Dramatic response to anti-PD-1 therapy in a patient of squamous cell carcinoma of thymus with multiple lung metastases
Introduction
Traditional cytotoxic chemotherapy or radiotherapy is still the standard first-line treatment for most advanced or metastatic malignancies. However, due to adverse events, such as myelosuppression and the subsequent risk of systemic infections, nausea, vomiting, and fatigue, patients are more likely to discontinue treatment. Recently, anti-programmed cell death-1 (PD-1)/PD-L1 therapy has demonstrated efficacy in patients with metastatic melanoma, non-small-cell lung cancer (NSCLC) and other kinds of solid tumor (1-4), which expands the treatment options, and might be a promising innovative therapy for advanced cancers. Here we present a case of squamous cell carcinoma of thymus with multiple lung metastases, who responded to anti-PD-1 therapy dramatically.
Case presentation
A 68-year-old non-smoking female came to our hospital for evaluation of a progressive squamous cell carcinoma of thymus. Approximately 1.5 years ago, the patient complained of recurrent cough and bloody sputum for weeks. A computed tomography (CT) scan revealed a large mass in the anterior mediastinum, with enlarged lymph nodes in the pulmonary hilum bilaterally and mediastinum, multiple nodules in the lungs and pericardial effusion. Positron emission computed tomography (PET-CT) confirmed above findings. Then an ultrasound-guided biopsy of the anterior mediastinum was performed. A histopathological evaluation revealed poorly differentiated, thymus-derived squamous cell carcinoma. The epidermal growth factor receptor (EGFR) mutation was wild. From September to November 2013, four cycles of cisplatin plus gemcitabine were administered. Sequential radiotherapy (350 cGy ×15) to thymus mass was also administered. The CT scan showed that the anterior mediastinum mass and the pericardial effusion disappeared, and the lung mass was significantly reduced. The response assessment showed partial remission (PR). In April 2014, chest CT scan suggested that the metastatic nodules were enlarged obviously. As a result, another five cycles of cisplatin plus gemcitabine were administered and response assessment showed stable disease (SD). In October 2014, chest CT scan showed that the metastatic nodules were enlarged again. Two cycles of docetaxel were administered, but the disease kept progressing. The patient showed significant bone marrow suppression with low level of leukocytes, platelets and hemoglobin. In March 2015, the patient admitted to our hospital again for dyspnea and fatigue. The physical examination revealed an anemic appearance and acropachy. Blood test showed pancytopenia and the performance status (PS) score was 3. The patient was not a candidate for chemotherapy due to the PS score and significant bone marrow suppression. Anti-PD-1 therapy was administered (pembrolizumab, 2 mg/kg, every 3 weeks) after obtaining patient’s consent. The patient was assessed by chest CT scan every 2 months after the start of treatment. After two cycles of treatment, the symptoms, including fatigue, dyspnea, and anemia, as well as the abnormalities in the laboratory test results, improved. Chest CT scan showed that nodules in the lung became smaller obviously. After eight cycles, chest CT scan showed that the metastatic nodules in the lung were totally disappeared and the assessment revealed complete remission (CR) (Figure 1). During the anti-PD-1 treatment, the patient had no severe adverse effects, with the exception of a grade 1 rash and itch [grading toxicity based on Common Toxicity Criteria (CTC)] on both legs following the first and second cycles, which spontaneously recovered without treatment. She had a transient fever 4 hours after second cycle infusion, and spontaneously resolved. She also had a grade 1 fatigue during the first two cycles. No immune-related pneumonia and diarrhea happened in this patient during the eight-cycle treatment.
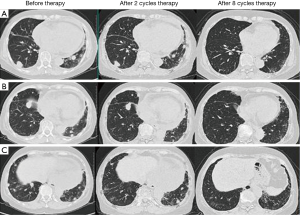
Discussion
Anti-PD-1/PD-L1 monoclonal antibody has been proven promising for advanced melanoma and NSCLC (1-3). It is now being tested in other malignancies, such as bladder cancer, kidney cancer and prostate cancer (4). Although still in controversy, PD-L1 expression might be used as a predictive biomarker to select patients who would benefit from anti-PD-1/PD-L1 therapy (5-7). Katsuya et al. evaluated PD-L1 expression in thymomas and thymic carcinomas, and suggested that the anti-PD-1/PD-L1 therapy could be used for unresectable or relapsed thymomas and thymic carcinomas (8). However, no study examined its efficacy in advanced thymus cancer. In current report, our case confirmed the efficacy of anti-PD-1 therapy in metastatic squamous cell carcinoma of thymus. In our case, only grade 1 rashes and itch occurred during the first and second cycles, which are in agreement with the report that rashes [skin toxicity due to ipilimumab (anti-CTLA4) treatment, another immune therapy drug] usually occur after the 3rd week of therapy, with a peak in the 6th week (9). Because of the mechanism of action underlying immune therapy, adverse events are anticipated due to a reduction in tolerance to antigens previously recognized as “self”. Most toxicity is considered grade 1 or 2 based on the CTC by the National Institute of Health and National Cancer Institute, and can be managed by monitoring or steroid taper treatment. Generally speaking, treatment should include symptom management for mild (grade 1 and grade 2) events and the use of steroids for severe (grade 3 and grade 4) cases.
In summary, we present a case of dramatic response to anti-PD-1 therapy in a patient of squamous cell carcinoma of thymus with multiple lung metastases, which suggests that anti-PD-1/PD-L1 monoclonal antibody may be effective in treating metastatic thymus carcinoma.
Acknowledgements
We thank the patient give us her consent for publication of this case.
Funding: This study was supported by a grant from the National Natural Science Foundation of China (No. 81200007).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Ivashko IN, Kolesar JM. Pembrolizumab and nivolumab: PD-1 inhibitors for advanced melanoma. Am J Health Syst Pharm 2016;73:193-201. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Alme AK, Karir BS, Faltas BM, et al. Blocking immune checkpoints in prostate, kidney, and urothelial cancer: An overview. Urol Oncol 2016;34:171-81. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Schmidt LH, Kümmel A, Görlich D, et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One 2015;10:e0136023. [Crossref] [PubMed]
- Carbognin L, Pilotto S, Milella M, et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One 2015;10:e0130142. [Crossref] [PubMed]
- Katsuya Y, Fujita Y, Horinouchi H, et al. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer 2015;88:154-9. [Crossref] [PubMed]
- Schmerling RA. Toxicity of checkpoint inhibitors. Chin Clin Oncol 2014;3:31. [PubMed]


