
Association between cigarette smoking history, metabolic phenotypes, and EGFR mutation status in patients with non-small cell lung cancer
Highlight box
Key findings
• The metabolic activity of 18F-fluoro-2-deoxy-D-glucose was found to be higher in smokers with primary non-small cell lung cancer (NSCLC) compared to never-smokers, while the incidence of epidermal growth factor receptor (EGFR) mutation exhibited a decrease.
What is known and what is new?
• Never-smokers diagnosed with NSCLC demonstrate a more favorable prognosis and exhibit a superior response to EGFR tyrosine kinase inhibitors compared to their smoking counterparts.
• In patients diagnosed with NSCLC, smokers exhibited a higher maximum standardized uptake value of primary tumors (pSUVmax) and a lower incidence rate of EGFR mutations in comparison to never-smokers.
What is the implication, and what should change now?
• Our findings provide insights into the potential impact of cigarette smoking and pSUVmax on the development and progression of NSCLC, which may have implications for personalized targeted EGFR therapies.
Introduction
Despite significant improvements in diagnosis and treatment, lung cancer remains one of the most common malignancies and the leading cause of cancer-related mortality worldwide (1,2). A large amount of evidence has demonstrated that cigarette smoking is a risk factor for lung cancer (3,4). Epidemiological and molecular genetic differences have been found between patients with non-smoking-associated lung cancer and tobacco-associated lung cancer (4-7). Never-smokers with non-small cell lung cancer (NSCLC) frequently have the following characteristics female gender, young age and a histological diagnosis of epidermal growth factor receptor (EGFR) mutated adenocarcinoma (ADC) (8,9). Moreover, this subgroup demonstrates a more favorable prognostic outlook when juxtaposed with their smoking counterparts. This clinical advantage is mainly determined by their elevated responsiveness to EGFR tyrosine kinase inhibitors (TKIs), such as osimertinib, afatinib, gefitinib, erlotinib and by the absence of smoking-related comorbidities (10,11). Lung ADC patients who had smoked more than 15 pack-years or quit less than 25 years ago have been shown to be less likely to have detectable EGFR mutations (12). In clinical practice, molecular detection of EGFR can be limited by invasive procedures, sampling errors, and long processing time (13). Liquid biopsy represents a non-invasive useful tool to identify EGFR alterations; nevertheless, it is worth noting that the sensitivity of this technique is around 65% (14). These data may help clinicians to assess the likelihood of EGFR mutations in patients with lung ADC when the results of EGFR mutation status are not available.
Positron emission tomography/computed tomography (PET/CT) with 18F-fluoro-2-deoxy-D-glucose (18F-FDG), a molecular imaging modality, has been widely used in clinical diagnosis, staging, response evaluation, and prognosis of lung cancer (15-17). Several metabolic parameters, including maximum standard uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), could reflect the metabolic activity of 18F-FDG (18,19). In patients with advanced or metastatic NSCLC, high MTV and TLG usually represent a lower progression-free survival (PFS) and overall survival (OS) (20). In addition, we previously reviewed the correlation between EGFR mutation status and metabolic activity of 18F-FDG in NSCLC (21). It has been reported that primary lung cancers with lower SUVmax may represent a higher incidence of EGFR mutations (22,23), but opposite results have also been observed (24,25). Accordingly, further studies are needed to verify these findings, so that clinicians can accurately predict EGFR mutations and evaluate the prognosis.
The effect of cigarette smoking on 18F-FDG metabolic activity of primary lung cancer was investigated in our previous study. Our results suggested that the metabolic activity of 18F-FDG in early primary lung cancer may be influenced by cigarette smoking (26). Accordingly, we speculate that there may be a significant causal relationship between them, which may guide clinicians in risk stratification and treatment strategy selection for patients with NSCLC. Thus, we performed a retrospective analysis to investigate the association and potential causation between cigarette smoking, metabolic phenotypes, and EGFR mutation status in patients with NSCLC. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1371/rc).
Methods
Patient selection
From September 2019 to March 2022, we performed a retrospective analysis of 1,104 consecutive patients with lung cancer who were initially diagnosed by 18F-FDG PET/CT imaging at Ningbo No.2 Hospital (Ningbo, China). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board (IRB) of Ningbo No.2 Hospital (No. YJ-NBEY-KY202108401). The requirement for written informed consent was waived due to the anonymous nature of the retrospective data.
Patients who met the following criteria were further analyzed in our study: (I) confirmation of NSCLC, and classification into ADC, squamous cell carcinoma (SCC), and NSCLC-others, by histopathology; (II) 18F-FDG PET/CT scanning had been performed before any treatment; (III) the status of EGFR mutations was determined by tissue-based analysis (27); (IV) a detailed cigarette smoking history was obtained. Finally, a total of 198 patients met our criteria and were analyzed in our study (Figure 1). The clinical characteristics [including age at diagnosis, sex, smoking status, tumor-node-metastasis (TNM) stage, and histopathological type] and metabolic features of 18F-FDG [including SUVmax of the primary tumors (pSUVmax), metastatic lymph nodes (nSUVmax), and distant metastases (mSUVmax)] based on EGFR mutation status are summarized in Table 1.

Table 1
| Characteristics | Total | EGFR-mutant | EGFR wild-type | P value |
|---|---|---|---|---|
| Age at diagnosis | 0.763 | |||
| No. of patients [%] | 198 [100] | 91 [46] | 107 [54] | |
| Mean ± SD, years | 66.3±8.9 | 66.5±9.0 | 66.1±9.0 | |
| Range, years | 34–86 | 36–85 | 34–86 | |
| Sex, n [%] | <0.001 | |||
| Male | 121 [61] | 39 [43] | 82 [77] | |
| Female | 77 [39] | 52 [57] | 25 [23] | |
| Smoking status, n [%] | <0.001 | |||
| Never smokers | 121 [61] | 73 [80] | 48 [45] | |
| Smokers | 77 [39] | 18 [20] | 59 [55] | |
| Clinical TNM stage, n [%] | 0.140 | |||
| I | 64 [32] | 35 [38] | 29 [27] | |
| II | 30 [15] | 15 [16] | 15 [14] | |
| III | 36 [18] | 11 [12] | 25 [23] | |
| IV | 68 [34] | 30 [33] | 38 [36] | |
| Histological types, n [%] | <0.001 | |||
| ADC | 152 [77] | 85 [93] | 67 [63] | |
| SCC | 34 [17] | 5 [5] | 29 [27] | |
| NSCLC-others | 12 [6] | 1 [1] | 11 [10] | |
| pSUVmax | <0.001 | |||
| No. of patients [%] | 198 [100] | 91 [46] | 107 [54] | |
| Mean ± SD | 10.9±6.2 | 8.9±4.5 | 12.7±6.9 | |
| Range | 0.5–37.7 | 0.6–20.7 | 0.5–37.7 | |
| nSUVmax | 0.934 | |||
| No. of patients [%] | 102 [52] | 42 [21] | 60 [30] | |
| Mean ± SD | 10.0±5.3 | 10.1±5.2 | 10.0±5.3 | |
| Range | 3.1–31.0 | 3.4–27.0 | 3.1–31.0 | |
| mSUVmax | 0.274 | |||
| No. of patients [%] | 69 [35] | 29 [15] | 40 [20] | |
| Mean ± SD | 9.7±5.7 | 8.8±5.9 | 10.3±5.5 | |
| Range | 0.5–29.8 | 0.5–29.8 | 2.6–27.4 |
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; SD, standard deviation; TNM, tumor-node-metastasis; ADC, adenocarcinoma; SCC, squamous cell carcinoma; pSUVmax, maximum standardized uptake value of primary tumor; nSUVmax, maximum standardized uptake value of metastatic lymph nodes; mSUVmax, maximum standardized uptake value of distant metastases.
Technique of PET/CT scan
All patients underwent PET/CT using a GE Discovery 710 PET scanner (GE Healthcare, Milwaukee, WI, USA). They were required to fast for more than 6 hours before PET/CT examination. The concentration of blood glucose was required to be less than 7.0 mmol/L before intravenous injection of 18F-FDG with 5.2–7.4 MBq/kg. We performed PET/CT scan 45–60 minutes after administration of 18F-FDG. Low-dose CT scan with 140 kV, 10 mA, 0.5 second rotation time, and 40 mm collimation was performed to evaluate the features of anatomy. In addition, CT scan data of an iterative algorithm was used for reconstruction. Subsequently, PET scanning in 3-dimensional (3D) mode was performed from the skull base to upper thigh at 2.5 minutes per bed position. Finally, the transverse, sagittal, and coronal PET, CT, and fused PET/CT images were obtained on a Xeleris workstation (GE Healthcare) for evaluation.
Analysis of PET/CT imaging
All PET and CT images were consistently evaluated by two senior nuclear physicians based on clinical data. The level of 18F-FDG uptake within the lesion was defined as abnormal when it was higher than the surrounding background. The SUVmax was used to quantify the uptake intensity of 18F-FDG. A 2-dimensional (2D) region of interest (ROI) was manually drawn at the edge of the tumor focus and placed in the tumor area with the highest 18F-FDG uptake. The peak SUV on the pixel with the highest count in the ROI was defined as SUVmax. The calculation formula was as follows: SUV = [radioactive concentration in the ROI (MBq/g)]/[injected dose (MBq)/patient’s total body weight (g)]. Based on visual qualitative analysis, metastatic lymph nodes were considered when the uptake of 18F-FDG was greater than the background mediastinal blood pool (28).
Assessment of cigarette smoking status
All patients underwent a face-to-face interview prior to the PET/CT scan, during which detailed information regarding their smoking history was obtained. The patients were classified into two groups based on cigarette smoking status: smokers and never-smokers. Patients were strictly defined as smokers if they had smoked ≥100 cigarettes in their lifetime, regardless of whether they had quit or not, and the remaining others were defined as never-smokers (8,12). The pack-year index is a parameter reflecting the cumulative smoking dose, which is calculated by multiplying the smoking period (year) by the number of cigarettes smoked every day (29). The number of pack-years is the number of packs smoked per day multiplied by the number of years smoked, with 1 pack-year being 1 day and 1 pack of accumulated smoking for 1 year, and so on.
Statistical analysis
We used descriptive statistics to express the demographic data of our included patients. The quantitative data were analyzed as mean ± standard deviation (SD). The clinical characteristics such as age, gender (male vs. female), histopathological subtypes (ADC, SCC, and NSCLC-others), clinical stage (I, II, III, and IV), and smoking status (never-smokers vs. smokers), were compared between patients with and without EGFR mutations by Fisher’s exact test analysis or chi-squared test. PET/CT parameters, including pSUVmax, nSUVmax, and mSUVmax, were compared between patients with or without EGFR mutations by Mann-Whitney test. Receiver operating characteristic (ROC) curves were constructed using factors that differed significantly between patients with and without EGFR mutations. The area under the ROC curve (AUC) was calculated to evaluate the predictive value. A two-sided P value <0.05 was considered statistically significant. All statistical analyses and graphic designs were performed using GraphPad Prism 9.0 software (GraphPad Software, San Diego, CA, USA).
Results
Patient characteristics
Patient characteristics stratified according to the status of EGFR mutations are summarized in Table 1. EGFR mutation was observed in 73 (60.3%) of 121 never-smokers and 18 (23.4%) of 77 smokers (P<0.001). Of the 198 patients, significant differences of sex (male vs. female), smoking status (smokers vs. never-smokers), and histopathological subtypes (ADC, SCC, and NSCLC-others) were observed between patients with and without EGFR mutations, but the age and clinical TNM stage showed no significant difference.
Association between EGFR mutation status and cigarette smoking history
In terms of cigarette smoking history, never-smokers had a higher rate of EGFR mutations than smokers [60.3% (73/121) vs. 23.4% (18/77), P<0.001]. With the increase of cigarette smoking dose, the incidence of EGFR mutations decreased significantly. EGFR mutation rates were 20% in patients who smoked >20 pack-years, 35% in patients who smoked 1–20 pack-years, and 60% in patients who never smoked (Table 2).
Table 2
| No. of pack-years | Patients | P value | ||
|---|---|---|---|---|
| Total No. | No. with mutations | % with mutations | ||
| 0 (never smokers) | 121 | 73 | 60 | NA |
| 1–20 | 17 | 6 | 35 | 0.067 |
| 21–30 | 15 | 3 | 20 | 0.005 |
| 31–40 | 20 | 4 | 20 | 0.001 |
| >40 | 25 | 5 | 20 | <0.001 |
P values compare pack-year categories with mutation rates in never smokers. EGFR, epidermal growth factor receptor; NA, not applicable.
Association between metabolic parameters and cigarette smoking status
The correlations between metabolic parameters (pSUVmax, nSUVmax, and mSUVmax) and the status of cigarette smoking (smokers and never-smokers) are presented in Table 3. We found that smokers had a higher pSUVmax than never-smokers (12.5±6.4 vs. 9.9±5.9, P=0.004), but no significant differences were observed between them for nSUVmax (10.8±4.9 vs. 9.5±5.5, P=0.229) and mSUVmax (10.1±5.6 vs. 9.5±5.8, P=0.651). In addition, with the increase of cigarette smoking dose, the pSUVmax increased significantly (r=0.198, P=0.005, Figure 2).
Table 3
| Metabolic parameters | Patients | P value | ||
|---|---|---|---|---|
| Total No. | Smokers | Never-smokers | ||
| pSUVmax | 0.004 | |||
| No. of patients [%] | 198 [100] | 77 [39] | 121 [61] | |
| Mean ± SD | 12.5±6.4 | 9.9±5.9 | ||
| Range | 0.5–35.2 | 0.6–37.7 | ||
| nSUVmax | 0.229 | |||
| No. of patients [%] | 102 [52] | 43 [22] | 59 [30] | |
| Mean ± SD | 10.8±4.9 | 9.5±5.5 | ||
| Range | 3.2–24.5 | 3.1–31.0 | ||
| mSUVmax | 0.651 | |||
| No. of patients [%] | 69 [35] | 25 [13] | 44 [22] | |
| Mean ± SD | 10.1±5.6 | 9.5±5.8 | ||
| Range | 2.6–27.4 | 0.5–29.8 | ||
pSUVmax, maximum standardized uptake value of primary tumor; SD, standard deviation; nSUVmax, maximum standardized uptake value of metastatic lymph nodes; mSUVmax, maximum standardized uptake value of distant metastases.

Association between metabolic phenotype, cigarette smoking, and EGFR mutation status
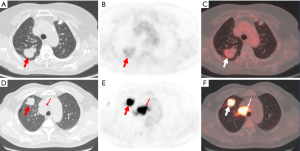
A significantly lower pSUVmax was observed in patients with EGFR mutant NSCLC than those with EGFR wild-type (8.9±4.5 vs. 12.7±6.9, P<0.001). There was no significant difference between nSUVmax and mSUVmax in patients with or without EGFR mutations (Table 1). In addition, we provided representative PET/CT images of two patients with EGFR mutations and EGFR wild-type NSCLC (Figure 3).
Based on these results, we performed ROC curve analysis to assess the value of predicting EGFR mutations in patients with NSCLC. The AUC was 0.688 [95% confidence interval (CI): 0.614–0.762] for cumulative smoking dose with sensitivity of 60.7% and specificity of 77.6% (Figure 4A), and 0.673 (95% CI: 0.599–0.747) for pSUVmax with sensitivity of 59.1% and specificity of 64.6% (Figure 4B). A multivariate logistic regression analysis was performed which showed that the AUC was 0.753 (95% CI: 0.685–0.821) when we combined cumulative smoking dose and pSUVmax with sensitivity of 66.3% and specificity of 74.0% (Figure 4C).

Discussion
In the present study, we observed a potential association between cigarette smoking and increased pSUVmax as well as decreased EGFR mutations in patients with NSCLC, thereby suggesting a plausible link between low pSUVmax and EGFR mutations. Based on cumulative smoking dose and pSUVmax, we obtained a moderate predictive value of EGFR mutations in patients with NSCLC.
The molecular genetic features of NSCLC have been evaluated by many studies, and patients with EGFR mutations usually benefit from targeted therapy with TKIs (30-32). However, almost all patients with NSCLC treated with EGFR-TKIs will inevitably develop drug resistance (33). Unfortunately, it is difficult to predict when resistance will occur during treatment. Thus, it is of great significance to evaluate EGFR mutations and prognosis in patients with lung cancer. Shigematsu et al. reported that EGFR mutations were found in 45% of never-smokers and only 7% of smokers (32); the frequency of EGFR mutations was negatively correlated with cigarette smoking exposure. Pham et al. (12) reported that among tobacco-related lung cancer patients with EGFR mutations, 39% were exposed for ≤15 pack-years and 7.48% were exposed for >15 pack-years. In our findings, EGFR mutations occurred in 60% of never smokers, compared with 35% of those who smoked 1–20 pack-years and 20% of those who smoked >20 pack-years. When we predicted EGFR mutations based on cumulative smoking dose, the AUC was 0.688 with a sensitivity of 60.7% and a specificity of 77.6%. These data may help clinicians to assess the likelihood of EGFR mutations in patients with NSCLC, but their predictive value is relatively low and further studies are needed.
Metabolic phenotypes, namely, pSUVmax, nSUVmax, and mSUVmax, have been used to predict EGFR mutation status in patients with NSCLC (34-36). Ko et al. retrospectively analyzed the predictive value of 18F-FDG PET/CT for EGFR mutation status, and the results showed that patients with SUVmax ≥6 were more likely to have EGFR mutations (35). However, Lee et al. found that 18F-FDG uptake in NSCLC had no significant clinical value in predicting EGFR mutation status (36). Conversely, Lv et al. demonstrated that low pSUVmax <7.0 was associated with mutant EGFR status in patients with NSCLC (34). In addition, the association between SUVmax and EGFR mutation status in lung ADC may be influenced by smoking status, particularly among individuals with a positive smoking history (37). These conflicting findings prompted us to further evaluate the association between metabolic activity and EGFR mutation status. In our results, we found that patients with EGFR mutations had a lower pSUVmax than those without EGFR mutation. In addition, there were no significant differences of nSUVmax and mSUVmax between patients with EGFR mutant NSCLC and EGFR wild-type. The AUC was 0.673 when we used pSUVmax to predict EGFR mutation status with sensitivity of 59.1% and specificity of 64.6%.
It has been shown that patients with lung cancer who have smoked and have high pSUVmax generally have lower survival rates than those who have never smoked and have low pSUVmax (16,20,38). EGFR mutations in lung cancer patients are commonly found in never-smokers (35). To our knowledge, a paradoxical association between EGFR mutation status and metabolic phenotype has been observed in NSCLC patients (22,24,25), but this situation still needs further research and verification. Moreover, the direct relationship between cigarette smoking, metabolic phenotypes, and EGFR mutation status has not been reported in NSCLC patients. In this study, we found that smoking patients had a higher pSUVmax and a lower rate of EGFR mutations than never-smokers, and patients with EGFR mutations had a lower pSUVmax than those with EGFR wild-type. Thus, we performed a multivariate logistic regression analysis using cumulative smoking dose and pSUVmax to predict EGFR mutation status. Our results showed a moderate predictive value, and the AUC was 0.753 with a sensitivity of 66.3% and a specificity of 74.0%. Based on these results, we suggest that cigarette smoking may be one of the inducements for the increase of pSUVmax and the decrease of EGFR mutation, and further confirm that EGFR mutation is related to the low pSUVmax, which may provide additional information to guide clinicians in risk stratification and treatment strategy selection for patients with NSCLC.
Although we elucidated the association between cigarette smoking, metabolic phenotypes, and EGFR mutation status in patients with NSCLC, our study had some limitations. First, the number of patients we retrospectively analyzed was relatively small, and the results need to be confirmed by extensive prospective analysis. Second, not all patients were tested for EGFR mutations, and there may have been bias in the selection of patients. Third, it would be of great significance if we could monitor the therapeutic response of patients with NSCLC and assess their prognosis based on the results of cigarette smoking and metabolic phenotypes. Overall, further studies are needed to verify these findings.
Conclusions
Our study comprehensively analyzed the association between cigarette smoking, metabolic phenotype, and the status of EGFR mutations in patients with NSCLC. Compared to never-smokers and with the increase of cumulative smoking dose, the metabolic activity of 18F-FDG in primary tumors increased significantly and the incidence of EGFR mutations decreased notably. We also observed that the status of EGFR mutations correlates with low pSUVmax. A moderate predictive value of EGFR mutations was found based on cumulative smoking dose and pSUVmax. These observations may guide clinicians to stratify the risk of NSCLC patients and select treatment strategies. Further prospective and retrospective studies are needed to confirm our findings and extend our study.
Acknowledgments
The authors are grateful to Dr. Haibo Shen, Gang Hua, Jie Li, Yiting Shi, Zhenyue Ye, and Junyong Zou in Ningbo No.2 Hospital for providing detailed clinical and molecular genetic information regarding our cases.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1371/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1371/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1371/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1371/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Ningbo No.2 Hospital (No. YJ-NBEY-KY202108401). Due to the retrospective nature of this study, the need for written informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584-90. [Crossref] [PubMed]
- Wang F, Tan F, Shen S, et al. Risk-stratified Approach for Never- and Ever-Smokers in Lung Cancer Screening: A Prospective Cohort Study in China. Am J Respir Crit Care Med 2023;207:77-88. [Crossref] [PubMed]
- Bates JHT, Hamlington KL, Garrison G, et al. Prediction of lung cancer risk based on age and smoking history. Comput Methods Programs Biomed 2022;216:106660. [Crossref] [PubMed]
- Wang X, Wang T, Hua J, et al. Histological types of lung cancer attributable to fine particulate, smoking, and genetic susceptibility. Sci Total Environ 2023;858:159890. [Crossref] [PubMed]
- Black GB, van Os S, Whitaker KL, et al. What are the similarities and differences in lung cancer symptom appraisal and help-seeking according to smoking status? A qualitative study with lung cancer patients. Psychooncology 2022;31:2094-103. [Crossref] [PubMed]
- Ko HW, Shie SS, Wang CW, et al. Association of smoking status with non-small cell lung cancer patients harboring uncommon epidermal growth factor receptor mutation. Front Immunol 2022;13:1011092. [Crossref] [PubMed]
- Kawaguchi T, Takada M, Kubo A, et al. Gender, histology, and time of diagnosis are important factors for prognosis: analysis of 1499 never-smokers with advanced non-small cell lung cancer in Japan. J Thorac Oncol 2010;5:1011-7. [Crossref] [PubMed]
- Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5:620-30. [Crossref] [PubMed]
- Miller VA, Kris MG, Shah N, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol 2004;22:1103-9. [Crossref] [PubMed]
- Cappuzzo F, Ligorio C, Jänne PA, et al. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the ONCOBELL trial. J Clin Oncol 2007;25:2248-55. [Crossref] [PubMed]
- Pham D, Kris MG, Riely GJ, et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol 2006;24:1700-4. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn 2013;15:415-53. [Crossref] [PubMed]
- Wang N, Zhang X, Wang F, et al. The Diagnostic Accuracy of Liquid Biopsy in EGFR-Mutated NSCLC: A Systematic Review and Meta-Analysis of 40 Studies. SLAS Technol 2021;26:42-54. [Crossref] [PubMed]
- Bedetti B, Schnorr P, May S, et al. Multidisciplinary Postoperative Validation of (18)F-FDG PET/CT Scan in Nodal Staging of Resected Non-Small Cell Lung Cancer. J Clin Med 2022;11:7215. [Crossref] [PubMed]
- Dondi F, Albano D, Bellini P, et al. Prognostic role of baseline (18)F-FDG pet/CT in stage I and stage ii non-small cell lung cancer. Clin Imaging 2023;94:71-8. [Crossref] [PubMed]
- Nomura K, Fukui M, Hattori A, et al. Diagnostic Value of Nodal Staging of Lung Cancer With Usual Interstitial Pneumonia Using PET. Ann Thorac Surg 2022;114:2073-9. [Crossref] [PubMed]
- Chung HW, Lee KY, Kim HJ, et al. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol 2014;140:89-98. [Crossref] [PubMed]
- Moon SH, Cho SH, Park LC, et al. Metabolic response evaluated by 18F-FDG PET/CT as a potential screening tool in identifying a subgroup of patients with advanced non-small cell lung cancer for immediate maintenance therapy after first-line chemotherapy. Eur J Nucl Med Mol Imaging 2013;40:1005-13. [Crossref] [PubMed]
- Ling T, Zhang L, Peng R, et al. Prognostic value of (18)F-FDG PET/CT in patients with advanced or metastatic non-small-cell lung cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front Immunol 2022;13:1014063. [Crossref] [PubMed]
- Jiang M, Zhang X, Chen Y, et al. A Review of the Correlation Between Epidermal Growth Factor Receptor Mutation Status and (18)F-FDG Metabolic Activity in Non-Small Cell Lung Cancer. Front Oncol 2022;12:780186. [Crossref] [PubMed]
- Cho A, Hur J, Moon YW, et al. Correlation between EGFR gene mutation, cytologic tumor markers, 18F-FDG uptake in non-small cell lung cancer. BMC Cancer 2016;16:224. [Crossref] [PubMed]
- Gao XC, Wei CH, Zhang RG, et al. (18)F-FDG PET/CT SUV(max) and serum CEA levels as predictors for EGFR mutation state in Chinese patients with non-small cell lung cancer. Oncol Lett 2020;20:61. [Crossref] [PubMed]
- Wang Y, Han R, Wang Q, et al. Biological Significance of (18)F-FDG PET/CT Maximum Standard Uptake Value for Predicting EGFR Mutation Status in Non-Small Cell Lung Cancer Patients. Int J Gen Med 2021;14:347-56. [Crossref] [PubMed]
- Kanmaz ZD, Aras G, Tuncay E, et al. Contribution of 18Fluorodeoxyglucose positron emission tomography uptake and TTF-1 expression in the evaluation of the EGFR mutation in patients with lung adenocarcinoma. Cancer Biomark 2016;16:489-98. [Crossref] [PubMed]
- Jiang M, Guo X, Zhang X, et al. Effects of cigarette smoking on metabolic activity of lung cancer on baseline (18)F-FDG PET/CT. PeerJ 2022;10:e13352. [Crossref] [PubMed]
- Jiang M, Chen P, Guo X, et al. Identification of EGFR mutation status in male patients with non-small-cell lung cancer: role of (18)F-FDG PET/CT and serum tumor markers CYFRA21-1 and SCC-Ag. EJNMMI Res 2023;13:27. [Crossref] [PubMed]
- Lee EY, Khong PL, Lee VH, et al. Metabolic phenotype of stage IV lung adenocarcinoma: relationship with epidermal growth factor receptor mutation. Clin Nucl Med 2015;40:e190-5. [Crossref] [PubMed]
- Alberg AJ, Ford JG, Samet JM, et al. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:29S-55S.
- Subramanian J, Govindan R. Molecular genetics of lung cancer in people who have never smoked. Lancet Oncol 2008;9:676-82. [Crossref] [PubMed]
- Fakhruddin N, Mahfouz R, Farhat F, et al. Epidermal growth factor receptor and KRAS mutations in lung adenocarcinoma: a retrospective study of the Lebanese population. Oncol Rep 2014;32:2223-9. [Crossref] [PubMed]
- Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer 2006;118:257-62. [Crossref] [PubMed]
- Li S, Zhu S, Wei H, et al. The prospect of combination therapies with the third-generation EGFR-TKIs to overcome the resistance in NSCLC. Biomed Pharmacother 2022;156:113959. [Crossref] [PubMed]
- Lv Z, Fan J, Xu J, et al. Value of (18)F-FDG PET/CT for predicting EGFR mutations and positive ALK expression in patients with non-small cell lung cancer: a retrospective analysis of 849 Chinese patients. Eur J Nucl Med Mol Imaging 2018;45:735-50. [Crossref] [PubMed]
- Ko KH, Hsu HH, Huang TW, et al. Value of 18F-FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol Imaging 2014;41:1889-97. [Crossref] [PubMed]
- Lee SM, Bae SK, Jung SJ, et al. FDG uptake in non-small cell lung cancer is not an independent predictor of EGFR or KRAS mutation status: a retrospective analysis of 206 patients. Clin Nucl Med 2015;40:950-8. [Crossref] [PubMed]
- Gao J, Shi Y, Niu R, et al. Association Analysis of Maximum Standardized Uptake Values Based on (18)F-FDG PET/CT and EGFR Mutation Status in Lung Adenocarcinoma. J Pers Med 2023;13:396. [Crossref] [PubMed]
- Maguire FB, Movsisyan AS, Morris CR, et al. Evaluation of Cancer Deaths Attributable to Tobacco in California, 2014-2019. JAMA Netw Open 2022;5:e2246651. [Crossref] [PubMed]


