
Efficacy and safety of anti-programmed cell death protein 1 antibody combination therapy in patients with advanced experienced epidermal growth factor receptor-tyrosine kinase inhibitor-resistant lung adenocarcinoma: a retrospective cohort study
Highlight box
Key findings
• Immune combination chemotherapy and immune combination anti-angiogenic therapy have equivalent efficacy in the treatment of programmed cell death ligand 1 positive patients with advanced epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI)-resistant lung adenocarcinoma (LUAD).
What is known and what is new?
• The effectiveness of combining immune checkpoint inhibitors and chemotherapy has been evaluated as superior to that of chemotherapy alone in patients with advanced EGFR-TKI-resistant non-small cell lung cancer.
• This study compared the efficacy of immunotherapy with or without anti-angiogenic or chemotherapy in the advanced EGFR-TKI-resistant LUAD patients.
What is the implication, and what should change now?
• This data may provide theoretic groundwork for avoiding unnecessary chemotherapy in EGFR-resistant patients and also highlight the role of programmed cell death protein 1 pathway inhibitors in immune combination therapy.
Introduction
The use of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) is a prevailing initial treatment for advanced EGFR-mutated non-small cell lung cancer (NSCLC); although it has displayed remarkable efficacy (1,2), the acquired resistance and progression of disease are ineluctable (3-5). Resistance to EGFR-TKIs can occur in three forms: first- and second-generation resistance, third-generation resistance at first line, and third-generation resistance at second and above line. Chemotherapy is considered the principal approach for managing EGFR-TKI resistance, but its effectiveness is suboptimal (6-9). These findings underscore the necessity for new therapeutic strategies. To address this issue, several immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) have been approved by the U.S. Food and Drug Administration for the therapeutic intervention of advanced NSCLC. Among these ICIs are nivolumab and pembrolizumab (10,11). However, the application of immunotherapy is controversial in the EGFR-TKI-resistant population; certain clinicians have recommended exploring ICIs-based combination therapy as a possible alternative (12,13), especially given the lackluster results yielded by ICI monotherapy.
Previous research conducted by our team has revealed that the combination of ICIs and an anti-angiogenic agent contributes to a surge in the duration of response, especially in cases of EGFR-TKI resistance (14). Hence, we aimed to assess the safety and effectiveness of varied immune-combination therapies in patients with advanced EGFR-TKI-resistant lung adenocarcinoma (LUAD). We present this article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1399/rc).
Methods
Patient selection and procedures
The tumor tissue samples and clinical treatment data of stage IV LUAD patients who visited The First Affiliated Hospital of Anhui Medical University between January 2020 and June 2022 were analyzed retrospectively in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the First Affiliated Hospital of Anhui Medical University (No. Quick-PJ 2023-04-34). The requirement for informed consent was waived due to the retrospective nature of this study. The study enrolled individuals between the ages of 18 and 75, with locally advanced or metastatic LUAD (stages III B–C or IV) according to the American Joint Committee on Cancer Staging Manual, eighth edition. Participants with EGFR sensitizing mutations confirmed by tumor histology, cytology, or circulating tumor DNA (ctDNA), including exon 19 deletions (E19del) and exon 21 L858R missense mutations (L858R) were eligible. In addition, participants were required to have experienced disease progression after receiving EGFR-TKI conforming to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1).
Disease progression was defined as follows: (I) progression following treatment with first- or second-generation EGFR-TKIs, and either having a negative EGFR Thr790Met (T790M) mutation status confirmed by tissue samples or receiving third-generation EGFR-TKI as first-line treatment; (II) progression following treatment with first- or second-generation EGFR-TKI, and having been treated with third-generation EGFR-TKIs for at least 6 months. Participants who progressed after third-generation treatment did not require re-biopsy. Additional inclusion criteria consisted of at least 1 measurable lesion (in accordance with RECIST 1.1), Eastern Cooperative Oncology Group performance status (ECOG-PS) 0 or 1, and an estimated life span of at least 3 months.
Patients who had small cell lung cancer (SCLC) histology or symptomatic metastasis of the central nervous system were excluded from the study. Those who had previously undergone systemic anti-tumor therapy, including cytotoxic chemotherapy, except for EGFR-TKIs for advanced NSCLC, immunotherapy (such as anti-PD-1, anti-PD-L1, anti-PD-L2, or anti-CTLA-4) antibodies, or agents that affect T-cell co-stimulation and other immune checkpoints, were also excluded.
Patients who met all the inclusion and exclusion criteria had their medical histories collected retrospectively, including information on the generation of EGFR-TKIs resistance, the use of ICIs and combined treatments, EGFR mutation detection results, and PD-L1 expressions.
Efficacy and safety
RECIST 1.1 was used to evaluate tumor response. The data of objective response rate [ORR; the combination of complete response (CR) and partial response (PR) rates], disease control rate [DCR; the combination of CR, PR, and stable disease (SD) rates], progression-free survival (PFS), and overall survival (OS) were analyzed. Immunohistochemistry (IHC) was used to analyze expression PD-L1 using the Dako 22C3 (Monoclonal Mouse Anti-Human PD-L1, Clone 22C3; Dako, Carpenteria, CA, USA) on the available tumor biopsy samples. Efficacy rates were compared according to treatment groups (anti-PD-1 antibody combined with or without anti-angiogenic or chemotherapy) and PD-L1 expression. Safety and tolerability were assessed using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 throughout the study.
Statistical analysis
Medians (ranges) were used to summarize continuous data, frequencies (percentages) were used for categorical data. PFS and OS were evaluated using the Kaplan-Meier method. All significance tests were two-sided, with a P value <0.05. Statistical analyses were conducted using the software SPSS 26.0 (IBM Corp., Armonk, NY, USA), as well as GraphPad Prism version 9 (GraphPad Software Inc., San Diego, CA, USA) and the Hiplot platform (https://hiplot.com.cn/).
Results
Patient characteristics
Over the course of January 2020 to June 2022, there were 43 patients who participated in this study. In the screening with a cut-off value of 1% of PD-L1 tumor proportion score (TPS), 14/20 (70.0%) patients were reported positive, and more specifically, 4/6 (66.7%) patients received immunotherapy combined with chemotherapy, 8/10 (80.0%) patients received immunotherapy combined with antiangiogenic, 2/4 (50.0%) patients received immunotherapy in combination with antiangiogenic and chemotherapy. In the study, the presence of brain metastases at baseline was reported in nearly one-third of participants. Table 1 provides an overview of the baseline demographic and clinical features.
Table 1
| Characteristics | Patients (N=43) |
|---|---|
| Sex | |
| Male | 27 (62.8) |
| Female | 16 (37.2) |
| Age (years) | 61 [32–75] |
| Histology | |
| Adenocarcinoma | 43 (100.0) |
| Smoking status | |
| Current or former smoker | 9 (20.9) |
| Never smoked | 34 (79.1) |
| EGFR mutation | |
| Edel19 | 22 (51.2) |
| L858R | 21 (48.8) |
| PD-L1 expression | |
| Positive | 14 (32.6) |
| Negative | 6 (14.0) |
| Not reported | 23 (53.5) |
| The generation of EGFR-TKIs resistance | |
| First generation | 15 (34.9) |
| Second generation | 2 (4.7) |
| Third generation as first-line treatment | 3 (7.0) |
| Third generation as second or above-line treatment | 23 (53.5) |
| Brain metastases | 13 (30.2) |
Data are shown as n (%) or median [range]. EGFR, epidermal growth factor receptor; PD-L1, programmed death ligand 1; TKIs, tyrosine kinase inhibitors.
Treatment distribution
In this study, 39.6% (17/43) of cases received immunotherapy in combination with antiangiogenic and chemotherapy, whereas 30.2% (13/43) received immunotherapy in combination with only antiangiogenic therapy, and the remaining 30.2% (13/43) received immunotherapy combined with only chemotherapy. Sintilimab (51.2%) and camrelizumab (48.8%) were the main anti-PD-1 drugs, which have been approved for NSCLC treatment in China (15,16). As for chemotherapy, the proportions of pemetrexed-platinum-based chemotherapy and paclitaxel-platinum-based chemotherapy were similar, at 53.8% and 46.2%, respectively. With respect to the anti-angiogenic drugs, anlotinib predominated (76.9%) in immune combination with an anti-angiogenic strategy, whereas bevacizumab was mainly used in immune combination with anti-angiogenic plus chemotherapy (82.4%).
Survival of EGFR-TKI-resistant patients
All 43 patients were evaluated for treatment efficacy: the overall ORR was 23.3% and the overall DCR was 90.7%. Among the 43 samples whose dates of PFS or OS were available, the median PFS (mPFS) and OS (mOS) were 6.5 and 10.6 months, respectively. No significant differences were detected in terms of the mPFS between anti-PD-1 antibody combined with anti-angiogenic drugs and chemotherapy, anti-PD-1 antibody combined with chemotherapy, and anti-PD-1 antibody combined with anti-angiogenic therapy (6 vs. 6.5 vs. 11.8 months, respectively, P=0.48, Figure 1A). Additionally, no statistically significant difference was observed in terms of mOS in above-mentioned 3 anti-PD-1 antibody combination therapies (8.2 vs. 11.8 vs. 10.9 months, respectively, P=0.12, Figure 1B).

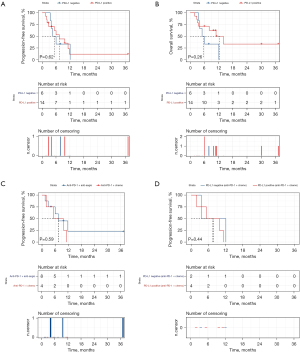
To assess the prognostic significance of PD-L1 expression, PFS and OS were analyzed according to the expression of PD-L1. In the overall cohort, patients with positive PD-L1 expression (mPFS 7.5 months) showed an improved PFS prognosis compared to those with negative PD-L1 expression (mPFS 5.25 months, Figure 2A), but the difference was not significant (P=0.62; log-rank test), similar results were also observed in terms of OS (12.45 vs. 5.50 months, respectively, P=0.26; log-rank test, Figure 2B). Furthermore, in the PD-L1 positive subgroup, no statistically significant difference was observed in terms of PFS between the use of immune combination chemotherapy and immune combination anti-angiogenic therapy (7.55 vs. 7.50 months, respectively, P=0.59; log-rank test, Figure 2C). Besides, in the population receiving immune combination chemotherapy, there was a trend towards prolonged PFS regardless of the PD-L1 expression status being positive or negative (7.55 vs. 7.80 months, respectively, P=0.44; log-rank test, Figure 2D).
Safety
Table 2 shows the immune-related adverse events (irAEs) observed in all study participants. Of the 43 patients, 26 (60.5%) experienced at least 1 irAE. Among these patients, 7 (16.3%) experienced irAEs that were grade 3–4, whereas 3 (7.0%) reported severe cases. Immune hepatitis and myelosuppression were the most typically reported irAEs, with incidence rates of 30.2% and 27.9%, respectively. As shown in Table 2, in different immunotherapy regimens, the incidence of treatment-related adverse events (TRAEs) was relatively high in anti-PD-1 antibody combined with chemotherapy (with or without anti-angiogenic therapy), specifically, fatigue (23.1%), reactive cutaneous capillary endothelial proliferation (46.2%) and immune hepatitis (46.2%) predominated in immune combination with chemotherapy, whereas immune combination with anti-angiogenic plus chemotherapy was mainly manifested in myelosuppression (41.2%).
Table 2
| Adverse events | All patients (N=43), n (%) | Anti-PD-1 + chemo (N=13), n (%) | Anti-PD-1 + anti-angio (N=13), n (%) | Anti-PD-1 + anti-angio + chemo (N=17), n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grades | Grade 3–4 | Any grades | Grade 3–4 | Any grades | Grade 3–4 | Any grades | Grade 3–4 | ||||
| irAEs leading to interruption | 3 (7.0) | 3 (7.0) | 1 (7.7) | 1 (7.7) | 1 (7.7) | 1 (7.7) | 1 (5.9) | 1 (5.9) | |||
| irAEs leading to discontinuation | 1 (2.3) | 1 (2.3) | 1 (7.7) | 1 (7.7) | 0 | 0 | 0 | 0 | |||
| irAEs requiring steroids | 2 (4.7) | 2 (4.7) | 1 (7.7) | 1 (7.7) | 0 | 0 | 1 (5.9) | 1 (5.9) | |||
| Treatment-related deaths | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| TRAEs | |||||||||||
| Fatigue | 5 (11.6) | 0 | 3 (23.1) | 0 | 1 (7.7) | 0 | 1 (5.9) | 0 | |||
| Rash | 2 (4.7) | 0 | 0 | 0 | 1 (7.7) | 0 | 1 (5.9) | 0 | |||
| Pruritus | 2 (4.7) | 0 | 0 | 0 | 1 (7.7) | 0 | 1 (5.9) | 0 | |||
| Diarrhea | 2 (4.7) | 0 | 1 (7.7) | 0 | 0 | 0 | 1 (5.9) | 0 | |||
| Nausea | 3 (7.0) | 0 | 1 (7.7) | 0 | 1 (7.7) | 0 | 1 (5.9) | 0 | |||
| Decreased appetite | 3 (7.0) | 0 | 1 (7.7) | 0 | 1 (7.7) | 0 | 1 (5.9) | 0 | |||
| RCCEP | 10 (23.3) | 0 | 6 (46.2) | 0 | 2 (15.4) | 0 | 2 (11.8) | 0 | |||
| Immune pneumonitis | 1 (2.3) | 1 (2.3) | 1 (7.7) | 1 (7.7) | 0 | 0 | 0 | 0 | |||
| Immune cystitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Urinary tract infection | 2 (4.7) | 0 | 1 (7.7) | 0 | 0 | 0 | 1 (5.9) | 0 | |||
| Immune hepatitis | 13 (30.2) | 0 | 6 (46.2) | 0 | 2 (15.4) | 0 | 5 (29.4) | 0 | |||
| Myelosuppression | 12 (27.9) | 3 (7.0) | 5 (38.5) | 2 (15.4) | 0 | 0 | 7 (41.2) | 1 (5.9) | |||
| Hypothyroidism | 5 (11.6) | 0 | 1 (7.7) | 0 | 3 (23.1) | 0 | 1 (5.9) | 0 | |||
PD-1, programmed cell death protein 1; anti-PD-1 + chemo, anti-PD-1 antibody in combination with chemotherapy; anti-PD-1 + anti-angio, anti-PD-1 antibody in combination with anti-angiogenic therapy; anti-PD-1 + anti-angio + chemo, anti-PD-1 antibody in combination with antiangiogenic and chemotherapy; irAEs, immune-related adverse events; TRAEs, treatment-related adverse events; RCCEP, reactive cutaneous capillary endothelial proliferation.
Only 1 patient was unable to receive anti-PD-1 therapy due to severe immune pneumonitis. Throughout the observation period, the causes of death were disease progression and respiratory failure. It is noteworthy that none of the patients died as a result of an irAE.
Discussion
EGFR-TKIs are regarded as the first option for NSCLC patients with EGFR mutations. However, although they can lead to ORRs, progression of the disease, and certain resistance often accompanies their use (17-19). Due to the challenges associated with the use of EGFR-TKIs, ICIs have garnered attention as an important area of research for improving survival rates among patients with advanced NSCLC who lack driver gene mutations (20-22). Research results have been inconsistent concerning the potency of immunotherapy in treating EGFR-TKI-resistant NSCLC. EGFR mutation is associated with an uninflamed phenotype and weak immunogenicity in the extracranial lesions (23). The efficacy of ICIs as a single therapy has proven to be unsatisfactory for patients with EGFR mutations, given that ICI combination therapies have been promoted by several studies, and some preclinical studies have provided a novel and powerful rationale for immune combination therapy (24,25). The results of ORIENT-31 demonstrated a significant increase in tumor control period when sintilimab was combined with bevacizumab, pemetrexed, and cisplatin compared with pemetrexed and cisplatin alone. Furthermore, the combination therapy was well-tolerated overall (26). However, in the ultimate analysis of the KEYNOTE-789 (NCT03515837) trial, although there was a slight improvement in OS for progressive disease following TKI therapy for metastatic non-squamous NSCLC patients receiving pembrolizumab combined with pemetrexed and platinum chemotherapy compared to the pemetrexed and platinum chemotherapy only group, these improvements did not reach statistical significance according to pre-specified statistical values. Such divergent results are thought-provoking. Although benefits were observed in the PFS/OS when immunotherapy was combined with anti-angiogenic therapy plus chemotherapy, especially for patients with EGFR mutations, the comparison among different immune combination therapies had not been assessed, emphasizing the need to further investigate whether the PFS/OS are different between chemotherapy and anti-angiogenesis therapy.
In this study, no statistical difference was observed in terms of mPFS between the use of immune combination chemotherapy and immune combination anti-angiogenic therapy in the PD-L1 positive subgroup. This data may provide theoretic groundwork for avoiding unnecessary chemotherapy in EGFR-resistant patients and also highlights the importance of PD-1 pathway inhibitors in immune combination therapy. Moreover, the PFS was prolonged regardless of the PD-L1 expression status being positive or negative in the population receiving immune combination chemotherapy, which provides some treatment options for patients who were not screened for PD-L1 expression.
In addition, no notable distinction was observed in mPFS and mOS among patients receiving three anti-PD-1 antibody combination therapies. Patients with positive PD-L1 expression showed an improved median PFS compared to those with negative PD-L1 expression. Similar results have been reported in other studies, showing that PD-1 pathway inhibitors are effective in EGFR-mutant patients with high expression of PD-L1 (27,28). Regarding safety, our results align with the established safety profiles of either ICIs or the combined therapy, and no novel safety signals were detected. Grade 3 or higher TRAEs were observed in 16.3% of patients, inclusive of TRAEs in immunotherapies that contained chemotherapy.
Due to the small sample size and retrospective setting, no significant results were found when compared mPFS and mOS among patients receiving 3 anti-PD-1 antibody combination therapies. These results need to be further strengthened by extending coverage or partnering with multiple institutions to increase the number of eligible patients.
Conclusions
Our study indicated that combination immunotherapy is feasible in post-TKI resistant individuals with LUAD harboring EGFR mutations. Immune combination chemotherapy and immune combination anti-angiogenic therapy have equivalent efficacy in the PD-L1 positive population. PD-L1 expression can be used as a reference for screening patient suitability for combination immunotherapy which suggests the potential for further improvement in patient prognosis.
Acknowledgments
The authors are grateful for the assistance from the research conductors and cooperators. We thank Shanghai Tengyun Biotechnology Co., Ltd. for developing the Hiplot Pro platform (https://hiplot.com.cn/) and providing technical assistance and valuable tools for data analysis and visualization.
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1399/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1399/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1399/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1399/coif). All authors thank Shanghai Tengyun Biotechnology Co., Ltd. for developing the Hiplot Pro platform (https://hiplot.com.cn/) and providing technical assistance and valuable tools for data analysis and visualization for free. All authors report that this research was supported by the Department of Science and Technology of Anhui Province (No. 2022e07020044) and the Chinese Thoracic Oncology Group (CTONG) (No. CTNOG-YC20220113). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the First Affiliated Hospital of Anhui Medical University (No. Quick-PJ 2023-04-34). The requirement for informed consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines® Insights: Non-Small Cell Lung Cancer, Version 2.2023. J Natl Compr Canc Netw 2023;21:340-50. [Crossref] [PubMed]
- Hanna N, Johnson D, Temin S, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- Del Re M, Crucitta S, Gianfilippo G, et al. Understanding the Mechanisms of Resistance in EGFR-Positive NSCLC: From Tissue to Liquid Biopsy to Guide Treatment Strategy. Int J Mol Sci 2019;20:3951. [Crossref] [PubMed]
- Fogli S, Polini B, Del Re M, et al. EGFR-TKIs in non-small-cell lung cancer: focus on clinical pharmacology and mechanisms of resistance. Pharmacogenomics 2018;19:727-40. [Crossref] [PubMed]
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29:i10-9. [Crossref] [PubMed]
- Han B, Yang L, Wang X, et al. Efficacy of pemetrexed-based regimens in advanced non-small cell lung cancer patients with activating epidermal growth factor receptor mutations after tyrosine kinase inhibitor failure: a systematic review. Onco Targets Ther 2018;11:2121-9. [Crossref] [PubMed]
- Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015;16:990-8. [Crossref] [PubMed]
- Yoshida T, Kuroda H, Oya Y, et al. Clinical outcomes of platinum-based chemotherapy according to T790M mutation status in EGFR-positive non-small cell lung cancer patients after initial EGFR-TKI failure. Lung Cancer 2017;109:89-91. [Crossref] [PubMed]
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. [Crossref] [PubMed]
- Pai-Scherf L, Blumenthal GM, Li H, et al. FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond. Oncologist 2017;22:1392-9. [Crossref] [PubMed]
- Jiang T, Wang P, Zhang J, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct Target Ther 2021;6:355. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Zhang Y, Zhao M, Cao S, et al. Unexpected favorable outcome to sintilimab plus bevacizumab in an EGFR-mutated non-small cell lung cancer patient: A case report. Thorac Cancer 2020;11:2717-22. [Crossref] [PubMed]
- Song H, Liu X, Jiang L, et al. Current Status and Prospects of Camrelizumab, A Humanized Antibody Against Programmed Cell Death Receptor 1. Recent Pat Anticancer Drug Discov 2021;16:312-32. [Crossref] [PubMed]
- Yang Y, Wang Z, Fang J, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol 2020;15:1636-46. [Crossref] [PubMed]
- Ohashi K, Maruvka YE, Michor F, et al. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol 2013;31:1070-80. [Crossref] [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Duan H, Wang T, Luo Z, et al. Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable non-small cell lung cancer: an open-label, multicenter, single-arm study. Transl Lung Cancer Res 2021;10:1020-8. [Crossref] [PubMed]
- Shi J, Li J, Wang Q, et al. The safety and efficacy of immunotherapy with anti-programmed cell death 1 monoclonal antibody for lung cancer complicated with Mycobacterium tuberculosis infection. Transl Lung Cancer Res 2021;10:3929-42. [Crossref] [PubMed]
- Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology 2017;6:e1356145. [Crossref] [PubMed]
- Leonetti A, Wever B, Mazzaschi G, et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist Updat 2019;46:100644. [Crossref] [PubMed]
- Wu J, Zhao X, Sun Q, et al. Synergic effect of PD-1 blockade and endostar on the PI3K/AKT/mTOR-mediated autophagy and angiogenesis in Lewis lung carcinoma mouse model. Biomed Pharmacother 2020;125:109746. [Crossref] [PubMed]
- Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2022;23:1167-79. [Crossref] [PubMed]
- Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018;19:521-36. [Crossref] [PubMed]
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]


