
The utility of quantitative computed tomography in cohort studies of chronic obstructive pulmonary disease: a narrative review
Introduction
Chronic obstructive pulmonary disease (COPD) represents a significant global health issue, due to its high morbidity and mortality rates, and it is projected to rank as the third leading cause of death globally by 2030 (1). Smoking is strongly linked to the incidence of COPD, particularly in Asian regions like China where high tobacco consumption contributes to a higher prevalence. In China, more than 13% of people over the age of 40 years develop COPD, and the total number of patients is estimated to be approximately 99.9 million in 2018 (2). The disease is characterized by incompletely reversible airflow obstruction and can affect different levels of the respiratory system with heterogeneous clinical presentations (3). Although spirometry remains the traditional method of COPD diagnosis and staging, imaging modalities such as quantitative computed tomography (QCT) may offer more comprehensive diagnostic and therapeutic insights. Especially during the coronavirus disease 2019 (COVID-19) pandemic, pulmonary function test procedures should be used with caution, due to the risk of aerosolized virus transmission (4). As a result, innovative diagnostic tools like QCT have gained popularity. QCT can safely evaluate COPD severity, including emphysema and airway obstruction, offering potential as a preliminary screening tool and guide for personalized COPD treatment (5).
The development of QCT enables rapid anatomical localization and quantitative detection of morphological and functional changes in the respiratory system, offering a globally standardized measurement method and advancing our understanding of the disease pathogenesis and prognosis (6-8). Identifying population at risk and effective biomarkers for disease outcome prediction represent important challenges in COPD management. In particular, cohort studies can be implemented to analyze lung anatomical changes in patients using computed tomography (CT) indicators, improve early COPD recognition, increase awareness of clinical phenotype, identify appropriate screening methods, and investigate treatment effectiveness and prognosis. CT indicators are frequently utilized as biomarkers for personalized treatment and prognosis assessment (9). In this paper, we provide a comprehensive summary of the significant findings of QCT in large-scale cohort studies of COPD and related researches conducted in the past decade, with particular emphasis on potential future directions for QCT research encompassing image features related to COPD progression and clinical outcomes. This article is presented in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1421/rc).
Methods
Publications in the PubMed database over the past few decades have provided comprehensive descriptions of the contribution of QCT in COPD cohort studies. A total of 1,181 papers were retrieved that had been published between January 1, 2013, and April 20, 2023, with an optimized search strategy. Rigorous scrutiny of inclusion criteria resulted in the selection of 74 publications that align closely with the topic of COPD cohort studies and QCT contribution. Additional articles were discovered by conducting a thorough review of the reference lists in relevant publications. Further details on this method are provided in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | April 20, 2023 |
| Databases and other sources searched | PubMed (NLM) |
| Search terms used | (“Tomography, X-Ray Computed”(MeSH) OR “Tomography, Spiral Computed”(MeSH) OR “Tomography, X-Ray Computed”(Title/Abstract) OR “CT scan”(Title/Abstract) OR “Computed tomography”(Title/Abstract) OR “QCT”(Title/Abstract) OR “Quantitative computed tomography”(Title/Abstract)) AND (“Cohort studies”(MeSH) OR “Prospective studies”(MeSH) OR “Longitudinal studies”(MeSH) OR “Observational studies”(MeSH) OR “Follow-up studies”(MeSH) OR “Cohort studies”(Title/Abstract)) AND (“Pulmonary disease, chronic obstructive”(MeSH) OR “Chronic bronchitis”(MeSH) OR “Emphysema”(MeSH) OR “COPD”(Title/Abstract) OR “Chronic obstructive pulmonary disease”(Title/Abstract)) |
| Timeframe | January 1, 2013–April 20, 2023 |
| Inclusion and exclusion criteria | Inclusion criteria |
| • Population: including patients diagnosed with COPD | |
| • Study design: cohort studies with >2-year follow-up | |
| • CT application: quantitative CT for COPD, such as lung structure, airway diameter, lung function, etc. | |
| • Outcome measurement: quantifiable data regarding CT results, such as the extent of lung lesions, progression of pathological changes, etc. | |
| • Publication date: prioritize recent studies to include the latest discoveries and technologies | |
| Exclusion criteria | |
| • Study design: includes fewer than 500 individuals, or don’t meet the cohort study requirements | |
| • No COPD diagnosis: without patients clearly diagnosed with COPD | |
| • No involvement of QCT application: don’t involve QCT applications for COPD | |
| • Incomplete data: insufficient data and information to support objectives | |
| • Publication quality: published in non-authoritative and low credibility journals | |
| Selection process | The selection of literature was conducted by Qi Dai, Xiaoxiao Zhu. All authors collaborated in establishing inclusion and exclusion criteria, reaching a consensus on the finalized list of literature |
| Any additional considerations, if applicable | Newly published papers with robust study designs and large sample sizes are preferred for citation among similar content |
NLM, National Library of Medicine; MeSH, Medical Subject Headings; CT, computed tomography; QCT, quantitative computed tomography; COPD, chronic obstructive pulmonary disease.
Overview of COPD cohort studies
In recent decades, cohort studies using clinical questionnaires, spirometry, chest QCT, and longitudinal follow-up, have been conducted. Internationally significant COPD cohort studies, including COPD Genetic Epidemiology (COPDGene) (10), Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-Points (ECLIPSE) (11), and Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) (12) have been conducted in recent years. In China, Investigation of the Clinical, Radiological and Biological Factors, Humanistic and Healthcare Utilization Burden Associated with Disease Progression, Phenotypes, and Endotypes of COPD (COMPASS) (13) is one of the largest research projects on COPD. Each cohort study has specific distinct objectives and observation indexes (Table 2). COPDGene aims to identify susceptibility genes and key factors, and establish a novel COPD classification system based on clinical data, genetic epidemiology, and CT imaging data of smokers or former smokers in the United States. ECLIPSE, a longitudinal observational study, was carried out across 46 clinical centers located in Europe and the United States to identify genetic factors and biomarkers for COPD progression by defining clinically relevant subtypes and predicting disease progression. SPIROMICS is a multicenter longitudinal study supported by clinical drug trials to discern genetic factors and biomarkers linked to clinical COPD subtypes and to forecast disease progression. Furthermore, SPIROMICS classifies COPD subtypes and predicts biomarkers related to treatment benefits, based on the extent of patients’ response to specific treatments. COMPASS aims to determine the factors associated with the progression of COPD, as well as its phenotypes and endotypes in China by analyzing clinical, radiological, and biological factors by 41 clinical centers in China.
Table 2
| Category | COPDGene | ECLIPSE | SPIROMICS | COMPASS |
|---|---|---|---|---|
| Area | United States | Europe, North America | United States | China |
| Centers | 21 | 12 | 6 | 41 |
| Participants | ||||
| Age (years) | 45–80 | 40–75 | 40–80 | 40–80 |
| Male (%) | 51 | 64 | 52 | N/A |
| Female (%) | 49 | 36 | 48 | N/A |
| Non-smoking | N/A | 245 | 200 | 120 |
| Smoking | N/A | 337 | 900 | N/A |
| PRISm | 1,323 | N/A | N/A | N/A |
| GOLD 0 | 4,378 | N/A | N/A | 180 |
| GOLD I | 788 | N/A | 1,500 (I & II) | 700 |
| GOLD II | 1,925 | 915 | 700 | |
| GOLD III | 1,164 | 861 | 600 (III & IV) | 200 |
| GOLD IV | 606 | 278 | 100 | |
| Total | 10,198 | 2,746 | 3,200 | 2,000 |
| Evaluation features | ||||
| Symptom questionnaire | √ | √ | √ | √ |
| Quality of life score | √ | √ | √ | √ |
| Biphasic chest CT | √ | √ | √ | √ |
| Spirometry | √ | √ | √ | √ |
| DLCO | √ | × | √ | √ |
| IOS | × | √ | × | × |
| 6-minute walking test | √ | √ | √ | √ |
| Sputum and blood biomarkers | √ | √ | √ | √ |
| Genome sequencing | √ | × | × | × |
| Lung microbiota | × | × | √ | √ |
| Follow-up | Long-term since 2008 | 3-year-long since 2008 | 3-year-long since 2010 | 2.5-year-long since 2019 |
COPD, chronic obstructive pulmonary disease; COPDGene, COPD Genetic Epidemiology; ECLIPSE, Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-Points; SPIROMICS, Subpopulations and Intermediate Outcomes in COPD Study; COMPASS, Investigation of the Clinical, Radiological and Biological Factors, Humanistic and Healthcare Utilization Burden Associated with Disease Progression, Phenotypes, and Endotypes of COPD; N/A, not applicable; PRISm, Preserved Ratio Impaired Spirometry; GOLD, Global Initiative for Chronic Obstructive lung Disease; CT, computed tomography; DLCO, diffusion capacity of carbon monoxide; IOS, Impulse Oscillometry System.
CT quantification of emphysema
Emphysema is a chronic lung disease characterized by permanent dilation of terminal bronchioles, destruction of alveolar walls and bronchioles, that primarily occurs due to smoking. The use of CT scans to define the emphysema region via Hounsfield units (HU) lower than −950 HU is a widely accepted threshold (14). The CT threshold utilized to quantify lung low-density areas (LLA) is useful for providing objective measures of emphysema severity (Figure 1). The SPIROMICS study (15) has shown that a significant proportion of emphysema can be detected on chest CT scans of smokers with no airflow limitations, emphasizing that the impact of smoking can be severely underestimated by lung function tests alone. A study examining 4,542 participants from the COPDGene cohort demonstrated that emphysema severity was associated with both impaired lung capacity and poor airflow limitation, as indicated by markedly. The ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) and FEV1 (correlation coefficient r=20.76 and 20.67 respectively) (16). A recent multicenter study (17) involving 4,211 participants revealed that emphysema progression in smokers could be measured accurately via the adjusted CT lung density (ALD) technique over a 5-year duration, thus highlighting the limited utility of measuring emphysema progression based on FEV1, which is less than 10% accurate compared to CT imaging. Emphysema is associated with a multidimensional score that predicts mortality risk in patients with COPD based on body mass index (BMI), presence of airflow obstruction, dyspnea, and exercise tolerance, known as the BODE index (18). Importantly, emphysema has a more profound effect on BODE score than respiratory illnesses (19,20). Although yielding potential limitations regarding sensitivity to longitudinal follow-up changes, visual evaluation remains the CT evaluation standard for emphysema. Using deep learning algorithms, Oh et al. (21) analyzed sequential chest CT scans of smokers and graded emphysema severity. Results of this study showed that an increase in emphysema score was associated with severe lung dysfunction and elevated mortality risk. Moreover, difference in CT equipment and scanning protocols have shown to affect the accuracy of prognostic evaluation.

There are ethnic disparities in emphysema. Hansel et al. (22) conducted a study involving 1,063 patients with moderate and severe COPD, which revealed that lung function impairment, airway wall thickness, and gas retention among African Americans and non-Hispanic Whites were similar, with only minimal emphysema detected by CT scans. A survey of 515 Black urbanites in a SPIROMICS cohort demonstrated a relatively high incidence of emphysema in segregated residents, as well as a significantly frequent occurrence of severe exacerbations of COPD (23). There are also gender differences in development of emphysema. Female patients with early onset COPD have a significantly lower smoking burden than men; however, their emphysema degrees are generally similar to those of men. This finding suggests that women might be more susceptible to smoking-related lung damage (24). The recent study has identified differences in the prognosis of patients with varying emphysema subtypes. CT scans performed on 399 patients from two distinct observational groups revealed that patients with moderate-to-severe centrilobular emphysema (CLE) exhibited faster declines in diffusion capacity and higher long-term mortality rates. These results suggest that CLE and paraseptal emphysema (PSE) may have varying clinical and physiological effects on patients (25).
Other factors, such as CT scan parameters, can affect the quantification of emphysema by CT value. CT value can be converted to calculate lung mass using the following formula: lung mass (g) = (HU + 1,024/1,024) × voxel volume × voxel quantity. Lung quality can supplement pulmonary air content in COPD quantitative CT imaging. A 5-year follow-up study of 1,623 cases from the COPDGene cohort identified that patients with early-onset COPD had higher lung mass compared to smokers with normal lung function and patients with intermediate and advanced COPD. This finding indicates a biphasic process of lung mass development throughout the course of COPD, with persistence of inflammation in early stages leading to increased lung mass followed by predominant tissue destruction and reduced lung mass (26,27).
CT quantification of abnormal airway
For the diagnosis and management of COPD, using quantitative CT imaging of the airways necessitates standardized technical criteria. Furthermore, high-quality evidence is required to establish clinical benefits for management guided by airway quantitative CT imaging (28). COPD airflow resistance occurs mainly in small airways with diameters of less than 2 mm, ranging from grade 4 to grade 12, which are characterized by inflammation. The gradual airway wall thickening and lumen narrowing in COPD result from hypertrophied mucus glands, smooth muscle cell proliferation, and fibrosis of the airway wall. Although The processes of inflammation and remodeling were previously thought to occur exclusively in small airways, recent research has shown that these processes can also occur simultaneously in larger, segmental, and subsegmental airways (29,30).
Several indices may be utilized for estimating airway size, with the proximal airway being directly observed and measured through CT technology. Utilizing threshold-based analysis and the half-height full-width algorithm, the airway tree could be segmented and the airway wall diameter line can be quantified after a 3-dimensional (3D) reconstruction of the tracheobronchial tree (31). Bronchial wall thickness, tracheal lumen area, tracheal lumen diameter, wall area percent (WA%), and internal perimeter (Pi) are among the direct quantitative indices of the airway. A significant correlation with histological measurements of the airway (32) was found in relation to these measurements. Due to their perpendicular position to the axial orientation and minimal exposure to heartbeats, the bronchus and its branches in the upper lobe of the right lung are preferred for airway measurement. The WA% can be calculated using the following formula: WA% = (total airway area − airway lumen area)/total airway area × 100. To enable inter-individual comparison, the standardized bronchial wall thickness is frequently represented by the square root of airway wall area. Pi10 and Pi15 are reference values for small airway disease assessment, representing the square root of theoretical airway wall area with an inner circumference of 10 and 15 mm, respectively (33). Continuous development in CT technology has transformed airway measurement from 2-dimensional (2D) to 3D, resolving the errors caused by the non-vertical geometric construction between the measured bronchial and the 2D cross section. With the aid of specialized software, automatic measurement can be conducted at positions perpendicular to the airways for those bronchial segments. Measured airway levels can extend to level 5 or 6, allowing for a more realistic depiction of small COPD airway changes (34,35). A prospective COPD cohort study routine CT scan concluded that standardization of Pi10 airway wall thickness can potentially serve as an imaging biomarker for lung function decline, increased mortality risk, and disease severity (36).
The COPDGene study (18) findings revealed that CT airway quantitative indices are significantly and independently related to respiratory quality of life based on St. George’s Respiratory Questionnaire (SGRQ) assessment. Following standardized treatment, airway wall thickness, WA%, and Pi10 values in the lung segment increased by 1 unit, whereas corresponding SGRQ scores were elevated by 1.9, 1.5, and 2.8 units, respectively. The measured value of airway wall thickness is correlated with chronic bronchitis (37), and normal or abnormal reactions to bronchodilator use (38,39), which can predict airway reactivity. With the advancement of artificial intelligence, unsupervised machine learning offers a peripheral bronchial identification accuracy of up to 96% in CT, enabling fully automated tube wall thickness measurement and high-throughput analysis (40). Analysis of CT scans evaluating central airway wall area, lumen and total bronchial area in 5,179 smokers and 92 nonsmoking individuals in the COPDGene cohort revealed an increase in WA% due to the combined effect of airway reduction and wall thickening (41). The SPIROMICS cohort study also confirmed that the duct wall area of grade 1–6 decreased linearly as COPD severity increased (42). It is essential to acknowledge that although WA% has a close relationship with respiratory system function and diseases. The provided information is insufficient to comprehensively understand the relative remodeling happening within the airway wall and lumen. Moreover, the limited range of variation between normal and severe lung disease restricts its effectiveness in assessing longitudinal changes over time and treatment response (43).
Notable gender and individual differences have been observed in natural airway anatomy. Analysis of CT images from the multi-center COPDGene cohort found that female smokers had a higher WA% than male smokers. However, these individuals exhibited smaller dimensions in terms of lumen area, inner diameter, and airway thickness when compared to anatomically similar airways (44). Those who had previous incidents of asthma during childhood are more likely to have smaller airways, heightening their susceptibility to long-lasting airflow blockage in adulthood (45). Other studies have revealed a significant interdependence between airway diameter and lung parenchyma (46,47). Furthermore, there is an inverse correlation between the degree of emphysema and the size of the airway, particularly in COPD patients with a bronchitis phenotype. As the emphysema phenotype’s severity increases, the change in airway diameter will gradually diminish, which partly explains why emphysema has low responsiveness to bronchodilators. Furthermore, airway collapse is common in smokers, especially in COPD patients, due to weakened tracheal cartilage or reduced wall elasticity. In a study of 8,830 COPDGene cohort cases (48) approximately 5% of smokers experienced airway collapse during exhalation, with a worsening quality of life and a higher likelihood of having acute respiratory disease.
CT quantification of air trapping
Air retention is an early CT sign of small airway disease. Since CT cannot directly display small airways, quantitative and indirect evaluation can be performed through dual-phase scanning during expiration and inspiration. The primary indexes of measurement are as follows: (I) the percentage of emphysema area in the total lung area during the expiratory phase with a lung density below −856 HU (LAA% −856); (II) the ratio of mean lung density during expiration to inspiration (E/I-ratio MLD); (III) the RVC, which stands for relative volume change in respiration, between −856 and −950 HU (i.e., the difference between the lung volume with an inspiratory phase lung density between −856 and −950 HU and the lung volume with an expiratory phase lung density between −856 and −950 HU); and (IV) air trapping index (ATI), the area of low attenuation where the density of the inspiratory and expiratory phases remains unchanged (Figure 2). Currently, the recommended ATI threshold is 60 HU. Air retention was associated with respiratory disease scales, such as dyspnea, breath-related quality of life, along with 6-minute walking distance (6MWD) (49). In another study, among 8,034 cases (50), 26.3% of smokers without airflow obstruction had an increased E/I ratio, which was associated with FEV1, SGRQ score, 6MWD, and BODE index.

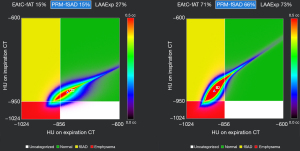
Parameter response mapping (PRM) is the registration of inspiratory phase and expiratory phase CT image voxels. Each voxel consists of a pair of CT values sharing the same geometric space. The voxels are divided into three categories based on CT density threshold: (I) inspiratory phase <−950 HU and expiratory phase <−856 HU, representing the emphysema region marked in red on the image and recorded as PRM emphysema (PRMEmph); (II) inspiratory phase >−950 HU and expiratory phase <−856 HU, representing the small airway lesion area displayed in yellow on the image and recorded as PRM functional small airways disease (PRMfSAD); (III) inspiratory phase ≥−950 HU and expiratory phase ≥−856 HU, representing the normal area denoted in green on the image and recorded as PRMNorm. The results are displayed as graphs, and the percentage of each category is calculated to locate lesions in COPD patients locally (Figure 3). McDonough et al. (51) and Galbán et al. (52) confirmed that the narrowing and disappearance of small airways occur prior to the development of emphysema in the progression of COPD. PRM, as a biomarker to distinguish air retention caused by emphysema and small airway lesions, provides abundant imaging information for COPD phenotypic classification (53) and facilitates accurate assessment of the heterogeneity in the progression of COPD (43). In a study involving 1,508 participants from the COPDGene cohort, it was found that PRMfSAD measured at baseline correlated with decreased FEV1 in both smokers without airflow blockages and patients in the early stages of the disease. PRMEmph and PRMfSAD demonstrated links to altered lung function in smokers with more severe disease (54). Martinez et al. (55) found that PRMfSAD increased by 2.7% every 10 years in the normal population, regardless of gender, age, smoking history, and asthma history. Every 1% increase in PRMfSAD would lead to a minimal increase of 9.4 mL in FVC and a decrease of approximately 0.15% in FEV1/FVC, consistent with the results obtained by Boudewijn et al. (56). Bhatt et al. (54) conducted a 5-year follow-up of 1,508 smokers and found that the increase of PRMfSAD was significantly correlated with the decrease of FEV1 in non-COPD smokers. Furthermore, both PRMfSAD and PRMEmph were correlated with the decrease of FEV1 in COPD patients; the correlation between PRMfSAD and weakened lung function was more significant among individuals diagnosed with mild-to-moderate COPD. Thus, PRMfSAD can be employed as supplementary information for FEV1 to evaluate the response to drug therapy in COPD patients, suggesting that PRM can be an effective tool for long-term assessment of COPD disease progression. Advances in artificial intelligence technology have allowed PRM to be based on machine learning classification models. This results in a stronger correlation with pulmonary function tests that enables better distinction between high-risk and normal COPD patients. Additionally, by determining the optimal FEV1% prediction value, this approach lays a foundation for redefining the diagnosis of high-risk COPD (57).
CT quantification of pulmonary vascular injury
Pathological pulmonary vascular remodeling is prevalent in COPD patients, and sometimes even observed in non-smokers with normal lung function. Quantitative evaluation of pulmonary vascular conditions through CT has implications for the study of COPD and changes in the cardiovascular system (58). Cross-sectional area percentage (%CSA) is a developing indicator for COPD evaluation. Specifically, %CSA <5 mm (i.e., the percentage of the sum of small blood vessels with a pulmonary vascular area <5 mm to the total lung area) and %CSA 5–10 mm (i.e., the percentage of the sum of small blood vessels with a pulmonary vascular area of 5–10 mm of the total lung area) are currently the most commonly used %CSA indicators (59,60). Wang et al. (61) suggested that QCT can evaluate the %CSA of pulmonary small vessels and predict the existence of COPD acute exacerbations and severity. Additionally, Takayanagi et al. (62) showed that despite the gradual progression of emphysema in COPD patients who quit smoking, there was no distinct trend of increase in %CSA <5 mm. Therefore, it can be inferred that smoking-induced COPD vascular remodeling is reversible to some extent.
Individuals diagnosed with COPD frequently exhibit mild-to-moderate pulmonary hypertension (63), characterized by changes in pulmonary great vessels, which are often imperceptible at this stage. The evaluation of COPD combined with pulmonary hypertension by measuring %CSA represents a valuable prospective approach. The possible physiological mechanism of this method encompasses 2 stages: (I) COPD patients experience pulmonary parenchyma destruction and excessive expansion of lung tissues, which cause compression and damage to peri-alveolar pulmonary small vessels, resulting in a decreased number of these vessels (64); and (II) factors such as smoking, inflammation, and hypoxia stimulate pulmonary vascular endothelial cells, leading to smooth muscle cell hypertrophy, fibroblast phenotype change, pulmonary vascular remodeling, tube wall thickening, lumen stenosis, and even occlusion (65). To assess the presence of severe pulmonary hypertension in COPD, Coste et al. (66) proposed a scoring method that combines arterial oxygen partial pressure, bronchial wall thickness, and %CSA, yielding a sensitivity of 75% and specificity of 80%. Moreover, the same research team demonstrated the feasibility of this method in determining whether patients with COPD should undergo right heart catheterization. Besides, Jo et al. (67) suggested that %CSA measurement and pulmonary small vessel changes have the theoretical potential for useful diagnostic applications aimed at early-stage assessment of COPD with pulmonary hypertension. In addition, COPD patients with emphysema and functional small-airway disorders may have a correlation between the total pulmonary vascular volume (TPVV), as measured by CT, and exercise-induced dyspnea symptoms. A recent investigation found that smokers with only minor/mild emphysema exposed to cigarette smoke and presenting with pulmonary vascular attenuation necessitated more ventilation during exercise, leading to increased dyspnea and unpleasant respiratory sensations. Specifically, the development of emphysema and functional small-airway diseases directly lead to decreased vascularization and smaller pulmonary blood vessels in COPD (68).
Incidental interstitial lung abnormality (ILA) in CT
Incidental ILA is defined as the presence of over 5% of independent lung abnormalities being incidentally detected by CT without previous clinical suspicion of the disease. It comprises one or more of the following lesions: some of the abnormalities observed in the lungs include ground glass or reticular patterns, traction bronchiectasis, honeycomb changes, non-emphysematous cysts and lung distortion. At least 5% of the lung area, divided into upper, middle, and lower areas by the inferior aortic arch and the right lower pulmonary vein, should be involved (69). The incidence of ILA was estimated to be 7–10%, and it was significantly and positively correlated with age and smoking (70-72). One out of every 12 scans from a high-resolution computed tomography (HRCT) analysis of 2,416 smokers from the COPDGene cohort showed the presence of ILA, which was independently associated with a comparatively short 6MWD (73). ILA subjects were shown to have an 80% and 77% increase in the probability of walking distance ≤500 meters and 250 meters, respectively, compared to those without ILA (74). In another study involving 8,345 participants (75), it was found that a 5% increase in ILA correlated with a decline of 2.5% in the predicted percentage of FVC and a reduction of 2.7% in the predicted percentage of FEV1. Putman et al. (72) in a study encompassing 6,827 participants, a 5% increase in ILA was linked to a 29% higher risk of mortality. Furthermore, in a pooled analysis of four independent cohorts with 11,691 participants, ILA was found to increase the risk of all-cause mortality over a median follow-up period of 3 to 9 years.
Subjective visual analysis of subtle changes in ILA is time-consuming and insensitive. However, an image processing tool that combines local histogram analysis and distance from the pleural surface was developed and tested in a CT-image based COPDGene cohort study that involved 2,257 heavy smokers, enabling the objective and automatic recognition of ILA-related CT image features (76). Furthermore, the objective analysis tool for ILA was used in another COPDGene cohort study of 8,266 participants (77). This revealed that the co-existence of ILA and emphysema was associated with disease severity and high mortality compared to emphysema alone.
CT predicts acute exacerbation
Acute exacerbations of COPD can result in decreased respiratory quality of life, accelerated lung function decline, and elevated mortality rates. The frequency of these episodes can vary widely, and predicting them, especially the severe episodes that may require hospitalization, can help to identify high-risk patients and conduct targeted treatment. A correlation analysis involving 1,002 participants from the COPDGene cohort (78) revealed that there is a relationship between bronchial wall thickness, the total percentage of emphysema, and the frequency of COPD exacerbations. It was observed that for each additional 1 mm of bronchial wall thickening, there was a 1.84-fold increase in the annual exacerbation rate. Meanwhile, in patients with a total emphysema percentage of 35% or higher, every 5% increase in emphysema was associated with a 1.18-fold rise in the exacerbation rate. Another study of 3,464 moderate-to-severe COPD participants revealed that a ratio of pulmonary artery diameter (PA) to aortic diameter (A) greater than 1 was significantly associated with a higher frequency of acute exacerbations (79). Furthermore, a study in the COPDGene cohort found that expiratory tracheal collapse increased the frequency of exacerbations by 1.5 times and the risk of severe exacerbations by 1.8 times (48). An analysis of COPD patients based on imaging and clinical features found that low attenuation area at −950 HU (LAA-950) was higher in individuals with frequent exacerbations, in comparison to those with non-frequent exacerbations. Age, smoking status, BMI, predicted FEV1, and LAA-950 were associated predictors for frequent exacerbations of COPD (80). Retrospective models using quantitative CT can identify COPD patients at high risk of severe exacerbation, whereas newly discovered CT biomarkers may reveal the underlying disease mechanisms that lead to COPD exacerbation.
CT assessment of extrapulmonary disease
COPD progression is associated with comorbidities that share common pathophysiological pathways. Although CT scans of the chest cannot systematically assess all comorbidities, they can be useful in evaluating the extrapulmonary presentation of these diseases (81). Mortality from heart disease in COPD patients surpasses that associated with respiratory failure (82). Ventricular morphometry estimates that have been derived from conventional chest CT plain scanning show strong associations with echocardiography and magnetic resonance imaging findings relating to both structural and functional data (83). Another study utilized the COPDGene cohort consisting of 3,436 individuals with moderate-to-severe COPD. Findings revealed an increase in the sphericity of both the upper-right and left ventricles, corresponding to the progression of disease severity (84). Kim et al. have also found that airflow obstruction, which is a primary characteristic of COPD, is associated with higher prevalence, range, and severity of coronary atherosclerosis. Therefore, it offers autonomous prognostic insights into cardiac risk (85). Finally, abdominal visceral and subcutaneous adipose tissues were estimated in a cohort of 1,267 COPD participants. The results revealed that individuals with a prior history of myocardial infarction (MI) exhibited a significantly greater mean area of visceral adipose tissue in comparison to those without a history of MI (86).
The presence of skeletal muscle dysfunction and muscle wasting in individuals with COPD suggests the potential for musculoskeletal complications associated with the disease. Though the loss of skeletal muscle mass may occur without a loss of adipose tissue, it could more accurately reflect COPD severity than BMI (87). In a cohort comprising 966 participants from the COPDGene study, the size of the pectoralis major muscle was more strongly correlated to lung function, dyspnea, and a 6MWD than BMI. Notably, even after adjusting for BMI, these correlations remained significant, highlighting the ongoing relevance of the pectoralis major muscle (88). Intercostal muscles play a vital role in alveolar ventilation, especially with increased ventilation demands in COPD patients. Intercostal atrophy is common in COPD patients and is associated with airway obstruction severity, aging, and BMI (89). Although current osteoporosis guidelines do not include smoking or COPD as risk factors, Bon et al. showed that emphysema and high bone resorption markers are independently linked to progressive bone mineral density loss in smokers (90). Another cohort of 3,321 smokers selected from the COPDGene study, 58% of participants had low-volume bone mineral density (vBMD), and 37% showed vertebral fractures (91). A Korean COPD cohort study (92) showed that a decrease in thoracic bone density measured by chest CT was significantly linked to mortality and disease severity in patients. Therefore, for smokers with or without COPD, radiologists are advised to consider possible osteopenia and fractures. A Chinese cohort study found that pectoralis muscle area (PMA) was linked to the severity of COPD airflow obstruction and respiratory outcomes. Lower PMA was noted in patients with mild-to-moderate airflow restriction. Measuring PMA could potentially aid in assessing COPD in the early stages (93).
QCT in COPD prediction models
COPD evaluation aims to identify the degree of airflow limitation, assess the impact of the disease on the patient’s overall well-being, and evaluate the potential for future occurrences such as disease exacerbation, hospitalization and death, which can aid clinical decision-making. QCT studies on COPD have developed several models that predict lung function, acute exacerbations, and mortality. Gawlitza et al. (94) established five machine learning-based lung function prediction models by extracting QCT parameters (e.g., mean lung density, total lung volume, volume of low-attenuation area, full width at half maximum, and the difference between the inspiratory and expiratory phases) for 75 participants. Results of this analysis indicate that pulmonary function values of COPD patients can be predicted based on QCT parameters with a reasonable degree of accuracy. González et al. (95) demonstrated that direct training of convolutional neural networks with CT imaging data can identify COPD patients among smokers and predict acute respiratory diseases and mortality. Nonetheless, this approach has high computational costs and memory requirements, which surpasses the processing capacity of current graphics processing units when handling massive datasets. Cho et al. (96) examined the use of radiomics to predict mortality in COPD patients. They derived imaging features from four indicators (emphysema, airway remodeling, pulmonary vascular disease, and air trapping) on chest CT, and used least absolute shrinkage and selection operator (LASSO) and Cox regression to choose five vital features as independent predictors of mortality in COPD patients.
Currently, quantitative imaging prediction models for COPD heavily rely on parameters limited to lung parenchyma or airways and lack close connections with clinical parameters, leading to potential biases. The extensive collection of CT images from a substantial number of clinically and spirometrically stable cases in the COPD cohort offers valuable insights into the underlying disease mechanisms and processes. Future research may investigate the potential of different parameter types and their interactions in predicting COPD outcomes to enhance the accuracy of predictive evaluation models.
Conclusions
COPD, a progressive respiratory condition, affects millions worldwide. Cohort studies that investigate the disease mechanisms and effective interventions require accurate and reliable measurements of lung morphology and physiology. These major COPD cohorts around the world are pre-exploratory and well-designed, focusing on smokers with respiratory symptoms, early disease recognition, smokers with mild interstitial abnormalities, risk assessment of acute exacerbations, and extrapulmonary comorbidities. In addition to statistics on score of life quality, breathlessness, physical performance, exacerbation, reduced lung function, and mortality, QCT is a non-invasive imaging technique that enables precise measurements of lung density, volume, and airway dimensions, and supplements important and objective evidence for disease risk assessment in populations at risk for early disease or with identified early stages of COPD. QCT may offer several advantages over traditional pulmonary function tests, including the ability to differentiate between various types of COPD and to monitor disease progression over time. As a results, QCT may become an important tool to help optimize the diagnosis and treatment of COPD (Figure 4).

Future research will also prioritize the investigation of emphysema and small airway disease phenotypes using imaging techniques. This will enhance our comprehension of the intricate interplay between structural, functional, and biological alterations that manifest in individuals with COPD. Moreover, the development of automated QCT analysis tools can improve the efficiency and reproducibility of measurements, facilitating large-scale studies across different populations. Overall, QCT represents a valuable tool for investigating COPD and has the potential to enhance medical decision-making and optimize patient care (97).
However, the use of QCT in clinical settings is currently not widespread, possibly due to technical barriers, high costs, and challenges. The lack of image analysis methods and support for equipment poses a major obstacle, with numerous medical institutions and clinicians struggle to allocate the necessary resources and time to engage in QCT. Additionally, variations in manufacturers and devices can affect the reliability and comparability of QCT results. Despite the significant value of QCT in COPD research, promoting its application requires standardizing the technology, improving cost-effectiveness, and conducting further research and implementation. Future research should focus on developing and standardizing QCT technology, gathering clinical evidence, conducting cost-effectiveness analyses, providing education and training, and offering policy support and insurance coverage. These efforts will advance the use of QCT in COPD management, leading to more accurate and personalized diagnosis and treatment.
Acknowledgments
The authors sincerely acknowledge the contributions to this review of Ms. Jiazhen Zhu from Ningbo No.2 Hospital.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1421/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1421/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1421/coif). EP serves as an unpaid Editorial Board Member of Journal of Thoracic Disease from February 2023 to January 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global strategy for the diagnosis, management and prevention of COPD; 2023. (EB/OL). Available online: https://goldcopd.org/2023-gold-report-2/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf. Accessed February 27, 2023.
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health CPH study): a national cross-sectional study. Lancet 2018;391:1706-17. [Crossref] [PubMed]
- Kim WJ, Gupta V, Nishimura M, et al. Identification of chronic obstructive pulmonary disease subgroups in 13 Asian cities. Int J Tuberc Lung Dis 2018;22:820-6. [Crossref] [PubMed]
- Hull JH, Lloyd JK, Cooper BG. Lung function testing in the COVID-19 endemic. Lancet Respir Med 2020;8:666-7. [Crossref] [PubMed]
- Teramoto S. The Quantitative Computed Tomography Techniques are Alternate Modalities of Assessing the Disease Profile of COPD Instead of Pulmonary Function Testing Under COVID-19 Pandemic. Int J Chron Obstruct Pulmon Dis. 2022;17:2485-6. [Crossref] [PubMed]
- Pistenmaa CL, Washko GR. Computerized Chest Imaging in the Diagnosis and Assessment of the Patient with Chronic Obstructive Pulmonary Disease. Clin Chest Med 2020;41:375-81. [Crossref] [PubMed]
- Bhatt SP, Washko GR, Hoffman EA, et al. Imaging Advances in Chronic Obstructive Pulmonary Disease. Insights from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) Study. Am J Respir Crit Care Med 2019;199:286-301. [Crossref] [PubMed]
- El Kaddouri B, Strand MJ, Baraghoshi D, et al. Fleischner Society Visual Emphysema CT Patterns Help Predict Progression of Emphysema in Current and Former Smokers: Results from the COPDGene Study. Radiology 2021;298:441-9. [Crossref] [PubMed]
- Vestbo J, Janson C, Nuevo J, et al. Observational studies assessing the pharmacological treatment of obstructive lung disease: strengths, challenges and considerations for study design. ERJ Open Res 2020;6:e00044-2020. [Crossref] [PubMed]
- Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010;7:32-43. [Crossref] [PubMed]
- Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J 2008;31:869-73. [Crossref] [PubMed]
- Couper D, LaVange LM, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax 2014;69:491-4. [Crossref] [PubMed]
- Liang Z, Zhong N, Chen R, et al. Investigation of the Clinical, Radiological and Biological Factors Associated with Disease Progression, Phenotypes and Endotypes of COPD in China (COMPASS): study design, protocol and rationale. ERJ Open Res 2021;7:e00201-2021. [Crossref] [PubMed]
- Gevenois PA, de Maertelaer V, De Vuyst P, et al. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995;152:653-7. [Crossref] [PubMed]
- Woodruff PG, Barr RG, Bleecker E, et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med 2016;374:1811-21. [Crossref] [PubMed]
- Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol 2013;201:W460-70. [Crossref] [PubMed]
- Pompe E, Strand M, van Rikxoort EM, et al. Five-year Progression of Emphysema and Air Trapping at CT in Smokers with and Those without Chronic Obstructive Pulmonary Disease: Results from the COPDGene Study. Radiology 2020;295:218-26. [Crossref] [PubMed]
- Martinez CH, Chen YH, Westgate PM, et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax 2012;67:399-406. [Crossref] [PubMed]
- Naval E, González MC, Giraldós S, et al. Frailty Assessment in a Stable COPD Cohort: Is There a COPD-Frail Phenotype? COPD 2021;18:525-32. [Crossref] [PubMed]
- Baraghoshi D, Strand M, Humphries SM, et al. Quantitative CT Evaluation of Emphysema Progression over 10 Years in the COPDGene Study. Radiology 2023;307:e222786. [Crossref] [PubMed]
- Oh AS, Baraghoshi D, Lynch DA, et al. Emphysema Progression at CT by Deep Learning Predicts Functional Impairment and Mortality: Results from the COPDGene Study. Radiology 2022;304:672-9. [Crossref] [PubMed]
- Hansel NN, Washko GR, Foreman MG, et al. Racial differences in CT phenotypes in COPD. COPD 2013;10:20-7. [Crossref] [PubMed]
- Woo H, Brigham EP, Allbright K, et al. Racial Segregation and Respiratory Outcomes among Urban Black Residents with and at Risk of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2021;204:536-45. [Crossref] [PubMed]
- Hardin M, Foreman M, Dransfield MT, et al. Sex-specific features of emphysema among current and former smokers with COPD. Eur Respir J 2016;47:104-12. [Crossref] [PubMed]
- Shiraishi Y, Tanabe N, Shimizu K, et al. Stronger Associations of Centrilobular Than Paraseptal Emphysema With Longitudinal Changes in Diffusing Capacity and Mortality in COPD. Chest 2023;164:327-38. [Crossref] [PubMed]
- Hoffman EA, Newell JD Jr. Lung Mass as the Complement to Lung Air Content in Quantitative CT of the COPD Lung. Acad Radiol 2017;24:383-5. [Crossref] [PubMed]
- Washko GR, Kinney GL, Ross JC, et al. Lung Mass in Smokers. Acad Radiol 2017;24:386-92. [Crossref] [PubMed]
- Kirby M, Smith BM. Quantitative CT Scan Imaging of the Airways for Diagnosis and Management of Lung Disease. Chest 2023;S0012-3692(23)00314-8. doi:
10.1016/j.chest.2023.02.044 . - Eapen MS, McAlinden K, Tan D, et al. Profiling cellular and inflammatory changes in the airway wall of mild to moderate COPD. Respirology 2017;22:1125-32. [Crossref] [PubMed]
- Charbonnier JP, Pompe E, Moore C, et al. Airway wall thickening on CT: Relation to smoking status and severity of COPD. Respir Med 2019;146:36-41. [Crossref] [PubMed]
- Karayama M, Inui N, Yasui H, et al. Clinical features of three-dimensional computed tomography-based radiologic phenotypes of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2019;14:1333-42. [Crossref] [PubMed]
- Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 2005;171:142-6. [Crossref] [PubMed]
- Aamli Gagnat A, Gjerdevik M, Gallefoss F, et al. Incidence of non-pulmonary cancer and lung cancer by amount of emphysema and airway wall thickness: a community-based cohort. Eur Respir J 2017;49:1601162. [Crossref] [PubMed]
- Ostridge K, Williams NP, Kim V, et al. Relationship of CT-quantified emphysema, small airways disease and bronchial wall dimensions with physiological, inflammatory and infective measures in COPD. Respir Res 2018;19:31. [Crossref] [PubMed]
- Nambu A, Zach J, Schroeder J, et al. Quantitative computed tomography measurements to evaluate airway disease in chronic obstructive pulmonary disease: Relationship to physiological measurements, clinical index and visual assessment of airway disease. Eur J Radiol 2016;85:2144-51. [Crossref] [PubMed]
- Kahnert K, Jörres RA, Kauczor HU, et al. Standardized airway wall thickness Pi10 from routine CT scans of COPD patients as imaging biomarker for disease severity, lung function decline, and mortality. Ther Adv Respir Dis 2023;17:17534666221148663. [Crossref] [PubMed]
- Bhatt SP, Bodduluri S, Kizhakke Puliyakote AS, et al. Structural airway imaging metrics are differentially associated with persistent chronic bronchitis. Thorax 2021;76:343-9. [Crossref] [PubMed]
- Fortis S, Comellas A, Make BJ, et al. Combined Forced Expiratory Volume in 1 Second and Forced Vital Capacity Bronchodilator Response, Exacerbations, and Mortality in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc 2019;16:826-35. [Crossref] [PubMed]
- Hansen JE, Dilektasli AG, Porszasz J, et al. A New Bronchodilator Response Grading Strategy Identifies Distinct Patient Populations. Ann Am Thorac Soc 2019;16:1504-17. [Crossref] [PubMed]
- Moll M, Qiao D, Regan EA, et al. Machine Learning and Prediction of All-Cause Mortality in COPD. Chest 2020;158:952-64. [Crossref] [PubMed]
- Washko GR, Diaz AA, Kim V, et al. Computed tomographic measures of airway morphology in smokers and never-smoking normals. J Appl Physiol (1985) 2014;116:668-73. [Crossref] [PubMed]
- Smith BM, Hoffman EA, Rabinowitz D, et al. Comparison of spatially matched airways reveals thinner airway walls in COPD. The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax 2014;69:987-96. [Crossref] [PubMed]
- Konietzke P, Wielpütz MO, Wagner WL, et al. Quantitative CT detects progression in COPD patients with severe emphysema in a 3-month interval. Eur Radiol 2020;30:2502-12. [Crossref] [PubMed]
- Bhatt SP, Bodduluri S, Nakhmani A, et al. Sex Differences in Airways at Chest CT: Results from the COPDGene Cohort. Radiology 2022;305:699-708. [Crossref] [PubMed]
- Hayden LP, Cho MH, Raby BA, et al. Childhood asthma is associated with COPD and known asthma variants in COPDGene: a genome-wide association study. Respir Res 2018;19:209. [Crossref] [PubMed]
- Yamashiro T, Moriya H, Tsubakimoto M, et al. Continuous quantitative measurement of the proximal airway dimensions and lung density on four-dimensional dynamic-ventilation CT in smokers. Int J Chron Obstruct Pulmon Dis 2016;11:755-64. [Crossref] [PubMed]
- Almeshari MA, Alobaidi NY, Sapey E, et al. Small Airways Response to Bronchodilators in Adults with Asthma or COPD: A Systematic Review. Int J Chron Obstruct Pulmon Dis 2021;16:3065-82. [Crossref] [PubMed]
- Bhatt SP, Terry NL, Nath H, et al. Association Between Expiratory Central Airway Collapse and Respiratory Outcomes Among Smokers. JAMA 2016;315:498-505. [Crossref] [PubMed]
- Hamakawa Y, Tanabe N, Shima H, et al. Associations of pulmonary and extrapulmonary computed tomographic manifestations with impaired physical activity in symptomatic patients with chronic obstructive pulmonary disease. Sci Rep 2022;12:5608. [Crossref] [PubMed]
- Bodduluri S, Reinhardt JM, Hoffman EA, et al. Signs of Gas Trapping in Normal Lung Density Regions in Smokers. Am J Respir Crit Care Med 2017;196:1404-10. [Crossref] [PubMed]
- McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365:1567-75. [Crossref] [PubMed]
- Galbán CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 2012;18:1711-5. [Crossref] [PubMed]
- Labaki WW, Gu T, Murray S, et al. Voxel-Wise Longitudinal Parametric Response Mapping Analysis of Chest Computed Tomography in Smokers. Acad Radiol 2019;26:217-23. [Crossref] [PubMed]
- Bhatt SP, Soler X, Wang X, et al. Association between Functional Small Airway Disease and FEV1 Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2016;194:178-84. [Crossref] [PubMed]
- Martinez CH, Diaz AA, Meldrum C, et al. Age and Small Airway Imaging Abnormalities in Subjects with and without Airflow Obstruction in SPIROMICS. Am J Respir Crit Care Med 2017;195:464-72. [Crossref] [PubMed]
- Boudewijn IM, Postma DS, Telenga ED, et al. Effects of ageing and smoking on pulmonary computed tomography scans using parametric response mapping. Eur Respir J 2015;46:1193-6. [Crossref] [PubMed]
- Pu Y, Zhou X, Zhang D, et al. Re-Defining High Risk COPD with Parameter Response Mapping Based on Machine Learning Models. Int J Chron Obstruct Pulmon Dis 2022;17:2471-83. [Crossref] [PubMed]
- Synn AJ, Zhang C, Washko GR, et al. Cigarette Smoke Exposure and Radiographic Pulmonary Vascular Morphology in the Framingham Heart Study. Ann Am Thorac Soc 2019;16:698-706. [Crossref] [PubMed]
- Tang G, Wang F, Liang Z, et al. Correlations of Computed Tomography Measurement of Distal Pulmonary Vascular Pruning with Airflow Limitation and Emphysema in COPD Patients. Int J Chron Obstruct Pulmon Dis 2022;17:2241-52. [Crossref] [PubMed]
- Park SW, Lim MN, Kim WJ, et al. Quantitative assessment the longitudinal changes of pulmonary vascular counts in chronic obstructive pulmonary disease. Respir Res 2022;23:29. [Crossref] [PubMed]
- Wang Z, Chen X, Liu K, et al. Small pulmonary vascular alteration and acute exacerbations of COPD: quantitative computed tomography analysis. Int J Chron Obstruct Pulmon Dis 2016;11:1965-71. [Crossref] [PubMed]
- Takayanagi S, Kawata N, Tada Y, et al. Longitudinal changes in structural abnormalities using MDCT in COPD: do the CT measurements of airway wall thickness and small pulmonary vessels change in parallel with emphysematous progression? Int J Chron Obstruct Pulmon Dis 2017;12:551-60. [Crossref] [PubMed]
- Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008;32:1371-85. [Crossref] [PubMed]
- Ruffenach G, Hong J, Vaillancourt M, et al. Pulmonary hypertension secondary to pulmonary fibrosis: clinical data, histopathology and molecular insights. Respir Res 2020;21:303. [Crossref] [PubMed]
- García-Lucio J, Argemi G, Tura-Ceide O, et al. Gene expression profile of angiogenic factors in pulmonary arteries in COPD: relationship with vascular remodeling. Am J Physiol Lung Cell Mol Physiol 2016;310:L583-92. [Crossref] [PubMed]
- Coste F, Benlala I, Dournes G, et al. Quantitative CT assessment of bronchial and vascular alterations in severe precapillary pulmonary hypertension. Int J Chron Obstruct Pulmon Dis 2019;14:381-9. [Crossref] [PubMed]
- Jo HH, Park MJ, Shin HS, et al. Adverse effect of smoking on cross-sectional area of small pulmonary vessel and arterial stiffness in healthy smokers without COPD. Clin Respir J 2019;13:368-75. [Crossref] [PubMed]
- Elbehairy AF, Vincent SG, Phillips DB, et al. Pulmonary Vascular Volume by Quantitative CT in Dyspneic Smokers with Minor Emphysema. COPD 2023;20:135-43. [Crossref] [PubMed]
- Hatabu H, Hunninghake GM, Richeldi L, et al. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med 2020;8:726-37. [Crossref] [PubMed]
- Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013;268:563-71. [Crossref] [PubMed]
- Araki T, Putman RK, Hatabu H, et al. Development and Progression of Interstitial Lung Abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med 2016;194:1514-22. [Crossref] [PubMed]
- Putman RK, Hatabu H, Araki T, et al. Association Between Interstitial Lung Abnormalities and All-Cause Mortality. JAMA 2016;315:672-81. [Crossref] [PubMed]
- Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med 2011;364:897-906. [Crossref] [PubMed]
- Doyle TJ, Washko GR, Fernandez IE, et al. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med 2012;185:756-62. [Crossref] [PubMed]
- Ash SY, Harmouche R, Putman RK, et al. Clinical and Genetic Associations of Objectively Identified Interstitial Changes in Smokers. Chest 2017;152:780-91. [Crossref] [PubMed]
- Ash SY, Harmouche R, Ross JC, et al. The Objective Identification and Quantification of Interstitial Lung Abnormalities in Smokers. Acad Radiol 2017;24:941-6. [Crossref] [PubMed]
- Ash SY, Harmouche R, Ross JC, et al. Interstitial Features at Chest CT Enhance the Deleterious Effects of Emphysema in the COPDGene Cohort. Radiology 2018;288:600-9. [Crossref] [PubMed]
- Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology 2011;261:274-82. [Crossref] [PubMed]
- Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012;367:913-21. [Crossref] [PubMed]
- Zhu D, Dai H, Zhu H, et al. Identification of frequent acute exacerbations phenotype in COPD patients based on imaging and clinical characteristics. Respir Med 2023;209:107150. [Crossref] [PubMed]
- Chaudhary MFA, Hoffman EA, Guo J, et al. Predicting severe chronic obstructive pulmonary disease exacerbations using quantitative CT: a retrospective model development and external validation study. Lancet Digit Health 2023;5:e83-92. [Crossref] [PubMed]
- André S, Conde B, Fragoso E, et al. COPD and Cardiovascular Disease. Pulmonology 2019;25:168-76. [Crossref] [PubMed]
- Rahaghi FN, Vegas-Sanchez-Ferrero G, Minhas JK, et al. Ventricular Geometry From Non-contrast Non-ECG-gated CT Scans: An Imaging Marker of Cardiopulmonary Disease in Smokers. Acad Radiol 2017;24:594-602. [Crossref] [PubMed]
- Bhatt SP, Vegas-Sánchez-Ferrero G, Rahaghi FN, et al. Cardiac Morphometry on Computed Tomography and Exacerbation Reduction with β-Blocker Therapy in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2017;196:1484-8. [Crossref] [PubMed]
- Kim JJ, Kim DB, Jang SW, et al. Relationship between airflow obstruction and coronary atherosclerosis in asymptomatic individuals: evaluation by coronary CT angiography. Int J Cardiovasc Imaging 2018;34:641-8. [Crossref] [PubMed]
- Diaz AA, Young TP, Kurugol S, et al. Abdominal Visceral Adipose Tissue is Associated with Myocardial Infarction in Patients with COPD. Chronic Obstr Pulm Dis 2015;2:8-16. [Crossref] [PubMed]
- Engelke K, Museyko O, Wang L, et al. Quantitative analysis of skeletal muscle by computed tomography imaging-State of the art. J Orthop Translat 2018;15:91-103. [Crossref] [PubMed]
- McDonald ML, Diaz AA, Ross JC, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc 2014;11:326-34. [Crossref] [PubMed]
- Ju S, Lee SJ, Park MJ, et al. Clinical importance of cross-sectional area of intercostal muscles in patients with chronic obstructive pulmonary disease. Clin Respir J 2018;12:939-47. [Crossref] [PubMed]
- Bon J, Zhang Y, Leader JK, et al. Radiographic Emphysema, Circulating Bone Biomarkers, and Progressive Bone Mineral Density Loss in Smokers. Ann Am Thorac Soc 2018;15:615-21. [Crossref] [PubMed]
- Jaramillo JD, Wilson C, Stinson DS, et al. Reduced Bone Density and Vertebral Fractures in Smokers. Men and COPD Patients at Increased Risk. Ann Am Thorac Soc 2015;12:648-56. [Crossref] [PubMed]
- Hwang HJ, Lee SM, Seo JB, et al. Quantitative Vertebral Bone Density Seen on Chest CT in Chronic Obstructive Pulmonary Disease Patients: Association with Mortality in the Korean Obstructive Lung Disease Cohort. Korean J Radiol 2020;21:880-90. [Crossref] [PubMed]
- Zhou K, Wu F, Zhao N, et al. Association of pectoralis muscle area on computed tomography with airflow limitation severity and respiratory outcomes in COPD: A population-based prospective cohort study. Pulmonology 2023;S2531-0437(23)00039-9.
- Gawlitza J, Sturm T, Spohrer K, et al. Predicting Pulmonary Function Testing from Quantified Computed Tomography Using Machine Learning Algorithms in Patients with COPD. Diagnostics (Basel) 2019;9:33. [Crossref] [PubMed]
- González G, Ash SY, Vegas-Sánchez-Ferrero G, et al. Disease Staging and Prognosis in Smokers Using Deep Learning in Chest Computed Tomography. Am J Respir Crit Care Med 2018;197:193-203. [Crossref] [PubMed]
- Cho YH, Seo JB, Lee SM, et al. Radiomics approach for survival prediction in chronic obstructive pulmonary disease. Eur Radiol 2021;31:7316-24. [Crossref] [PubMed]
- Wang JM, Ram S, Labaki WW, et al. CT-Based Commercial Software Applications: Improving Patient Care Through Accurate COPD Subtyping. Int J Chron Obstruct Pulmon Dis 2022;17:919-30. [Crossref] [PubMed]


