
Vein graft preservation with an endothelial damage inhibitor in isolated coronary artery bypass surgery: an observational propensity score-matched analysis
Highlight box
Key findings
• The use of an endothelial damage inhibitor (EDI) for vein flushing and preservation resulted in a significant decrease in major adverse cardiac events (MACEs) at 3 years post-procedure (10.0% vs. 21.1%, log-rank P=0.035). This benefit was more pronounced in diabetic patients (7.1% vs. 20.9%; log-rank P=0.041) and in patients who had more than one vein graft used (4.0% vs. 30.3%; log-rank P=0.015).
What is known and what is new?
• Saphenous vein grafts may experience accelerated atherosclerosis and graft failure is often linked to non-fatal events.
• The utilization of an EDI significantly decreases the incidence of MACE. This protective effect is particularly notable in diabetics and after multiple vein grafts.
What is the implication, and what should change now?
• The use of an EDI should be recommended for vein preservation and flushing, particularly in diabetic patients or when multiple vein grafts are used.
Introduction
One of the main benefits of coronary artery bypass grafting (CABG) is its durability, with a significant reduction in long-term adverse events when compared to percutaneous coronary interventions (PCI) (1). However, graft failure is often linked to non-fatal events such as acute coronary syndromes (ACSs), recurring angina, and the need for additional revascularization (2). Saphenous vein grafts may experience accelerated atherosclerosis compared to arterial grafts, leading to stenosis and blockage (3). The expected occurrence of saphenous vein graft failure is roughly 50% of the grafts blocked 5–10 years after the procedure (4).
Nearly 95% of patients who undergo CABG worldwide receive at least one vein graft (5). This widespread use of vein grafts emphasizes the importance of implementing strategies to enhance their patency. Factors that affect vein graft patency include the surgical technique during harvesting (6), preservation methods (7), and postoperative medical therapy with antiplatelet medication and statins (8).
Recently, DuraGraft® (Marizyme, Jupiter, FL, USA), an endothelial damage inhibitor (EDI) solution, has been used for vein preservation. This solution has been shown to decrease oxidative stress and maintain endothelial integrity after harvesting with improved graft patency (9). Using an EDI may be associated with improved long-term event-free survival after CABG (10,11).
The objective of this research is to examine the potential effect of using an EDI on the reduction of major adverse cardiac events (MACEs) 3 years post-surgery, in comparison to a group of patients treated with the conventional preservation solution. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-636/rc) (12).
Methods
Ethical considerations
The EDI employed in this study is a Conformité Européenne (CE) marking approved product since October 2014 (13) and was used in its labeled indication. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Board of Hospital Universitario Ramón y Cajal (No. 005-01). Each patient in whom the EDI was used provided written informed consent for the use of the preservation solution. Due to the observational nature of the study, and the fact that data were anonymized, the need for informed consent in the control group was waived.
Study population
This is a single-center, prospective, observational cohort study that includes all patients who underwent isolated CABG and received at least one saphenous vein graft during surgery between 2014 and 2019. As per our institutional protocol, an EDI was used in every CABG patient between 2016 and 2018. Our institution (Hospital Universitario Ramón y Cajal), a public university hospital, serves patients referred from the Madrid metropolitan area and surrounding regions.
Therefore, the treatment group consists of patients who received the EDI used to intraoperatively flush and store the vein grafts. The control group was selected from all patients who underwent isolated CABG between 2014 and 2019 and vein harvest and preservation were conducted with the conventional technique (Figure 1). The control group was balanced through propensity score (PS) matching. Out of the 103 isolated CABG patients in whom the EDI was used, the PS algorithm identified 90 pairs that could be matched to the control cohort. The 13 patients whose calculated PS fell outside the defined maximum caliper distance of 3% standard deviation were not included in the study sample due to potentially differing baseline characteristics from the control group.
Variables of the study and follow-up
All data during the hospital admission was extracted from the digital medical (HCIS, Dedalus) records of our institution. Follow-up was conducted by consulting digital records of the hospital and completing with regional level data if needed. All patients were contacted through an annual telephone interview. The follow-up was conducted up to 3 years after the procedure.
Three-year follow-up was complete for all the study cohort, except for one patient in the EDI cohort who moved to another country, and contact was not possible.
Vein harvesting and preservation technique
The conventional treatment for vein harvest at our institution is as follows: the vein was harvested by the open or endoscopic technique (Vasoview Hemopro®, Getinge, Gothenbourg, Sweden). After complete dissection of the desired graft length, all side branches were dissected and secured with automated hemoclips (Ligaclip®, Ethicon, Cincinnati, OH, USA). The vein was cut at its distal portion and connected to a cannula to infuse the vein preservation solution.
The standard preservation solution used was 100 mL of 0.9% saline solution, with 5,000 UI of heparin and 2.5 mL of intravenous (IV) nitroglycerin. The vein was flushed with the preservation solution, taking care not to over-distend the vein to avoid endothelial disruption and ensuring proper hemostasis of all side branches. The full graft was then preserved in the preservation solution until it was used as a coronary graft when required.
The treatment arm consisted of patients in whom the conventional preservation solution was substituted with the EDI. The EDI used in this study is a solution used to flush the vein after harvesting and as a preservation fluid for storage until it is used. In brief, it is an ionically and pH-balanced physiological solution containing L-glutathione, L-ascorbic acid, L-arginine, and other additives that protect the graft from the damaging effects of ischemia and handling during CABG.
All other aspects of the harvesting technique or surgical technique for bypass elaboration remained unchanged. Furthermore, it is important to note that in our institution, we routinely employed TTFM (Transit-Time Flow Measurement) for intraoperative graft assessment to evaluate graft flow and pulsatility in every CABG procedure. If the obtained measurements were deemed suboptimal, immediate graft revision was performed to ensure optimal graft function and patency.
Every CABG patient in our institution is discharged home with dual antiplatelet therapy (DAPT) for a duration of 3 months, along with high-dose statins. The DAPT regimen consists of aspirin in combination with clopidogrel. It is important to note that both study groups adhered to this institutional protocol, with a 100% completion rate.
Objectives of the study
The main objective of the study was to analyze the occurrence of MACEs up to 3 years after the procedure. MACE was defined as a combination of all-cause mortality, new ACS requiring hospitalization, or a new unplanned revascularization. All-cause mortality was considered to avoid potential bias in cause of death adjudication.
The secondary objective was to individually analyze all-cause postoperative mortality, new ACS requiring hospitalization, or new unplanned revascularization.
Two subgroups were pre-determined for subgroup analysis based on the study protocol due to their higher expected events during follow-up: diabetic patients and patients who received more than one vein graft.
Statistical analysis
Given the observational nature of the study, PS matching was used to control for confounding by indication and adjust for imbalances in preoperative characteristics of the study population that may be associated with early vein graft failure. The PS was calculated using the 1:1 nearest neighbor technique, without replacement, and with a caliper of 0.03 standard deviations.
The PS was estimated using logistic regression, with the dependent variable being the preservation solution used (EDI or conventional treatment), and the independent variables being pre-selected factors that could be related to early vein graft failure (14). The independent variables that were employed in the PS calculation were age at intervention, diabetes mellitus, smoking history, number of distal anastomoses, number of vein grafts, and use or non-use of off-pump coronary artery bypass (OPCAB) grafting technique.
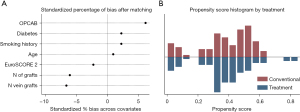
The matching algorithm identified 90 pairs of patients, distributed in the two cohorts (Figure 1). The imbalance in the independent variables was analyzed, and the percentage of bias was less than 10% in all the covariates after matching (Table 1), which suggests that the groups are well-matched. The overlap of calculated PS was also analyzed, and the PS matching was successful in balancing the distribution of covariates between the groups (Figure 2). Statistics for independent cohorts was used thereafter.
Table 1
| PS covariates | Treatment (n=90) | Conventional (n=90) | % bias |
|---|---|---|---|
| Age (years) | 69.6±9.0 | 69.5±9.6 | 0.9 |
| Diabetes | 42 (46.7) | 43 (47.8) | 2.2 |
| Smoking history | 46 (51.1) | 45 (50.0) | 2.2 |
| EuroSCORE 2 | 3.44±4.21 | 3.54±4.73 | −2.3 |
| No. of distal anastomosis | 3.04±0.72 | 3.08±0.74 | −6.1 |
| No. of saphenous grafts | 1.4±0.70 | 1.4±0.64 | −6.1 |
| OPCAB | 75 (83.3) | 77 (85.6) | 6.1 |
Data are presented as mean ± SD or n (%). PS, propensity score; EuroSCORE, European system for cardiac operative risk evaluation; OPCAB, off-pump coronary artery bypass; SD, standard deviation.
Continuous variables were presented as mean and standard deviation or median and interquartile range if they were significantly skewed. Normality was determined using the Shapiro-Wilk test. Equality of variances was assessed using Levene’s robust test, and comparisons were made using the Student’s t-test. Categorical variables were presented as frequencies and percentages, and comparisons were made using chi-squared test or Fisher’s exact test as appropriate.
Event-free survival was analyzed using Kaplan-Meier analysis. Differences in survival among study groups were evaluated using the log-rank test. Incidence-rate (IR) data was used to calculate point estimates and confidence intervals (CIs) for the incidence-rate ratio (IRR).
Results
During the study period, 424 patients were operated on isolated CABG in our institution with the use of at least one vein graft (Figure 1). The PS algorithm matched 90 patients in each group, ensuring a balanced distribution of all the selected variables (Table 1).
Table 2 presents the baseline characteristics of the study population. A high percentage of patients had risk factors for coronary disease, with nearly 50% being diabetic. European system for cardiac operative risk evaluation (EuroSCORE) 2 was similar in both groups and the surgical details were comparable. Both groups had a similar number of grafts per patient, and 32.2% of the patients had more than one vein graft. A high percentage of patients underwent off-pump CABG (84.4%) and endoscopic vein harvesting (20.2%). Anatomical complete revascularization was achieved in the 96.1% of the patients, without differences among both groups.
Table 2
| Study variables | Treatment (n=90) | Conventional (n=90) | P value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 69.6±9.0 | 69.5±9.6 | 0.95 |
| Male | 74 (82.2) | 81 (90.0) | 0.13 |
| Diabetes | 42 (46.7) | 43 (47.8) | 0.88 |
| Arterial HTN | 75 (83.3) | 74 (82.2) | 0.84 |
| Dyslipidemia | 67 (74.4) | 73 (81.1) | 0.60 |
| Smoking history | 46 (51.1) | 45 (50.0) | 0.88 |
| PERIF arterial disease | 21 (23.3) | 22 (24.4) | 0.83 |
| Active smoker | 12 (13.3) | 19 (21.1) | 0.19 |
| EuroSCORE 2 | 3.44±4.21 | 3.54±4.73 | 0.88 |
| No. of diseased vessels | 2.84±0.36 | 2.80±0.51 | 0.48 |
| Left main disease | 43 (47.8) | 42 (46.7) | – |
| Surgical procedure | |||
| No. of distal anastomosis | 3.04±0.72 | 3.08±0.74 | 0.68 |
| No. of saphenous grafts | 1.4±0.70 | 1.4±0.64 | 0.66 |
| More than 1 vein | 25 (27.8) | 33 (36.7) | 0.20 |
| No. of IMA per patient | 1.63±0.53 | 1.61±0.53 | 0.80* |
| OPCAB | 75 (83.3) | 77 (85.6) | 0.68 |
| EVH | 17 (18.9) | 19 (21.1) | 0.77 |
| Postoperative course | |||
| Days of ICU | 1 [0–2] | 2 [0–2] | 0.13** |
| Hospitalization days since surgery | 7 [6–9] | 7 [6–9] | 0.69** |
Data are presented as mean ± SD, n (%) or median [Q1–Q3]. *, unequal variance Student’s t-test; **, Wilcoxon rank-sum test. HTN, hypertension; PERIF, peripheral; EuroSCORE, European system for cardiac operative risk evaluation; IMA, internal mammary artery; OPCAB, off-pump coronary artery bypass; EVH, endoscopic vein harvest; ICU, intensive care unit; SD, standard deviation; Q, quartile.
The study found that in-hospital mortality and hospital length of stay were equivalent. In-hospital mortality was 2.2% and was lower than expected by EuroSCORE 2 (mean EuroSCORE 2 of 3.5%). After 3 years of follow-up, overall mortality was still equivalent among both groups (Table 3).
Table 3
| Follow-up | Treatment (n=90) | Conventional (n=90) | Log-rank P |
|---|---|---|---|
| Primary end-point | |||
| Overall MACE | 9 (10.0) | 19 (21.1) | 0.035* |
| Freedom MACE 1 year (%) | 93 [86–97] | 82 [72–89] | |
| Freedom MACE 2 years (%) | 91 [82–95] | 80 [70–87] | |
| Freedom MACE 3 years (%) | 89 [80–94] | 78 [68–86] | |
| Secondary end-point | |||
| In-hospital mortality | 2 (2.2) | 2 (2.2) | 0.96 |
| Three-year mortality | 8 (8.9) | 7 (7.8) | 0.20 |
| ACS | 5 (5.6) | 11 (12.2) | 0.10 |
| Revascularization | 1 (1.1) | 4 (4.4) | 0.14 |
| Clinical status at last follow-up | |||
| Angina | 15 (16.7) | 16 (17.8) | – |
| NYHA | – | ||
| NYHA I | 65 (72.2) | 59 (65.6) | |
| NYHA II | 14 (15.6) | 23 (25.6) | |
| NYHA III | 8 (8.9) | 6 (6.7) | |
| NYHA IV | 2 (2.2) | 2 (2.2) | |
| Pre-specified subgroup analysis | |||
| MACE/diabetic patients | |||
| Diabetics | 3 (7.1) | 9 (20.9) | 0.041* |
| No diabetics | 6 (12.8) | 10 (20.8) | 0.29 |
| MACE/multiple vein grafts | |||
| More than 1 vein | 1 (4.0) | 10 (30.3) | 0.015* |
| Single vein | 8 (12.3) | 9 (15.8) | 0.51 |
MACEs among both cohorts. The survival was analyzed using the Kaplan-Meier method and compared with log-rank test. Data are presented as n (%) or HR [95% CI]. *, statistically significant differences. In treatment group, there is one loss to follow up in the treatment arm. Pre-specified subgroup analysis was conducted in the diabetic patients (85 patients) and in those with multiple vein grafts (58 patients). MACE, major adverse cardiac event; ACS, acute coronary syndrome; NYHA, New York Heart Association; HR, hazard ratio; CI, confidence interval.
As shown in Table 3 and Figure 3A, after 3 years of follow-up the treatment arm had significantly less MACEs than the control group (10.0% vs. 21.1%, log-rank P=0.035) and a lower incidence rate of MACE during follow-up (4.0 events/100 patients/year in the EDI group compared to 9.8 events/100 patients/year in the control group). The use of an EDI had a significant protective effect against MACE at 3 years, with an IRR of 0.41 (95% CI: 0.16–0.96; P=0.026). Additionally, most patients had minimal symptoms of angina at last follow-up (Table 3).

In the prespecified subgroup analysis (Table 3), treatment significantly improved event free survival rates in diabetic patients (7.1% vs. 20.9%; log-rank P=0.041) (Figure 3B) and in patients with more than one vein used (4.0% vs. 30.3%; log-rank P=0.015) (Figure 3C).
Besides, we found that 3-year freedom from MACE was similar between patients who underwent surgery on-pump and off-pump (IRR =0.61; 95% CI: 0.24–1.84; P=0.29). We also found that endoscopic vein harvest (EVH) did not affect 3-year freedom from MACE (IRR =0.80; 95% CI: 0.2–2.3; P=0.73).
Discussion
The main finding of this study is that the use of an EDI for vein flushing and preservation in CABG resulted in a significant decrease in MACE at 3 years post-procedure. This benefit was more pronounced in diabetic patients and in patients who had more than one vein graft used.
CABG often requires multiple grafts, and although arterial grafts have a proven longer patency than venous ones (15), currently, more than 95% of patients undergoing CABG have one or more vein grafts (3,5). These vein grafts are prone to accelerated atherosclerosis (4), with an estimated 50% occlusion 5–10 years after the procedure. This accelerated atherosclerosis is thought to be related to various factors, such as damage during graft harvest and chronic inflammation (16,17), intimal hyperplasia caused by adaptation to arterial pressure (18), technical issues, and comorbidities such as dyslipidemia, hypertension, diabetes mellitus, and smoking (14). Accelerated atherosclerosis can lead to graft failure, which can result in non-fatal complications even years after the procedure (2), with the occurrence of new acute myocardial infarction, ACSs, and new unplanned revascularizations.
Medication after CABG is crucial for optimizing long-term outcomes and maintaining graft patency, therefore antiplatelet therapy (19) and statins should be prescribed to CABG patients to improve outcomes (8). Additionally, vein graft patency can be improved through various strategies such as no-touch vein harvest (20), preservation solutions (7), and external supports (21).
Preservation solutions are a particularly promising strategy and studies have shown that an EDI can maintain endothelial integrity and function after harvest (11,22), which may lead to less chronic inflammation and hyperplasia (11). This diminished intimal hyperplasia at 12 months after the procedure has been demonstrated by Perrault et al. (11), with uncertain clinical benefit. Besides, the use of preservation solutions during vein harvest is easy to implement, as it does not require any changes to the surgical technique and is readily available as a commercial product.
We conducted a study comparing 3-year event-free survival in a cohort of patients in whom the EDI was used, against a matched cohort of patients who received the conventional harvest and preservation technique. PS matching was used to account for potential biases, and several variables that were thought to influence graft patency were used in the matching algorithm (14).
The overall incidence of MACEs in the cohort was 15.6%, which aligns with previously reported rates among patients with similar characteristics (23). Our study found that the use of an EDI significantly enhanced event-free survival from MACEs with a 58.8% preventable fraction. Similar to other previous observational studies, our study also showed that the use of an EDI during CABG improves long-term prognosis. However, our study offers a unique advantage by utilizing PS matching to control for bias. Haime et al. (10) reported a 45% lower occurrence of non-fatal myocardial infarction and 19% lower risk of long-term MACE in the EDI cohort. However, a randomized controlled trial is needed to confirm the evidence suggested by the observational studies.
It is crucial to highlight that the use of an EDI in our study aimed to prevent long-term complications associated with intimal hyperplasia and inflammation. While the observed difference in recurrent angina and repeat revascularization in the first year may not be solely attributed to intimal hyperplasia, it is important to consider that short-term graft dysfunction can be influenced by factors related to graft preservation and endothelial integrity. By employing an EDI, we sought to maintain endothelial integrity and function, which has the potential to reduce short-term graft-related complications.
Furthermore, we analyzed two subgroups at higher risk of early vein graft failure: diabetic patients and those with multiple vein grafts. Our findings indicate that the use of an EDI is associated with a preventable fraction of MACE of 74% in diabetic patients and 89.5% in patients with multiple vein grafts. However, the effect of the EDI was not statistically significant in patients without diabetes or with only one vein graft, possibly due to low statistical power in a less frequent event. Therefore, the protective effect of an EDI is particularly pronounced in these subgroups of patients.
In conclusion, these results suggest that an EDI should be recommended for vein preservation and flushing in CABG, particularly in diabetic patients or when multiple vein grafts are used.
Limitations
The main limitation of this study is that it is observational in nature. While PS matching was used to control for variables and bias by indication, it is still possible that uncontrolled factors may have influenced the results. We acknowledge that our PS logistic model included a limited number of variables. However, it is important to note that the variables included were carefully selected based on their clinical relevance and potential impact on vein graft durability. Our aim was to capture the most significant factors while minimizing collinearity and potential confounding.
Besides, a potential selection bias cannot be ruled out, but as the EDI was used in every patient between 2016 and 2018, the use of the product was not influenced by any other aspect apart from the date of the surgical procedure.
Other important limitation is the low power of the study. The study was designed with a sample size of 90 patients in each group, resulting in a power of 54% (based on a 1:1 ratio, observed event rates of 10.0% and 21.1% for the primary endpoint). Despite the limitations of the sample size and low statistical power, we were able to identify significant differences. This suggests that the observed statistical significance may indeed reflect a clinically effective impact of the treatment arm. Additionally, we would like to clarify that the subgroup analyses performed in this study were prespecified as part of our research plan. These analyses were included to explore potential effects within specific patient subsets and provide additional insights. Nonetheless, we acknowledge that some of the subgroup sizes were relatively small, and caution should be exercised when interpreting the results in these subgroups.
Another limitation is the fact that vein patency was not assessed in every patient, therefore, the observed MACE cannot be directly related to vein graft occlusions. We have limited data available regarding vein graft patency. Among the 5 patients who required revascularization, it was observed that 3 of them had occluded vein grafts, with 2 cases in the conventional treatment arm. However, it is important to note that these numbers are relatively small, and drawing definitive conclusions based on this limited information would be inappropriate.
Finally, the control group received saline as conventional treatment. However, there is evidence that alternative preservation solutions, such as heparinized autologous blood or buffered solutions, may be more effective. These alternative solutions were not compared to the EDI in this study.
As this is an observational study, it generates hypotheses, but it is not conclusive. To confirm the findings, a randomized controlled trial is needed to provide stronger evidence.
Conclusions
In conclusion, the utilization of the EDI for vein flushing and storage after vein harvest in CABG procedures has been shown to significantly decrease the incidence of MACE at 3 years post-surgery. This protective effect is particularly notable in diabetic patients and in individuals who have multiple vein grafts.
These findings suggest that the use of an EDI may be a valuable tool in improving outcomes for CABG patients.
Acknowledgments
We thank the patients who participated in this study for their contribution to this project.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-636/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-636/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-636/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-636/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics board of Hospital Universitario Ramón y Cajal (No. 005-01). Each patient in whom the EDI was used provided written informed consent for the use of the preservation solution. Due to the observational nature of the study, and the fact that data were anonymized, the need for informed consent in the control group was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629-38. [Crossref] [PubMed]
- Gaudino M, Di Franco A, Bhatt DL, et al. The association between coronary graft patency and clinical status in patients with coronary artery disease. Eur Heart J 2021;42:1433-41. [Crossref] [PubMed]
- Royse A, Ren J, Royse C, et al. Coronary Artery Bypass Surgery Without Saphenous Vein Grafting: JACC Review Topic of the Week. J Am Coll Cardiol 2022;80:1833-43. [Crossref] [PubMed]
- Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. [Crossref] [PubMed]
- Caliskan E, de Souza DR, Böning A, et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat Rev Cardiol 2020;17:155-69. [Crossref] [PubMed]
- Souza DS, Dashwood MR, Tsui JC, et al. Improved patency in vein grafts harvested with surrounding tissue: results of a randomized study using three harvesting techniques. Ann Thorac Surg 2002;73:1189-95. [Crossref] [PubMed]
- Harskamp RE, Alexander JH, Schulte PJ, et al. Vein graft preservation solutions, patency, and outcomes after coronary artery bypass graft surgery: follow-up from the PREVENT IV randomized clinical trial. JAMA Surg 2014;149:798-805. [Crossref] [PubMed]
- Kulik A, Ruel M, Jneid H, et al. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation 2015;131:927-64. [Crossref] [PubMed]
- Aschacher T, Baranyi U, Aschacher O, et al. A Novel Endothelial Damage Inhibitor Reduces Oxidative Stress and Improves Cellular Integrity in Radial Artery Grafts for Coronary Artery Bypass. Front Cardiovasc Med 2021;8:736503. [Crossref] [PubMed]
- Haime M, McLean RR, Kurgansky KE, et al. Relationship between intra-operative vein graft treatment with DuraGraft® or saline and clinical outcomes after coronary artery bypass grafting. Expert Rev Cardiovasc Ther 2018;16:963-70. [Crossref] [PubMed]
- Perrault LP, Carrier M, Voisine P, et al. Sequential multidetector computed tomography assessments after venous graft treatment solution in coronary artery bypass grafting. J Thorac Cardiovasc Surg 2021;161:96-106.e2. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- DuraGraft for preserving vascular grafts. Medtech innovation briefing. 2019. Available online: https://www.nice.org.uk/advice/mib184
- Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation 1998;97:916-31. [Crossref] [PubMed]
- Taggart DP, Gaudino MF, Gerry S, et al. Effect of total arterial grafting in the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2022;163:1002-9.e6. [Crossref] [PubMed]
- Tekin I, Demir M, Özdem S. Effect of different storage solutions on oxidative stress in human saphenous vein grafts. J Cardiothorac Surg 2022;17:7. [Crossref] [PubMed]
- Pimentel MD, Lobo Filho JG, Lobo Filho HG, et al. Effect of preservation solution and distension pressure on saphenous vein's endothelium. Interact Cardiovasc Thorac Surg 2022;35:ivac124. [Crossref] [PubMed]
- Goldstein DJ, Puskas JD, Alexander JH, et al. External Support for Saphenous Vein Grafts in Coronary Artery Bypass Surgery: A Randomized Clinical Trial. JAMA Cardiol 2022;7:808-16. [Crossref] [PubMed]
- López J, Morales C, Avanzas P, et al. Long-term effect of dual antiplatelet treatment after off-pump coronary artery bypass grafting. J Card Surg 2013;28:366-72. [Crossref] [PubMed]
- Tian M, Wang X, Sun H, et al. No-Touch Versus Conventional Vein Harvesting Techniques at 12 Months After Coronary Artery Bypass Grafting Surgery: Multicenter Randomized, Controlled Trial. Circulation 2021;144:1120-9. [Crossref] [PubMed]
- Goldstein DJ. Device profile of the VEST for external support of SVG Coronary artery bypass grafting: historical development, current status, and future directions. Expert Rev Med Devices 2021;18:921-31. [Crossref] [PubMed]
- Thatte HS, Biswas KS, Najjar SF, et al. Multi-photon microscopic evaluation of saphenous vein endothelium and its preservation with a new solution, GALA. Ann Thorac Surg 2003;75:1145-52; discussion 1152. [Crossref] [PubMed]
- Head SJ, Holmes DR Jr, Mack MJ, et al. Risk profile and 3-year outcomes from the SYNTAX percutaneous coronary intervention and coronary artery bypass grafting nested registries. JACC Cardiovasc Interv 2012;5:618-25. [Crossref] [PubMed]



