
Etoposide soft capsule combined with anlotinib in the third-line treatment of advanced non-small cell lung cancer: a retrospective cohort study
Highlight box
Key findings
• Etoposide soft capsule combined with anlotinib has a certain clinical effect in the third-line treatment of advanced non-small cell lung cancer (NSCLC).
What is known and what is new?
• Anlotinib has a certain clinical effect in the third-line monotherapy of advanced NSCLC.
• This study retrospectively analyzed the efficacy and safety of etoposide soft capsules combined with anlotinib in the third-line treatment of advanced NSCLC.
What is the implication, and what should change now?
• For patients with third-line NSCLC who refuse intravenous chemotherapy or whose tolerance has declined, this option may be considered for treatment.
Introduction
Lung cancer has prevailed as a malignant tumor with high morbidity and mortality in China and worldwide. Non-small cell lung cancer (NSCLC) accounts for about 85% of lung cancer (1), and most cases of NSCLC have already reached the advanced stage by the time of diagnosis and have lost the opportunity for radical surgery. Patients with advanced NSCLC with positive driver genes are generally treated with corresponding targeted drugs in the first and second lines. Those patients with negative driver genes often receive chemotherapy combined with anti-angiogenesis therapy or immunotherapy in the first and second lines (2). When there is progression on second-line treatment, the physical tolerance in the patient often deteriorates, with a limited effective rate of the third-line treatment (1). Shao et al. have shown that the median progression-free survival (mPFS) values in the single chemotherapy agent, EGFR-TKIs and doublet chemotherapy groups were 2.30, 3.17 and 2.37 months. The rates of stage III–IV toxicities were 33.3%, 18.2% and 68.8% (3). With the emergence of more low-toxic chemotherapy and small-molecule targeted drugs, more patients still have the opportunity to receive third-line treatment after first-line and second-line treatment, and third-line treatment is superior to the best supportive treatment, which has been confirmed by a number of studies (4-6). We need more efficient and less toxic solutions.
Anlotinib was approved by the Chinese Food and Drug Authority (FDA) in 2018 for the third-line monotherapy of advanced NSCLC (7). In the ALTER0303 study, the mPFS in the anlotinib group was 5.4 months, the median overall survival (mOS) was 9.6 months, the objective response rate (ORR) was 9.2%, and the disease control rate (DCR) was 81.0% (8). Zhong et al. also showed that anlotinib demonstrated a marked effect on the clinical treatment of advanced NSCLC patients; it could effectively prolong the PFS of patients, was well tolerated and safe (9).
A study has shown that the ORR of anlotinib combined with chemotherapeutic drugs can reach 27%, and the DCR can reach 78% (10), but there are few related reports. Etoposide soft capsules are commonly used oral chemotherapeutic drugs in clinical practice. This is a clinically accessible combination of etoposide and anlotinib. This study retrospectively analyzed the efficacy and safety of etoposide soft capsules combined with anlotinib in the third-line treatment of advanced NSCLC. The detailed reports are as follows. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1412/rc).
Methods
Clinical data
A retrospective analysis was conducted of the clinical data of 46 patients with advanced NSCLC who received etoposide soft capsule combined with anlotinib in the third-line treatment from January 2019 to December 2020 in the Second Affiliated Hospital of Fujian Medical University. The inclusion criteria were as follows: (I) NSCLC was diagnosed by pathological tissue combined with imaging diagnosis as stage IV NSCLC; (II) disease progression or treatment intolerance after two previous systemic chemotherapy regimens or targeted therapies had been used; (III) the patient signed an informed consent and chose etoposide soft capsule combined with anlotinib for treatment; (IV) Eastern Cooperative Oncology Group (ECOG) performance status (PS) score ≤2 points, electrocardiogram, blood routine, biochemical, urine routine, and blood coagulation function had no treatment contraindications; (V) according to the response evaluation criteria in solid tumors version 1.1 (RECIST 1.1), there were clinically relevant lesions measurable by imaging [computed tomography (CT), positron emission tomography (PET)-CT, or magnetic resonance imaging (MRI)]. The exclusion criteria were as follows: (I) patients with active bleeding, including gastrointestinal bleeding, hemoptysis, brain metastasis bleeding, and so on; (II) previous history of poorly controlled hypertension [systolic blood pressure ≥150 mmHg (1 mmHg =0.133 kPa), diastolic blood pressure ≥100 mmHg]; (III) patients with urine protein ++ to +++ in urine routine; (IV) central type, cavity lung squamous cell carcinoma or imaging showing that the tumor had invaded important blood vessels, or those with tumors that the investigators considered very likely to invade important blood vessels and cause fatal hemorrhage in the follow-up study; (V) those who had experienced arterial/venous thrombosis within 6 months, such as cerebrovascular accident, deep vein thrombosis, and pulmonary embolism. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University (No. 435). Individual consent for this retrospective analysis was waived.
Treatment plan
Etoposide soft capsule (trade name: Wick) 50 mg each time, once a day, to be taken orally on an empty stomach in the morning, d1–10. Anlotinib (trade name: Focavi) 12 mg each time, once a day, to be taken orally before breakfast, d1–14. The program comprised a 3-week cycle. If there was a 3rd or 4th degree treatment-related toxicity, anlotinib could be reduced to 8 or 10 mg once a day. If the investigator interpreted that the patient was not suitable for continued medication or was evaluated as experiencing disease progression according to the RECIST 1.1, the medication was terminated.
Evaluation of curative effect and adverse reactions
The curative effect was evaluated every 2 cycles, and the adverse reactions were evaluated every cycle until an intolerable adverse reaction or disease progression occurred. According to the RECIST 1.1 standard, the efficacy evaluation was categorized into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). ORR = (CR + PR)/total number of cases × 100%, DCR = (CR + PR + SD)/total number of cases × 100%. Progression-free survival (PFS) was defined as the time from the beginning of the third-line treatment to the progression of the disease. Overall survival (OS) was defined as the time from the start of third-line treatment to death. Adverse reactions were classified into grade 0–IV according to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE) 4.0 standard.
Statistical methods
The statistical software SPSS 20.0 (IBM Corp., Armonk, NY, USA) was used for data analysis. The count data was expressed as a percentage (%). The survival analysis was performed by the Kaplan-Meier method. All statistical tests were two sided, and P<0.05 was considered to be statistically significant.
Results
Clinical features and efficacy
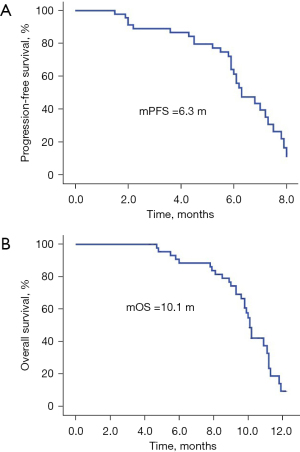
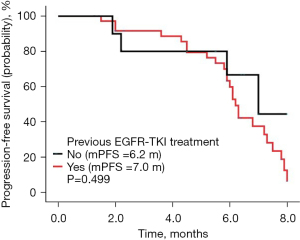
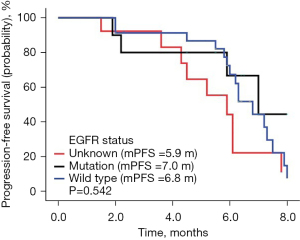
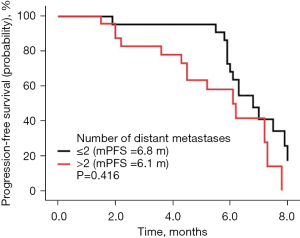
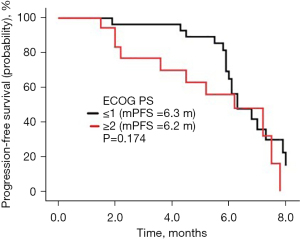
The follow-up lasted 2.5 years. By the end of the follow-up, all 46 cases had died. Among all cases, 32 were males and 14 were females, with a median age of 64 years. There were 31 cases with a history of smoking. The ECOG score was ≤1 in 28 cases and ≥2 in 18 cases. There were a total of 30 adenocarcinoma cases, 16 squamous cell carcinoma cases, and there were 10 cases of epidermal growth factor receptor (EGFR) type, 23 cases of wild type, and 13 cases were untested. There were 22 cases with ≤2 and 24 cases with >2 metastatic sites, respectively, and 11 cases of brain metastases; 19 cases had received bevacizumab treatment in the past, 10 cases of EGFR had been treated with TKI, and 9 cases had been treated with immune checkpoint inhibitors (Table 1). There were no cases of CR, 9 cases of PR, 29 cases of SD, and 8 cases of PD. The ORR was 19.57% (9/46), the DCR was 82.61% (38/46), the mPFS was 6.3 months (Figure 1A), and the mOS was 10.1 months (Figure 1B). Among them, univariate analysis showed that in the subgroup of patients with adenocarcinoma, who had previously received TKI treatment, ≤2 metastases, and an ECOG PS score ≤1, the mPFS had a tendency to prolong. Patients who had never used bevacizumab had significantly longer mPFS than those who had previously used bevacizumab (P<0.05) (Figures 2-7).
Table 1
| Characteristics | Patients (n=46) |
|---|---|
| Gender, n [%] | |
| Male | 32 [70] |
| Female | 14 [30] |
| Age (years), n [%] | |
| Median | 64 |
| ≤65 | 30 [65] |
| >65 | 16 [35] |
| Smoking history, n [%] | |
| Yes | 31 [67] |
| No | 15 [33] |
| ECOG PS | |
| ≤1 | 28 [61] |
| ≥2 | 18 [39] |
| Pathologic type, n [%] | |
| Adenocarcinoma | 30 [65] |
| Squamous cell carcinoma | 16 [35] |
| EGFR status, n [%] | |
| Mutation | 10 [22] |
| Wild type | 23 [50] |
| Unknown | 13 [28] |
| Number of distant metastases, n [%] | |
| ≤2 | 22 [48] |
| >2 | 24 [52] |
| Brain metastases, n [%] | |
| Yes | 11 [24] |
| No | 35 [76] |
| Previous bevacizumab treatment, n [%] | |
| Yes | 19 [41] |
| No | 27 [59] |
| Previous EGFR-TKI treatment, n [%] | |
| Yes | 10 [22] |
| No | 36 [78] |
| Previous checkpoint inhibitors treatment, n [%] | |
| Yes | 9 [20] |
| No | 37 [80] |
| Clinical efficacy, n [%] | |
| CR | 0 [0] |
| PR | 9 [20] |
| SD | 29 [63] |
| PD | 8 [17] |
ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease


Adverse reactions
Common adverse reactions included fatigue (80%), hypertension (63%), nausea (22%), stomatitis (30%), leukopenia (63%), hand-foot syndrome (80%), abnormal liver function (15%), proteinuria (35%), hemoptysis (17%), hypothyroidism (24%), and so on. Grade 3 adverse reactions included fatigue, hypertension, stomatitis, leukopenia, and hand-foot syndrome. No grade 4 adverse reactions occurred (Table 2).
Table 2
| Adverse event | Patients (n=46) [%] | |
|---|---|---|
| All grades | Grade 3 | |
| Fatigue | 37 [80] | 8 [17] |
| Hypertension | 29 [63] | 7 [15] |
| Nausea | 10 [22] | 0 |
| Oral mucositis | 14 [30] | 4 [9] |
| Leukopenia | 29 [63] | 5 [11] |
| Hand-foot syndrome | 37 [80] | 8 [17] |
| Abnormal liver function | 7 [15] | 0 |
| Proteinuria | 16 [35] | 3 [7] |
| Hemoptysis | 8 [17] | 0 |
| Hypothyroidism | 11 [24] | 6 [13] |
A total of 8 patients (4 cases of squamous cell carcinoma and 4 cases of adenocarcinoma) developed hemoptysis, 1 case was grade 2 and the remaining 7 cases were grade 1 and were resolved after timely withdrawal and administration of hemostatic drugs. No life-threatening hemoptysis occurred.
Drug reduction
Among all patients, the dose of anlotinib was reduced to 10 mg in 3 cases. The reason for the dose reduction in 2 of these cases was grade 3 hypertension. After reducing the dose and administering amlodipine besylate, the blood pressure was controlled below 140/90 mmHg; and the other patient manifested grade 3 fatigue, which improved after the dosage reduction. In another 3 patients, the dose of anlotinib was lowered to 8 mg, of which 2 were due to grade 3 hand-foot syndrome, which was reduced to grade 1 after the dosage reduction. The remaining case was due to grade 3 stomatitis; the dose was reduced and the mouthwash was used to relieve symptoms.
Discussion
In the current Chinese Society of Clinical Oncology (CSCO) NSCLC diagnosis and treatment guidelines, the first- and second-line treatments of stage IV driver gene-positive non-small cells are recommended to be oral treatment with corresponding target small molecule tyrosinase inhibitors, and when it comes to third-line treatment, monochemotherapy ± bevacizumab (non-squamous cell carcinoma) or anlotinib should be considered. For NSCLC with negative stage IV driver genes, nivolumab, docetaxel, pemetrexed, or anlotinib can be considered in the third-line treatment (2).
Continuous vascular support is essential for tumor growth and metastasis. A number of clinical trials have indicated that the addition of anti-angiogenic drugs, such as bevacizumab, to conventional chemotherapy can significantly improve the ORR, PFS, and OS of patients with advanced NSCLC (11-13). Anlotinib is an oral small molecule inhibitor of multi-receptor tyrosine kinases, and it targets VEGFR1, VEGFR2, VEGFR3, c-Kit, PDGFR-α, FGFR1, FGFR2, and FGFR3, which can inhibit tumor angiogenesis and cell proliferation (14-17). Wang et al. and others have shown that clinically, anlotinib combined with chemotherapy can further improve the efficacy (10,18,19). In the study of Zhu et al., it is more cost effective to use anlotinib for patients with advanced NSCLC. Patients with anlotinib treatment costs of ¥92,334 survived 1.04 years, and patients with placebo costs of ¥69,606 survived 0.85 years (20). Etoposide is an inhibitor of topoisomerase II. Clinically, it has certain effects on small cell lung cancer, lymphoma, malignant germ cell tumor, leukemia, neuroblastoma, rhabdomyosarcoma, ovarian cancer, NSCLC, gastric cancer, and esophageal cancer. It has been confirmed that there is no significant difference between oral and intravenous etoposide in the treatment of small cell lung cancer patients (21).
Conclusively, this study employed etoposide soft capsule combined with anlotinib for treatment of advanced NSCLC, expecting a certain therapeutic effect. In the previous ALTER0302 study by Baohui Han, the ORR of the anlotinib group was 9.2%, the DCR was 81.0%, the mPFS was 5.4 months, and the mOS was 9.6 months (22). In this study, the ORR of etoposide soft capsule combined with anlotinib was 19.57%, the DCR was 82.61%, and the mPFS was 6.3 months. The OS was 10.1 months, and PFS and OS were prolonged compared with the anlotinib group in the ALTER0303 study, which was consistent with the results of Wang et al.
The subgroup analysis of this study suggested that the mPFS of etoposide combined with anlotinib in those who had never used bevacizumab before was longer than that of those who had used bevacizumab before, and the difference was statistically significant (P=0.031). Preliminary analysis suggested that anlotinib has a stronger ability to inhibit angiogenesis and anti-tumor proliferation in patients who have never used bevacizumab, which needs further verification in cell experiments or animal models. It is commonly believed the clinical benefit from chemotherapy may be reduced substantially after EGFR-TKI treatment (23). But in the ALTER0302 study, patients with EGFR mutation who had disease progression after EGFR-TKI treatment and chemotherapy, anlotinib achieved a PFS of 5.6 months and an OS of 10.7 months (24). In our study, it was also shown that patients with EGFR mutation who had disease progression after EGFR-TKI treatment, our combination regimen achieved a PFS of 7.0 months. Monitoring ctDNA is important in the treatment of lung cancer. It can guide drug use and help analyze the mechanism of drug resistance (25). But this study is a retrospective study, the genetic test results of these patients with EGFR mutations are not complete, and further studies cannot be conducted to explore which patients are more likely to benefit from anlotinib.
The subgroup of patients with the number of metastatic tumors ≤2 and an ECOG PS score ≤1 had a tendency towards a prolonged mPFS when receiving the combined regimen, which also suggests that anlotinib combined with etoposide may achieve more beneficial effects when used in patients with a relatively small tumor burden or with better general conditions.
In the ALTER302 study, the main adverse reactions to anlotinib were hypertension (55.00%), hypothyroidism (36.67%), hand-foot syndrome (28.33%), and diarrhea (23.33%). The incidence of treatment-related grade 3 or 4 adverse reactions in the anlotinib group was 21.67%. The most common treatment-related grade 3/4 adverse reactions were hypertension (10.00%), elevated triglyceride (TG; 5.00%), and hand-foot syndrome (3.33%) (17). Common adverse reactions in this study were fatigue (80%), hypertension (63%), nausea (22%), stomatitis (30%), leukopenia (63%), hand-foot syndrome (80%), liver dysfunction (15%), proteinuria (35%), hemoptysis (17%), hypothyroidism (24%), and so on. The grade 3 adverse reactions included fatigue, hypertension, stomatitis, leukopenia, and hand-foot syndrome. No grade 4 adverse reactions occurred. Compared with single-agent group of anlotinib, the adverse reactions of combined etoposide soft capsules were not significantly worsened. They were well tolerated, especially in terms of low incidence of hemoptysis and hematological toxicity. When the dose was reduced due to toxicity, the adverse reactions were rapidly alleviated after the reduction, indicative of the safe and controllable features of the regimen.
The present study had limitations. The study did not include front-line treatment of immunotherapy patients. The efficacy of this combined regimen after immunotherapy could not be evaluated. And the sample size was small in subgroups, and it was difficult to draw firm conclusions in specific populations. In order to further verify the role of etoposide soft capsule combined with anlotinib, it is necessary to further accumulate clinical cases and conduct larger sample, multi-center, randomized, and controlled clinical trials.
Conclusions
In summary, the combination of etoposide and anlotinib has a definite curative effect, characteristic of high safety, convenient oral administration, and no hospitalization. For patients with third-line NSCLC who refuse intravenous chemotherapy or whose tolerance has declined, this option may be considered for treatment. Especially during proliferation of the new coronavirus pneumonia, it could be a worthy choice. However, given that the sample size of this study is small and that it is a retrospective study, a prospective study with a larger sample, multi-center, randomized, and controlled clinical trials can be conducted to further verify the above conclusions.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1412/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1412/prf
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1412/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1412/coif). All authors report that this study was supported by the Youth Project of Health and Family Planning Commission (No. 2018-2-26). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University (No. 435). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- He J, Li J, Ma J, et al. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Diagnosis and Treatment of Common Malignant Tumors 2020. First edition. Beijing: People’s Medical Publishing House; 2020:113-20.
- Shao L, Song Z, Hu L, et al. Analysis of the Efficacy and Survival of Third-line Treatment in Advanced Non-small Cell Lung Cancer. Chin J Lung Cancer 2012;15:369-74. [Crossref] [PubMed]
- Girard N, Jacoulet P, Gainet M, et al. Third-line chemotherapy in advanced non-small cell lung cancer: identifying the candidates for routine practice. J Thorac Oncol 2009;4:1544-9. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [Crossref] [PubMed]
- Sun JM, Lee KW, Kim JH, et al. Efficacy and toxicity of pemetrexed as a third-line treatment for non-small cell lung cancer. Jpn J Clin Oncol 2009;39:27-32. [Crossref] [PubMed]
- Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. [Crossref] [PubMed]
- Si X, Zhang L, Wang H, et al. Quality of life results from a randomized, double-blinded, placebo-controlled, multi-center phase III trial of anlotinib in patients with advanced non-small cell lung cancer. Lung Cancer 2018;122:32-7. [Crossref] [PubMed]
- Zhong Y, Wei Q, Lu Y, et al. Efficacy and safety of anlotinib in patients with advanced non-small cell lung cancer. J Thorac Dis 2020;12:6016-22. [Crossref] [PubMed]
- Wang HY, Chu JF, Zhao Y, et al. A Trial of the Safety and Efficacy of Chemotherapy Plus Anlotinib vs Chemotherapy Alone as Second- or Third-Line Salvage Treatment for Advanced Non-Small Cell Lung Cancer. Cancer Manag Res 2020;12:3827-34. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [Crossref] [PubMed]
- Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:4349-57. [Crossref] [PubMed]
- Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene 2018;654:77-86. [Crossref] [PubMed]
- Syed YY. Anlotinib: First Global Approval. Drugs 2018;78:1057-62. [Crossref] [PubMed]
- Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci 2018;109:1207-19. [Crossref] [PubMed]
- Zhong CC, Chen F, Yang JL, et al. Pharmacokinetics and disposition of anlotinib, an oral tyrosine kinase inhibitor, in experimental animal species. Acta Pharmacol Sin 2018;39:1048-63. [Crossref] [PubMed]
- Li J, Tian Y, Zheng M, et al. Anlotinib plus chemotherapy for T790M-negative EGFR-mutant non-sqNSCLC resistant to TKIs: A multicenter phase 1b/2 trial. Thorac Cancer 2022;13:3496-503. [Crossref] [PubMed]
- Zheng HR, Jiang AM, Gao H, et al. The efficacy and safety of anlotinib combined with platinum-etoposide chemotherapy as first-line treatment for extensive-stage small cell lung cancer: A Chinese multicenter real-world study. Front Oncol 2022;12:894835. [Crossref] [PubMed]
- Zhu Q, Ni R, Guan X. Cost-effectiveness analysis of anlotinib as a third-line or further treatment for advanced non-small cell lung cancer in China. Transl Lung Cancer Res 2023;12:1782-9. [Crossref] [PubMed]
- Rezonja R, Knez L, Cufer T, et al. Oral treatment with etoposide in small cell lung cancer - dilemmas and solutions. Radiol Oncol 2013;47:1-13. [Crossref] [PubMed]
- Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018;118:654-61. [Crossref] [PubMed]
- Zeng Z, Yan HH, Zhang XC, et al. Reduced chemotherapy sensitivity in EGFR-mutant lung cancer patient with frontline EGFR tyrosine kinase inhibitor. Lung Cancer 2014;86:219-24. [Crossref] [PubMed]
- Zhao Y, Wang Q, Zhang L, et al. The efficacy of anlotinib as third-line treatment for non-small cell lung cancer by EGFR mutation status: a subgroup analysis of the ALTER0303 randomized phase 3 study. Transl Lung Cancer Res 2022;11:776-85. [Crossref] [PubMed]
- Diebels I, Van Schil PEY. Diagnosis and treatment of non-small cell lung cancer: current advances and challenges. J Thorac Dis 2022;14:1753-7. [Crossref] [PubMed]


