
Safety and tolerability of combination treatment with pirfenidone and nintedanib in patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis
Highlight box
Key findings
• Combination therapy in idiopathic pulmonary fibrosis (IPF) was associated with relatively frequent incidence of discontinuation and adverse drug reactions (ADRs).
What is known and what is new?
• The clinical efficacy and safety of combination treatments with two antifibrotic agents, pirfenidone and nintedanib, has been not established in IPF.
• The pooled proportions of discontinuation treatments and the developments of serious and any ADRs was 24%, 10%, and 82%, respectively.
What is the implication, and what should change now?
• Our findings provide clinicians with data on the proportions of discontinuation and ADRs of combination treatments in patients with IPF based on the current evidence.
• Further large-scale, randomized, controlled trials with larger samples are needed to support the conclusion.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive interstitial lung disease of unknown etiology (1). The prevalence and incidence of IPF are increasing worldwide, and IPF occurs commonly in elderly adults. IPF has characteristic radiological and histopathologic findings (1) and is associated with a higher mortality rate than other fibrotic interstitial lung diseases. Therefore, treatments that inhibit IPF progression are needed (1).
Current guidelines recommend the use of two antifibrotic agents, pirfenidone and nintedanib, to manage IPF (2). Pirfenidone has anti-inflammatory and antifibrotic effects through down-regulation of the transforming growth factor-beta pathway and modulation of cellular oxidation (3,4). Previous studies demonstrated that use of pirfenidone resulted in a significant improvement over placebo in preventing decline of lung function (3,4). Nintedanib is a tyrosine kinase inhibitor with multiple cellular targets and was initially developed for its oncological effectiveness as an anti-vascularization agent (5). Based on mechanisms of action of this agent in inhibiting vascular and platelet-derived growth factor cell-signaling pathways, major randomized controlled trials (RCTs) were conducted and demonstrated the efficacy of nintedanib in IPF (5,6).
Although the pathogenesis of IPF remains unclear, several coactivated pathways are involved in the development of IPF (7). Multiple pathway modulation by the combination of the two antifibrotic agents may have additive or synergistic benefits in IPF. An in vitro study revealed that combination treatment reduced proliferation of fibroblastic cells relative to either drug alone (8). However, pirfenidone and nintedanib have similar adverse drug reaction (ADR) profiles with respect to gastrointestinal events and elevation of liver enzyme levels, raising concerns over the safety of combination therapy (3,9,10). The aim of the present study was to investigate the safety and tolerability of pirfenidone and nintedanib combination therapy in patients with IPF through a systematic review and meta-analysis of clinical trial data. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-946/rc).
Methods
Data sources and search strategy
A comprehensive search using electronic databases (PubMed, EMBASE, and Cochrane Central Register) was performed to identify potentially relevant articles published before January, 2023. The study was registered in PROSPERO with the registration number CRD42022375176 in which could be assessed the study protocol. The search terms were (“idiopathic pulmonary fibrosis” or “fibrosing alveolitis” or “usual interstitial pneumonia”) and ([“pirfenidone” and “nintedanib”] or [“anti-fibrotic” or “antifibrotic”]). A manual search of the references listed in relevant articles was performed.
Study selection, data extraction, and outcomes
We included studies that met these criteria in our systematic review and meta-analysis: (I) randomized controlled or observational trials for treatment of IPF patients defined by the current guidelines; (II) studies of patients who received combination therapy with pirfenidone (600–2,400 mg/day) and nintedanib (200–300 mg/day); and (III) studies with available clinical outcomes for ADRs and tolerability. The search was limited to human trials published in peer-reviewed, English language journals. We included all full-length studies or letters, while review articles, commentaries, and case reports were excluded. Studies published only in abstract form were also excluded because the methods and results could not be reviewed.
Two investigators independently retrieved potentially relevant studies, reviewed each study according to predefined criteria for eligibility, and extracted data. For each study, a pre-defined form was used to extract data including authors, year of publication, study design, sample size, patient age and gender, baseline forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide, treatment duration, drug dosing, intervention protocol, primary endpoint, and the proportions of ADRs and individuals discontinuing therapy.
The primary outcome was the proportion of discontinued therapy. The proportions of serious and any ADRs for combination treatment with the two antifibrotic agents were investigated as a secondary outcome. The rates of ADRs such as elevated liver enzymes, diarrhea, nausea, vomiting, decreased appetite, fatigue, weight loss, photosensitivity, upper abdominal pain, and headache or dizziness were analyzed. We also investigated the proportions of acute exacerbations (AEs) during combination therapy. An ADR was defined as an adverse event with a possible causal relationship to combination therapy as determined by the investigators. Serious ADRs were defined as those that caused death or life-threatening events, hospitalization related to ADRs, or disability or permanent damage (11).
Quality assessment
The methodological quality of included RCTs was evaluated through the Cochrane Handbook for Systemic Reviews of Interventions ‘risk of bias’ tool (12). A ‘low’, ‘high’, or ‘unclear’ risk of bias was assigned to domains of sequence generation/allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. The methodological quality of non-randomized Studies was evaluated using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool (13). The ROBINS-I was composed of seven distinct domains to evaluate the risk of bias in individual study due to confounding, selection of participants, classification of interventions, deviation from interventions, missing data, measurement of outcome, and selection of reported result. The risk of bias was categorized as low, moderate, serious, critical, or no information (13). Any disagreements in the study search, data extraction, and quality assessment were discussed and resolved through consensus.
Statistical analysis
For proportional meta-analysis, we extracted the numbers of discontinuations and ADRs either directly or through a recalculation based on the reported measures of sample size from the eligible studies. A proportion with 95% confidence interval (CI) was derived. We reported heterogeneity based on the I2 statistic on a scale of 0–100% to quantify the degree of heterogeneity among included studies. Moderate heterogeneity was defined as an I2 value between 30% and 60%, and >60% indicated significant between-study heterogeneity (14). If significant heterogeneity did not exist, a fixed effect model was used for analysis; otherwise, a random effect model was used. Publication bias was assessed by funnel plot, and asymmetry was assessed with the Egger regression test. A P value <0.05 was considered statistically significant in Stata version 14.2 (StataCorp LP, College Station, TX, USA).
Results
A total of 1,790 titles was initially identified through database searches. After removing duplicate articles, we obtained full published articles of 1,549 studies that were potentially eligible for inclusion. Of these articles, 1,529 were excluded based on title and abstract screening. The remaining 20 papers underwent full-text review, and four were included in the final analysis (Figure 1) (15-18). The baseline characteristics of the included studies are presented in Table 1. All selected articles were published between 2018 and 2022; two were RCTs and two were of retrospective observational design. The number of patients in the studies ranged from 3 to 89, and the total number of patients in our meta-analysis was 191.
Table 1
| Author, year | Study sites | Design | No. of patients | Age (years), mean | Male (%) | Baseline FVC (%), mean | Baseline DLco (%), mean | Treatment duration (weeks) | Dosing (mg/day) | The proportion of discontinuation treatment (%) | Intervention protocol | Primary endpoint |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flaherty, 2018 (15) | International, multicenter | Single-arm, open-label, phase IV study | 89 | 68.2 | 80 | 71.8 | 48.4 | 24 | Nintedanib: 200–300; pirfenidone: 1,602–2,403 |
18 | Adding nintedanib to stable pirfenidone treatment in patients with IPF | Proportion of patients who completed 24 weeks of combination treatment |
| Hisata, 2021 (16) | Japan, multicenter | Retrospective, observational study | 46 | 68 | 83 | 62.5 | 45.7 | 52 | Nintedanib: 200–300; pirfenidone: 600–1,800 |
30 | Adding another agent to preceding treatment with antifibrotic drugs | Percentage of adverse events and adverse drug reactions |
| Ikeda, 2022 (17) | Japan, single center | Randomized, open-label, selection design, phase II trial | 3 | 67.3 | 100 | 62.1 | 45.3 | 24 | Nintedanib: 200–300; pirfenidone: 1,200–1,800 |
67 | Adding nintedanib with or without pirfenidone for patients with IPF who experienced disease progression during previous pirfenidone therapy | Incidence of a ≥5% relative decline in FVC or death during the first 6 months |
| Vancheri, 2018 (18) | International, multicenter | Randomized, open-label, phase IV study | 53 | 68.9 | 79 | 83.1 | 45.6 | 12 | Nintedanib: 300; pirfenidone: 2,403 | 36 | Add-on pirfenidone compared with nintedanib alone in patients with IPF | Percentage of patients with treatment-related gastrointestinal adverse events |
FVC, forced vital capacity; DLco, diffusing capacity of the lung for carbon monoxide; IPF, idiopathic pulmonary fibrosis.
The daily doses administered during combination therapy were pirfenidone 600–2,403 mg/day and nintedanib 200–300 mg/day. Most subjects received the dosing of pirfenidone 1,200 mg/day or more and nintedanib 200 mg/day or more. Among 46 patients in one study, only 4 received low dose pirfenidone therapy of 600–1,200 mg/day (16). For intervention protocols, two studies added nintedanib to ongoing pirfenidone therapy in patients with IPF (15,17), one study added pirfenidone to nintedanib monotherapy (18), and the remaining study added another agent to preceding treatment with antifibrotic drugs (16). Assessment of quality for the included studies is shown in the Tables S1,S2. One trial was judged to be at high risk of bias because participants, researchers, and outcome assessments were not blinded (18).
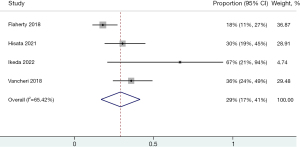
A forest plot of the discontinued rate for IPF combination treatment with two antifibrotic agents is shown in Figure 2 (15-18). In pooled estimates, 29% of patients discontinued treatment during the study period (95% CI: 17–41%). Because there was evidence of high statistical heterogeneity (I2=65.42%), the random effect model was used for the current meta-analysis. A funnel plot of the included studies did not show asymmetry of publication bias, and Egger regression tests indicated no significant publication biases (P=0.158) (Figure S1). Of the total population, the pooled proportions of discontinuation of combination treatments due to the development of ADRs was 24% (95% CI: 14–35%; I2=58.21%).
The pooled proportions of serious and any ADRs were 10% (95% CI: 1–19%; I2=79.13%) and 82% (95% CI: 75–90%; I2=39.20%), respectively (Figure 3A,3B). The details of pooled ADRs are presented in Table 2. In the pooled proportional analysis, gastrointestinal symptoms were frequently reported. Of these, diarrhea was the most frequent (42%), followed by nausea (33%), vomiting (18%), and upper abdominal pain (6%). Fatigue (15%), decreased appetite (21%), weight loss (12%), elevated liver function test (10%), headache or dizziness (7%), and photosensitivity (7%) were also reported during the follow-up period. IPF-AEs were reported in only two studies (16,17). The pooled proportion of IPF-AEs was 7% (95% CI: 0–14%; I2=0%).

Table 2
| Adverse drug reactions | The number of studies | The pooled proportion, % | 95% confidence intervals, % | I2, % |
|---|---|---|---|---|
| Diarrhea | 4 | 42 | 34–50 | 15.17 |
| Nausea | 4 | 33 | 20–46 | 40.41 |
| Decreased appetite | 4 | 21 | 9–34 | 74.14 |
| Vomiting | 3 | 18 | 2–34 | 89.97 |
| Fatigue | 3 | 15 | 10–20 | 0 |
| Weight loss | 3 | 12 | 2–21 | 47.01 |
| Elevated liver function test | 4 | 10 | 4–16 | 42.98 |
| Photosensitivity | 2 | 7 | 3–12 | 0 |
| Headache or dizziness | 2 | 7 | 3–11 | 0 |
| Acute exacerbations | 2 | 7 | 0–14 | 0 |
| Upper abdominal pain | 3 | 6 | 1–11 | 58.92 |
Discussion
In the present study, we investigated the pooled proportions of discontinuations and ADRs of combination treatment with two antifibrotic IPF agents. Because trials directly comparing the safety and tolerability of combination therapy with that of monotherapy of individual antifibrotic agents were very scarce, we performed a proportional meta-analysis for pooled estimates of combination treatment. In pooled estimates, approximately a quarter of patients receiving combination antifibrotic agents discontinued treatment. The pooled proportions of the development of serious ADRs any ADRs were 10% and 82%, respectively. Gastrointestinal ADRs were the most frequently reported.
Since our findings could not directly compare with the outcomes of antifibrotic monotherapy, we investigated previous data using individual antifibrotic agent monotherapy. First, the adherence outcomes associated with pirfenidone monotherapy have been reported through data collected from three multinational phase 3 trials (the ASCEND trial and the CAPACITY trials) (19). Approximately 16% of patients in the pirfenidone group discontinued study treatment (19). A recent systematic review of eight reports with nine RCTs for pirfenidone monotherapy in IPF reported that the majority of patients receiving pirfenidone had ADRs, mainly nausea, diarrhea, photosensitivity, and skin rashes; most of these ADRs were mild to moderate (20). Second, the INPULSIS trials as a major study of nintedanib monotherapy reported that 24.5 % of patients discontinued treatment and 95.5% of those had ADRs (21). Diarrhea was the most frequent ADR, experienced by 62.4% of patients in the nintedanib group (21). Several retrospective studies using clinical data reported that diarrhea developed in 32–79% of patients receiving nintedanib (22).
Only one trial directly compared the safety and efficacy between combination treatment and monotherapy of antifibrotic agent (18). An open-label randomized trial reported that nintedanib with add-on pirfenidone was associated with a significant increase in the incidence of discontinuation treatment compared to nintedanib alone (35.8% vs. 17.6%, P=0.036). Although the incidence of serious and any ADRs were similar between both groups (3.8% vs. 9.8%, P=0.220 and 89% and 88%, P=0.944, respectively), most of the reasons for discontinuation treatment in combination therapy group was ADRs. In the present study, 29% of patients discontinued combination treatment during the study period. Among these patients, the rate of discontinuation of combination treatments owing to the development of ADRs was 86.3%. The rate of discontinuation in combination therapy seemed to be relatively high compared to the rate reported in the previous major trials on pirfenidone or nintedanib individual therapy (19,21). Further large scale RCTs directly comparing the rate of discontinuation between combination treatments and antifibrotic monotherapy is needed in the future.
The expected efficacy for reduced risk of AEs is a major factor considered in the prescription of antifibrotic agents in IPF. A recent meta-analysis that included 12,956 patients from 26 studies revealed that antifibrotic agents decreased risk of AEs, with a pooled risk ratio of 0.63 (95% CI: 0.53–0.76; I2=0%), in patients with IPF (23). In our study, the pooled proportion of AEs during combination therapy was approximately 7% in patients with IPF. Because only two research studies reported IPF AEs, additional data to confirm our results are required.
Monotherapy with either pirfenidone and nintedanib has proven effectiveness in reducing IPF progression rates compared with placebo but often does not prevent disease progression as patients continue to experience a decrease in lung function despite maintenance of the antifibrotic agent (3,5). The different estimated action mechanisms of pirfenidone and nintedanib provide a rational basis for use of combination therapy of the two agents in an attempt to hamper lung function decline in IPF (8). At the beginning of our study, we questioned the clinical efficacy of combination treatment. Because trials designed to assess effectiveness of combination therapy were scarce, we could not analyze these outcomes. Two recent studies using combination treatment reported a change in lung function during the study (15,18). In a single-arm, open-label, phase IV study, exploratory efficacy analyses reported decreases in mean FVC and diffusing capacity for carbon monoxide by 0.4% and 1.9%, respectively, from baseline to week 24 of combination therapy (15). The other study discovered lower decline in FVC over 12 weeks in patients receiving combination treatment than in those receiving nintedanib alone. In that study, mean changes from baseline in FVC predicted at Week 12 were −0.3% and −1.3%, respectively (18). In addition, a retrospective study subjectively assessed the clinical efficacy of combination therapy based on pulmonary function, radiological findings, and pulmonary symptoms. The investigators reported that 39.2% of the IPF patients may have received additional positive effects with addition of a second agent to the preceding monotherapy (16).
To our knowledge, this is the first meta-analysis to examine clinical outcomes of IPF combination therapy. The present study has some limitations. First, as previously mentioned, we could not analysis data that directly compared the safety profiles between combination treatment and antifibrotic monotherapy because of the scarcity of trials. And the included studies collected data from relatively small sample sizes less than 100 patients. Second, a large degree of heterogeneity was present among the included studies (I2=65.42%), but we could not determine the source of heterogeneity because of the sample sizes and the method of descriptive integration. And the study design was inconsistent between researches. Although two studies were RCTs, the remaining studies were retrospective clinical and post-marketing studies that assessed the safety and tolerability of combination treatment in clinical practice. Third, study protocols were especially heterogeneous among the selected studies. The dosing and treatment durations of antifibrotic agents among the included trials varied, which could affect the prevalence and severity of ADRs.
Conclusions
Our systemic review and meta-analysis reports pooled proportions of discontinuations, serious ADRs, and any ADRs of combination treatment with IPF antifibrotic agents. The most frequently reported ADR in patients treated with combination treatment was diarrhea. Because of the absence of a control group and the small sample size, we could not draw solid conclusions. And further researches for the dosing strategy of combination therapy to see maximum benefit without any discontinuation are demanded.
Acknowledgments
Funding: This work was supported by a research grant from
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-946/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-946/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-946/coif). J.L. was supported by a research grant from the Jeju National University Hospital Research Fund of Jeju National University College of Medicine in 2023. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lederer DJ, Martinez FJ. Idiopathic Pulmonary Fibrosis. N Engl J Med 2018;378:1811-23. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18-47. [Crossref] [PubMed]
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083-92. [Crossref] [PubMed]
- Ley B, Swigris J, Day BM, et al. Pirfenidone Reduces Respiratory-related Hospitalizations in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2017;196:756-61. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- Costabel U, Inoue Y, Richeldi L, et al. Efficacy of Nintedanib in Idiopathic Pulmonary Fibrosis across Prespecified Subgroups in INPULSIS. Am J Respir Crit Care Med 2016;193:178-85. [Crossref] [PubMed]
- Wuyts WA, Antoniou KM, Borensztajn K, et al. Combination therapy: the future of management for idiopathic pulmonary fibrosis? Lancet Respir Med 2014;2:933-42. [Crossref] [PubMed]
- Lehtonen ST, Veijola A, Karvonen H, et al. Pirfenidone and nintedanib modulate properties of fibroblasts and myofibroblasts in idiopathic pulmonary fibrosis. Respir Res 2016;17:14. [Crossref] [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760-9. [Crossref] [PubMed]
- Richeldi L, Cottin V, du Bois RM, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: Combined evidence from the TOMORROW and INPULSIS(®) trials. Respir Med 2016;113:74-9. [Crossref] [PubMed]
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998;279:1200-5. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Flaherty KR, Fell CD, Huggins JT, et al. Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis. Eur Respir J 2018;52:1800230. [Crossref] [PubMed]
- Hisata S, Bando M, Homma S, et al. Safety and tolerability of combination therapy with pirfenidone and nintedanib for idiopathic pulmonary fibrosis: A multicenter retrospective observational study in Japan. Respir Investig 2021;59:819-26. [Crossref] [PubMed]
- Ikeda S, Sekine A, Baba T, et al. Randomized phase II study of nintedanib with or without pirfenidone in patients with idiopathic pulmonary fibrosis who experienced disease progression during prior pirfenidone administration. Medicine (Baltimore) 2022;101:e29232. [Crossref] [PubMed]
- Vancheri C, Kreuter M, Richeldi L, et al. Nintedanib with Add-on Pirfenidone in Idiopathic Pulmonary Fibrosis. Results of the INJOURNEY Trial. Am J Respir Crit Care Med 2018;197:356-63. [Crossref] [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J 2016;47:243-53. [Crossref] [PubMed]
- Zang C, Zheng Y, Wang Y, et al. The effects and safety of pirfenidone in the treatment of idiopathic pulmonary fibrosis: a meta-analysis and systematic review. Eur J Med Res 2021;26:129. [Crossref] [PubMed]
- Corte T, Bonella F, Crestani B, et al. Safety, tolerability and appropriate use of nintedanib in idiopathic pulmonary fibrosis. Respir Res 2015;16:116. [Crossref] [PubMed]
- Podolanczuk AJ, Cottin V. A Narrative Review of Real-World Data on the Safety of Nintedanib in Patients with Idiopathic Pulmonary Fibrosis. Adv Ther 2023;40:2038-50. [Crossref] [PubMed]
- Petnak T, Lertjitbanjong P, Thongprayoon C, et al. Impact of Antifibrotic Therapy on Mortality and Acute Exacerbation in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Chest 2021;160:1751-63. [Crossref] [PubMed]



