there is clinical significance in being able to recognize the “aggressive” members in these GGO-manifesting adenocarcinomas. In a background where either non-selective lymph node dissection or systematic nodal sampling is still advocated for non-small-cell lung cancers, the accurate recognition of nodal status will undoubtedly help to define a reasonable range for pursuing lymph node removal, especially for these GGO-representing adenocarcinomas. Therefore, this study aimed to determine related risk factors of lymph node metastasis in GGO cases based on a large-scale case series.
Methods
Inclusion criteria
All pulmonary resections for GGO lesions at Shanghai Pulmonary Hospital in China between January 2008 and December 2014 were reviewed. We reviewed among all CT scans for reports including the words “GGO”, “GGN”, “nonsolid nodule”, “part-solid nodule”, “ground-glass opacity” or “ground-glass nodule”. In addition, we established the pathology-database of adenocarcinomas for those patients, after reviewing each patients. The inclusion criteria in the present study was: (I) GGO lesions confirmed by high-resolution computed tomography (HRCT); (II) histologically confirmed adenocarcinomas; (III) either systematic lymph node dissection or sampling was performed; and (IV) ≤3 cm in tumor size. Patients were excluded if they had either distant metastasis, history of lung cancer or neoadjuvant chemotherapy. Ultimately, 867 patients were enrolled into the present study. This study was conducted in accordance with the principles of the Declaration of Helsinkiand approved by the Institutional Review Board of Shanghai Pulmonary Hospital (k16-167), and all patients provided written informed consent for surgery.
Preoperative examinations
All the patients underwent routine preoperative evaluations such as cardio-pulmonary tests, brain magnetic resonance imaging or enhanced CT, whole-body bone scan and abdominal ultrasonography or CT as well, to rule out possible metastasis. Serum tumor markers, including carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) were routinely examined.
High-resolution CT evaluation
The HRCT scans were performed by using Somatom Definition AS (Siemens Medical Systems, Germany) and Brilliance (Philips Medical Systems, Netherlands) with 120 kVp and 100–160 mAs. All image data were reconstructed with a section thickness of 0.7 to 1.5 mm. The lung was photographed with a window level of −500 to −700 Hounsfield units and a window width of 1,000 to 2,000 Hounsfield units. All the HRCT images were reviewed and related variables were measured and recorded by two observers, both have at least five years of experience, which included information on lesion size, pleural retraction, air bronchogram sign, proportion of solid components, etc. Air bronchogram is defined as air-filled bronchus is visible in GGN (12). A pleural retraction was defined as a linear attenuation heading toward the pleura or the major or minor fissure from a GGN (12-14). The evaluated the lesion on the lung window were the maximum diameters of the GGO and solid part (consolidation); the solid component was defined as an area of increased opacification that completely obscured the underlying vascular markings. GGO was defined as an area of a slight homogeneous increase in density that did not obscure the underlying vascular markings. We record the longest diameter of the solid part and the GGO lesion, then according to the different proportions of GGO (solid part/GGO size); all the lesions were reclassified into three groups: group I, pure GGO; group II, 1% to 50%; and group III, 50% to 79%, more than 80% were considered as solid inert, and don’t include in this study. The two observes were unaware of the lymph nodal status and pathologic staging. Discrepancies in interpretation between observers were resolved by consensus.
Operations
For peripheral nodules, a wedge resection was performed and then sent for frozen section. This was converted to subsequent segmentectomy or lobectomy when the frozen section showed a tumor of an invasive nature. For deeply localized lesions, preliminary segmentectomy or lobectomy was initially scheduled. Systematic lymph node dissection was performed in all patients with “invasive carcinomas” and optional systematic lymph node sampling if the frozen section showed “minimally-invasive adenocarcinomas” and AIS. At our institution, systematic lymph node dissection requested removal of at least three stations and more than six mediastinal lymph nodes; hilar nodes were also routinely harvested. Systematic lymph node dissection should remove all lymphatic tissues within the defined anatomic landmarks of stations 2, 4, and 7 to 12 on the right and stations 4 to 12 on the left, and optional systematic lymph node sampling was requested removal of at least three stations according to the classification of the American Thoracic Society.
Pathology
All tumor sizes were re-measured on the fresh specimens. Tumor histological subtypes were recorded by two pathologists independently, both have at least five years of experience. The histological subtypes standard was according to the new IASLC/ATS/ERS multidisciplinary lung adenocarcinoma classification, which were respectively AIS, minimally invasive adenocarcinoma (MIA), lepidic predominant adenocarcinoma (LPA), invasive mucinous adenocarcinoma (IMA), acinar predominant, papillary predominant, micropapillary predominant and solid predominant (3). The lesions from 2008–2011 were classified again. Further, visceral pleural invasion (VPI) was categorized into three groups by elastin staining (Victoria blue-van Gieson) (15): PL0 represents a tumor either within the sub-pleural lung parenchyma or invading superficially into the pleural connective tissue beneath the elastic layer; and PL1 refers to a tumor that invades beyond the elastic layer but is not exposed on the pleural surface, and PL2 was a tumor that is exposed on the pleural surface but does not involve adjacent anatomic structures. In this study, pathologic PL0 status was defined as without VPI, whereas pathologic PL1 and PL2 were defined as with VPI. Information regarding lymph node metastasis was recorded as well.
Follow-up
All patients were closely followed up after their operations. During the first post-operative two years, all the patients were re-visits every three months. The schedules were twice on the third post-operative year and then every year thereafter. Patients were contacted every six months between the intervals by phone call or mail to gather information on health status and disease recurrence.
Data collection and statistical analysis
All clinical information was collected from the original medical records. SPSS Statistics 20 software (IBM Corp, Armonk, NY, USA) was utilized for subsequent analysis. t-test was used to compare the serum tumor markers between lymph nodal normal and metastasis patients. In univariate analysis, relationships between the clinicopathologic factors and postoperative nodal metastasis were analyzed with Pearson’s chi-squared test or Fisher’s test. The receiver operating characteristic (ROC) analysis was performed to set the cut lines of different factors. Multivariate logistic regression was performed on those factors which were significant from univariate analysis. A P<0.05 was considered statistically significant.
Results
General information
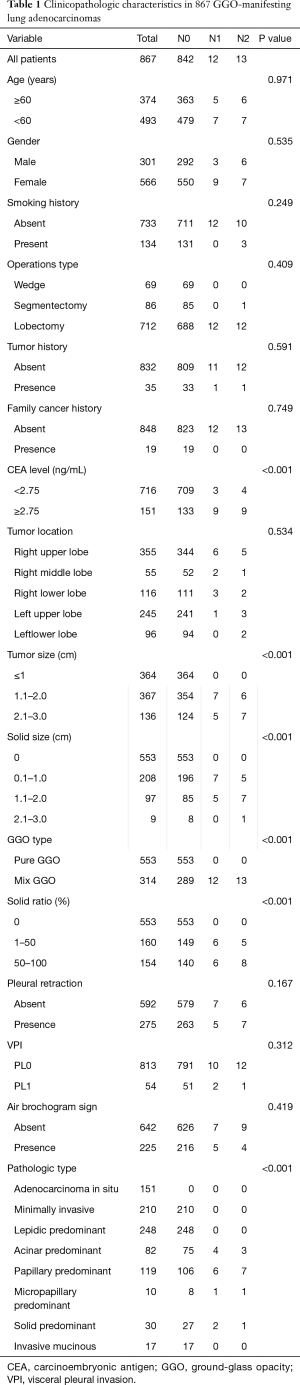
Of the 867 patients eligible for this study, there were 566 (65.3%) females and 301 (34.7%) males aged between 26 and 80 (median 56) years old. 134 (15.5%) patients had a history of smoking and the mean smoking index (pack years) was 326 [100–1,600] (Table 1). Tumor history was found in 35 patients, and among them 7 had breast cancers, 7 thyroid cancers, and one each for ovarian, prostate, stomach, rectal and colon cancer; 10 patients had hysteromyomas and 16 had benign thyroid nodules.
Full table
Pathology
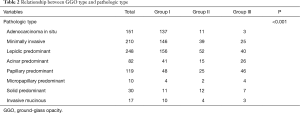
Histological subtypes of these 867 adenocarcinomas were 151 (17.4%) AIS; 210 (24.2%) MIA; and 506 (58.4%) invasive adenocarcinomas. Respectively by histology, these included: 248 (28.6%) cases of LPA, 82 (9.6%) acinar predominant, 119 (13.7%) papillary predominant, 10 (1.2%) micropapillary predominant, 30 (3.4%) solid predominant and 17 (2.0%) invasive mucinous. VPI was found in 54 (6.2%) of the total case series, where all were at a PL1 level; there were no PL2 cases. Histological subtypes of the pure GGO were 137 (24.8%) AIS; 146 (26.4%) MIA; and 506 (48.8%) invasive adenocarcinomas. Respectively by histology, these included: 156 (28.2%) cases of LPA, 41 (7.4%) acinar predominant, (8.7%) papillary predominant, 4 (0.7%) micropapillary predominant, 11 (2.0%) solid predominant and 10 (1.8%) invasive mucinous (Table 2). In total, 9,364 (average 10.8) lymph nodes were removed, which included 5,822 mediastinal nodes and 3,542 hilar nodes. There were in total 61 nodes (31 mediastinal and 30 hilar) in 25 patients that were positive, based on which these 25 tumors were staged as IIA in 12 and IIIA in 13 cases.
Full table
Among the 25 node positive cases, 7 were acinar-predominant, 13 papillary predominant, 2 micropapillary predominant and 3 solid predominant. Only 3 cases with VPI. The incidence of lymph node metastasis was zero in sub-centimeter tumors, 3.5% (13/367) in tumor sized 1–2 cm, and 8.8% (12/136) if the tumor size was 2–3 cm. Of the 13 pN2 patients, 9 had simultaneous N1 nodal involvement and 4 had nodal skip metastasis without hilar involvement. Additionally, single mediastinal nodal station involvement was seen in 8 patients, 2 stations in 4 patients, and multi-station involvement was seen in 1 case. Among all the metastatic mediastinal nodes, the most frequently involved was the subcarinal station (10 positive nodes in 30 harvested, dispersed to 5 patients).
Serum tumor markers
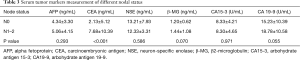
There was a significant difference in mean preoperative serum CEA levels between those with and without lymph node metastasis (7.7±10.4 vs. 2.1±5.1 ng/mL, P<0.001). No significant differences were found for the other serum tumor markers,, including AFP, neuron-specific enolase (NSE), β-2microglobulin (β-MG), carbohydrate antigen 15-3 (CA15-3), and carbohydrate antigen 19-9 (CA19-9) (Table 3). Ten out of these 25 cases had serum markers re-evaluation during follow-up, with all of them showing a reduction of postoperative serum CEA.
Full table
Radiological measurement
Of the cases, 553 (63.8%) presented as pure GGOs and 314 (36.2%) mix GGOs on thin-section CT scans. Among the latter, 160 cases were grouped into group II and 154 into group III. Pleural retraction was noticed in 275 (31.7%) patients, 225 (26.1%) had an air bronchogram sign. There were no patients with pure GGO that had lymph node metastasis. The incidence of lymph node metastasis was 5.8% (12/208) in solid sized 0.1–1 cm, 12.4% (12/97) in solid size 1.1–2 cm, and 11.1% if the solid size was 2.1–3 cm. The 25 patients with lymph node metastasis were all in the mixed GGO manifestations, among them 11 (11/160) were Group II and 14 (14/154) were Group III. And the lymph node metastasis between Group II and Group III have statistical significance (P=0.015), Additionally, 12 GGOs had pleural indentation and an air bronchogram sign was visible in 9 cases.
Follow-up outcomes
Follow up information was obtained from all patients. Of the 25 node positive cases, 16 patients had operations in recent 12 months, 8 patients in the last two years and only one did it at two years ago. Two of these patients, both at an N2 nodal status, died at the post-operative 23rd month and 36th month, respectively. They both presented as mixed GGO lesions with a >50% solid ratio and had abnormal serum CEA levels of 7.42 and 5.48 ng/mL, respectively. No recurrence was noticed in the rest of patients at the date of follow-up.
Univariate analysis toward lymph node metastasis
Univariate analyses showed that a solid ratio, tumor size, solid size and CEA level were significantly associated with lymph node metastasis (Table 1). Figure 1 showed the ROC curve generated based on distributions of each candidate variable toward lymph nodal metastasis. The area under the ROC curve values for preoperative serum CEA level, tumor size, and solid/GGO ratio were 0.809 [95% confidence interval (CI): 0.780–0.835], 0.770 (95% CI: 0.741–0.798) and 0.797 (95% CI: 0.769–0.823), respectively. The optimal cutoff points were: 2.75 ng/mL CEA level, 1.1 cm for tumor size of the entire lesion and 24% for solid GGO ratio, which represent a higher CEA level (>2.75 ng/mL), bigger size (>1.1 cm) and lesion with solid ratio larger than 24% has high risk for lymph node involvement. There were no significant differences in distribution of node status according to age, gender, smoking history, tumor location, pleural invasion, air bronchogram sign, tumor history and family history of cancer.

Multivariate analysis toward lymph node involvement
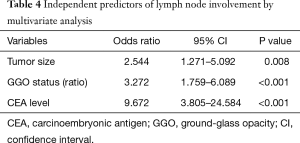
Multivariate analysis confirmed three independent predictors of nodal metastasis: preoperative serum CEA level (OR: 9.709, P<0.001), tumor size (OR: 2.554, P=0.008), and solid ratio of GGO (OR: 3.289, P<0.001) (Table 4).
Full table
Discussion
GGO-dominant lung cancers, although defined as malignant, have been unanimously considered inert based on the following observations. Firstly, the doubling time of this special category of tumor was longer than solid lung cancers. Hasegawa et al. suggested that pure GGO, semi-solid lesions and solid lesions had an average doubling time of 813, 457, and 149 days, respectively (16). Secondly, both lymphatic and remote metastatic rates were extremely low at the first time of diagnosis. In the case series of Hashizume and Ohde et al., there was no lymph node involvement when a GGO area occupied >50% of lesions (17,18). And thirdly, the long-term prognosis of these GGOs was optimal. Cho et al. even documented a 98.6% 5-year overall survival rate of pure GGO and 95.5% in a mixed GGO group (9).
The “aggressive” side, however, in the form of nodal metastasis was not rare in these GGO lesions. The nodal metastasis rate was 2.9% (25/867) in the present total case series. It was even higher in mixed GGO cases where the number reached 6.9% (11/160) if a solid part occupied a <50% area, and was 9.1% (14/154) if the solid component was of a>50% area. Several prior studies also show the same conclusion. Tsutani et al. noticed lymph node involvement in 2 (2/239) patients who had a >50% GGO component (11). Aoki et al. also listed one case of node metastasis in his 24 case series with a >50% GGO component (19). All these data highly suggest that consideration of lymph nodes is also a critical issue in a certain subgroup of these “inert” lung cancers.
One of the most prominent risk factors toward lymph node metastasis was tumor diameter, as demonstrated in both the present and past studies. In this study, no lymph nodal metastasis, after a comprehensive review of the literature showed that for sub-centimeter GGO lesions, there was no evidence of lymph node metastasis. This empirical finding now has additional support from the ROC curve in the present study, which statistically agreed with the inert nature of the sub-centimeter tumors. With an increase in entire size, the rate of lymph node metastasis increased to statistical significance. In our study, it was 3.5% (13/367) for 1–2 cm tumors and 8.8% (12/136) for 2–3 cm tumors (P<0.05).
Using serum CEA level as a predictor requires further investigation. The other preoperative serum tumor markers, such as AFP, etc., showed no statistical significance. In our study, the serum CEA level is related to the lymph node metastasis, and the cut-off value of serum CEA level is 2.75 ng/mL, even this level was within the normal range. serum CEA level has been as a predictive factor for lymph node metastasis in many studies (20-22). Prior studies from Kioke et al. have evaluated both a CEA level of >5.0 and >3.5 ng/mL were associated with lymph nodal metastasis in non-small-cell lung cancers, which included lung cancers of a GGO manifestation (20). In that study, the rate of lymph node metastasis was 13.8% and 6.3% when serum CEA was >5 and <5 ng/mL, respectively. Therefore, cancerous GGO lesions with an enhanced serum CEA level had a possibly higher prevalence of lymph node metastasis, even when the serum CEA level was within the normal range (0–5 ng/mL).
The relationship between postoperative histological type and lymph node involvement was also examined in the present study. In present study, all 25 cases with lymph node metastasis were pathologically invasive adenocarcinoma. Interestingly, no metastasis was observed in LPA and this finding was consistent with some previous studies (22-24). In addition, Russell et al. also proposed that there was no lymph node metastasis in AIS, MIA or IMA cases, which was exactly the same as found in our study. The underlying clinical significance is that such cases most possibly had very minimal chance of nodal involvement and therefore could be possibly waive nodal dissection. A definite conclusion warrants further investigation and evidence.
GGO density is obviously an important suggestion of invasiveness. In our study, all 25 patients with nodal metastasis were in mixed GGO manifestations and among them 11 (11/160, 6.9%) were group II and 14 (14/154, 9.1%) were group III, the lymph node metastasis between group II and group III have statistical significance (P=0.015). And according to the ROC curve, which represent that lesion with solid ratio larger than 24% has high risk for lymph node involvement. Cho and his colleges think that consolidation-to-tumor ratio less than 0.25 should be considered as pure GGO (9). Matsuguma et al. showed that a greater GGO ratio was associated with a lower nodal metastasis rate, the authors divided GGO into five groups according to the proportion of the GGO area: 0%, 1–25%, 26–50%, 51–75% and 76–100%, and found the rate was 44.4% (12/27), 18.2% (4/22), 22.2% (2/9), 0% (0/11) and 0% (0/15), respectively (25). Aoki et al. noted that the rate was 26.0% (19/73), 16.7% (5/30) and 4.2% (1/24) in accordance to the proportion of GGO area at <10%, 10–50% and >50% (19). Together, this data highly suggested that for mixed GGO lesions, the definite size and serum CEA level should be incorporated while planning nodal removal.
One of the most important clinical significances of finding nodal metastasis lies in possible survival prediction and subsequent medical intervention. During the relatively inadequate follow-up period of the present study (median 15 months), there were already two death cases subsequent to the “aggressive” lymph node involvement. In Cho and associates’ case series, the 5-year overall survival rate was 98.6% in the pure GGO group and 95.5% in the mixed GGO group (P=0.663) (9). We believe that among the prior studies, there was also heterogeneity among GGOs. As such, recognition of the aggressive biological behavior helps define and properly treat these cases.
The present study has several limitations. It was a retrospective study, with a relatively short follow-up time, and was only derived from one single center. Only a few patients underwent positron emission tomography, which is superior to CT for mediastinal staging of lung cancer, data analysis has been not available to us, because of the limited availability of positron emission tomography in China limits the ability to make comparisons with studies in other countries where positron emission tomography is performed routinely. We believe that the screening of a large case series in the present study may help in identifying lymph node status in GGO-manifesting lung cancers.
In conclusions, among the majority of “indolent” GGO lesions, lymph node metastasis occurs occasionally at 2.9%. Lymph node metastasis was absent in sub-centimeter GGO lesions and pure GGOs. Mixed GGO, elevated serum CEA level (>2.75 ng/mL) and a larger tumor diameter predicted a higher possibility of lymph node metastasis.
Acknowledgements
Funding: This study was supported by the Shanghai Committee of Science and Technology (grants 15411968400 and 13DZ1942805) and Shanghai hospital development center (SHDC12015116).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was conducted in accordance with the principles of the Declaration of Helsinkiand approved by the Institutional Review Board of Shanghai Pulmonary Hospital (k16-167), and all patients provided written informed consent for surgery.
References
- Austin JH, Müller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200:327-31. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Callol L, Roig F, Cuevas A, et al. Low-dose CT: a useful and accessible tool for the early diagnosis of lung cancer in selected populations. Lung Cancer 2007;56:217-21. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Suzuki K, Asamura H, Kusumoto M, et al. "Early" peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002;74:1635-9. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Yokouchi H, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer 2001;33:17-25. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007;245:267-75. [Crossref] [PubMed]
- Lee HJ, Goo JM, Lee CH, et al. Predictive CT findings of malignancy in ground-glass nodules on thin-section chest CT: the effects on radiologist performance. Eur Radiol 2009;19:552-60. [Crossref] [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [Crossref] [PubMed]
- Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. [Crossref] [PubMed]
- Hashizume T, Yamada K, Okamoto N, et al. Prognostic significance of thin-section CT scan findings in small-sized lung adenocarcinoma. Chest 2008;133:441-7. [Crossref] [PubMed]
- Ohde Y, Nagai K, Yoshida J, et al. The proportion of consolidation to ground-glass opacity on high resolution CT is a good predictor for distinguishing the population of non-invasive peripheral adenocarcinoma. Lung Cancer 2003;42:303-10. [Crossref] [PubMed]
- Aoki T, Nagata Y, Negoro Y, et al. Evaluation of lung injury after three-dimensional conformal stereotactic radiation therapy for solitary lung tumors: CT appearance. Radiology 2004;230:101-8. [Crossref] [PubMed]
- Koike T, Koike T, Yamato Y, et al. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. J Thorac Oncol 2012;7:1246-51. [Crossref] [PubMed]
- Bao F, Yuan P, Yuan X, et al. Predictive risk factors for lymph node metastasis in patients with small size non-small cell lung cancer. J Thorac Dis 2014;6:1697-703. [PubMed]
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014;98:217-23. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Zhang Y, Sun Y, Shen L, et al. Predictive factors of lymph node status in small peripheral non-small cell lung cancers: tumor histology is more reliable. Ann Surg Oncol 2013;20:1949-54. [Crossref] [PubMed]
- Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: A predictor of lymph node metastasis. J Thorac Cardiovasc Surg 2002;124:278-84. [Crossref] [PubMed]


