Factors influencing development and mortality of acute respiratory failure in hospitalized patient with active pulmonary tuberculosis: a 10-year retrospective review
Introduction
Pulmonary tuberculosis is one of the common pulmonary infections worldwide and is among one of the top ten causes of death in the populations throughout developing countries (1). The range of overall mortality rates of patients infected with Mycobacterium tuberculosis is 4% to 65% (2-7). From previous studies, acute respiratory failure was also indicated as a mortality risk in pulmonary tuberculosis. Although the incidence of acute respiratory failure induced by pulmonary tuberculosis from a previous observational study was approximately 1.5%, mortality of the patients with pulmonary tuberculosis who developed acute respiratory failure and required mechanical ventilation ranged between 47% and 80% (4,5,7-12). The mortality rate of active pulmonary tuberculosis requiring mechanical ventilation (TBMV) was double the mortality rate of patients with severe bacterial pneumonia who developed acute respiratory failure (13). In addition, pulmonary tuberculosis complicated by acute respiratory failure requires several modalities, manpower, and interventions to treat and prevent further spreading of organisms, which may burden the limited resources of some countries (14,15). The availability of influencing factors in the development and mortality of TBMV can be useful for early and aggressive management in these patients. Therefore, this retrospective study aims to determine the factors influencing the development of acute respiratory failure requiring mechanical ventilation in patients with active pulmonary tuberculosis and to define the mortality determinations in these patients.
Methods
Patients
The electronic medical records of adult patients admitted for management of active pulmonary tuberculosis or its complications between January 2003 and December 2012 were collected and reviewed at Songklanagarind Hospital which is a tertiary care university hospital in Songkhla, Thailand. The study protocol was approved by the ethic committee (EC) at Faculty of Medicine, Prince of Songkla University (EC number: 51-039-14-4-3). A waiver of consent was approved and the investigators assigned a confidentiality term.
Data collection
All demographic data including gender, age, underlying disease, clinical presentation, duration of illness, results of initial laboratory investigation, qualitative amounts of acid fast bacilli from sputum smear, and chest X-ray findings were recorded. The laboratory variables collected within the first 24 hours of admission were complete blood count (CBC), blood urea nitrogen (BUN), creatinine, and serum albumin. Causes of ventilator support, arterial blood gas results within the first 24 hours of ICU admission, acute physiologic and chronic health evaluation (APACHE) II, and acute lung injury index (PaO2/FiO2 ratio) were collected in the TBMV patients. The treatment related outcomes included time to start anti-tuberculosis agents, hospital length of stay, ICU length of stay, duration of mechanical ventilator days, and hospital mortality. The institutional review board of the Faculty of Medicine, Prince of Songkla University approved this study protocol and waived the requirement of informed consent.
Definitions
The diagnosis of active pulmonary tuberculosis was defined as positive sputum smear for acid fast bacilli with a new compatible abnormal infiltration on chest X-ray. The qualitative amount of acid fast bacilli from sputum smear was classified as 1+ to 4+ by the standard definition (16). The chest X-ray findings were reported by experienced radiologists as nodular infiltration, interstitial infiltration, consolidation, and lung cavity (17). The extension of the infiltration on chest X-ray was classified as confined in 2 lobes or greater than 2 lobes. Extrapulmonary tuberculosis was defined as positive for acid fast staining or positive Mycobacterium tuberculosis culture from tissue or other sites from respiratory specimens. TBMV was defined as a patient who required intubation and mechanical ventilator support. The causes of mechanical ventilator support were classified into respiratory decompensation, sepsis, cardiac causes, neurological causes, and perioperative intubation. The hospitalized patients with active pulmonary tuberculosis or its complications without mechanical ventilator support were classified as non-TBMV group. The outcomes of TBMV patients were classified into two groups: the survivor group if they were discharged alive from the hospital and the non-survivor group if the patients died in the hospital.
Statistical analysis
Continuous variables were expressed as mean and standard error of the mean (SEM) or median with a minimum and maximum depending on the distribution of data. Categorical variables were given in numbers and percentage. All statistical analyses used the SPSS software package version 17.0 (SPSS Inc, Chicago, IL, USA).
Factors influencing development of TBMV
All data from hospitalized patients with active pulmonary tuberculosis were compared between TBMV and non-TBMV group by the Chi-square test, or the Student’s t-test depending on the types and distributions of variable. Selected variables that were statistically significant with P values <0.1 were then introduced into a forward, stepwise, logistic regression model. Odds ratios (ORs) and their 95% confidence intervals (CIs) were used to identify the independent significant influencing factors in the development of TBMV.
Factors influencing mortality of TBMV
All data of TBMV patients were compared between the survivor and non-survivor groups by the Chi-square test or the Student’s t-test depending on the types and distributions of the variables. Selected variables that were statistically significant with P values <0.1 were then introduced into a forward, stepwise, logistic regression model. ORs and their 95% CIs were used to identify the independent significant influencing factors of mortality of TBMV.
Results
During the 10-year period, the electronic medical records of 268 active pulmonary tuberculosis patients were collected and reviewed. All demographic parameters, clinical presentations, chest X-ray findings, laboratory findings, and treatment outcomes are presented in Table 1. The mean age ± SD of all hospitalized patients with pulmonary tuberculosis was 59.58±19.86 years old. Of the 268 patients, 185 (69%) were male and 143 (53.4%) had comorbidities. The mean ± SEM for the duration of illness was 29.34±3.12 days. Productive cough (63.4%), fever (61.6%), and dyspnea (55.2%) were among the first three common presentations. Hemoptysis was found in only 17.5% of all hospitalized patients with pulmonary tuberculosis. Only 10.1% of the cases had extrapulmonary tuberculosis at presentation. The common chest X-ray findings were consolidation (70.5%), nodular infiltration (31.0%), cavity lesion (16.0%), and interstitial infiltration (14.2%). Almost 40% of the cases had extension of infiltration on chest X-ray greater than 2 anatomical lobes of lung and approximately 60% of the cases had qualitative acid fast stain on sputum smear of 1+ to 2+. The mean ± SEM of time to start anti-tuberculosis agents was approximately 4.81±0.66 days in both groups. Finally, the overall mortality of active pulmonary tuberculosis in this retrospective study was 30.2% (81/268).
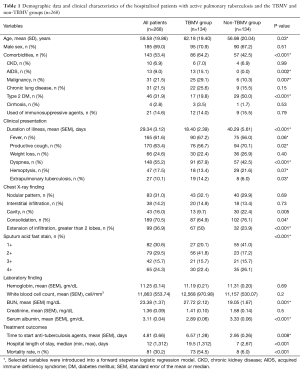
Full table
Clinical characteristics between TBMV and non-TBMV patients and independent factors to develop TBMV
Table 1 presents the characteristics of the TBMV and non-TBMV patients. From the univariate analysis, the TBMV group was significantly older and had significantly more comorbidities than the non-TBMV group. The occurrence of underlying diseases of AIDS and malignancies were significantly higher in the TBMV group, but type 2 diabetes mellitus occurred significantly less often. According to the clinical presentations, patients in the TBMV group presented significantly more often with dyspnea and had shorter durations of illness than the non-TBMV group. In addition, the extension of infiltration on chest X-ray greater than 2 anatomical lobes of lung and the larger amount of qualitative acid fast stain on sputum smear were significantly higher in the TBMV population. The TBMV group had a significantly higher mean BUN level and a significantly lower mean serum albumin level than the non-TBMV group. The time to start anti-tuberculosis agents was significantly delayed in TBMV patients. Eventually, the medians of hospital length of stay and mortality rate were significantly higher in the TBMV group. On multivariate regression analysis, the shorter duration of illness (OR, 0.99; 95% CI, 0.98–0.99), underlying disease of AIDS (OR, 14.55; 95% CI, 1.71–123.91), presentation of fever (OR, 2.11; 95% CI, 1.20–3.71) and dyspnea (OR, 3.51; 95% CI, 2.02–6.11), large amount of acid fast bacilli on sputum smear (OR, 3.76; 95% CI, 1.90–7.47), lower serum albumin level (OR, 0.39; 95% CI, 0.26–0.59), and delayed initiation of anti-tuberculosis agents (OR, 1.06; 95% CI, 1.00–1.12) were independent factors to develop TBMV (Table 2).
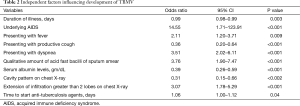
Full table
Clinical characteristics between non-survivor and survivor groups in TBMV patients and independent factors of mortality
Table 3 presents all demographic data and clinical characteristics of the TBMV group. Respiratory decompensation (59.7%), sepsis (14.2%), and perioperative (13.4%) were among the top three potential causes of mechanical ventilator dependence. The median and ranges of mechanical ventilator days and hospital length of stay were 10 [1, 176] days and 19.5 [1, 312] days, respectively. The overall mortality of this group was 54.5%. Nosocomial infection was the major cause of death (63/73, 86.3%). Ventilator associated pneumonia (55/63, 87.3%), primary bacteremia (6/63, 9.5%), and urinary tract infection (2/63, 3.2%) were among the nosocomial infections in this study. The variables of the non-survivor group had male gender, presentation of dyspnea, lobar consolidation on chest X-ray, lower serum albumin levels, and longer duration of mechanical ventilation than the survivors. After introducing the variables with a P value <0.1 into a forward, stepwise, logistic, regression model, male gender (OR, 2.16; 95% CI, 1.02–4.61), consolidation pattern on chest X-ray (OR, 2.41; 95% CI, 1.17–4.98), and lower serum albumin level (OR, 0.39; 95% CI, 0.21–0.71) showed a significant correlation to increased mortality in patients with active pulmonary tuberculosis complicated by acute respiratory failure (Table 4).
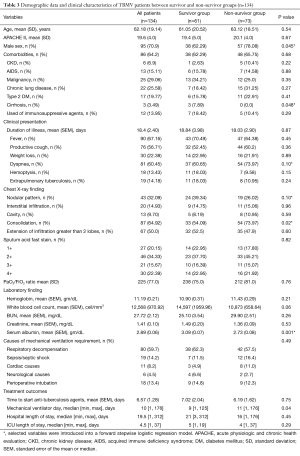
Full table
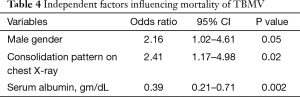
Full table
Discussion
Pulmonary tuberculosis is one of the common lung infections in Thailand that results in morbidity and mortality. The World Health Organization (WHO) classified Thailand as a high tuberculosis country in the year 2015 when the incidence and prevalence rates of tuberculosis were 171 and 236 per 100,000 population, respectively. The overall mortality rate was 11/100,000 of the population. Compared to the report from the previous year, the incidence rate of tuberculosis was gradually falling but the prevalence and mortality rate still did not achieve the WHO target. A national tuberculosis program has been conducted for decades and the burden on the national budget is around 32 million US dollars in 2015 (18). Furthermore, active pulmonary tuberculosis with acute respiratory failure will consume even more health care resources along with an increase in the required budget (12,14). The availability of independent factors in the development of acute respiratory failure and outcome would be helpful for early and aggressive management to prevent this catastrophe.
This retrospective study presents the clinical characteristics of 268 hospitalized patients with active pulmonary tuberculosis or its complications between January 2003 and December 2012. Half of the patients required mechanical ventilator support. The overall mortality rate was 30.2% which is comparable to previous reports (5,19-21). We found that shorter duration of illness, underlying disease of AIDS, development of fever and dyspnea on presentation, large amount of acid fast bacilli on sputum smear, lower serum albumin level, and delayed initiation of anti-tuberculosis agents were among the independent risk factors to develop acute respiratory failure requiring mechanical ventilator support. In addition, our findings also indicated that male patients, consolidation appearance on chest imaging, and low serum albumin level were independent factors of mortality in pulmonary tuberculosis patients who suffered from acute respiratory failure.
Pulmonary tuberculosis in the adult population normally originates by reactivation of dormant previous focus sites at the time of primary infection. From this pathogenesis of disease, the clinical presentation and duration of illness is typically insidious and could present for weeks or months before a diagnosis is made (22). A cavitary lesion on chest X-ray indicates the insidious disease pattern and is commonly found in reactivation of pulmonary tuberculosis (23-25). However, the reactivation of tuberculosis can cause a rapid onset of illness and develop into a severe disease, particularly in the elderly, AIDS patients, and patients with immunosuppressive agents (26). Acute tuberculous pneumonia, the severe form of reactivation of disease, is characterized by fever, dyspnea, and clinical signs mimicking severe bacterial pneumonia and sepsis. The aggressive immunologic responses to tuberculoprotein or a massive number of tubercle bacilli from reactivation of disease induces the hypersensitivity reaction and eventually develops into a systemic inflammatory response similar to a common systemic bacterial infection (27,28). The duration of illness usually is less than one month, which is different from common reactivation of disease (7,10,29,30). Dense consolidation with multiple anatomical lobes of lung infiltration on chest X-ray was seen in this pathophysiology (31).
In our study, short duration of illness, fever and dyspnea, and multiple-lobe infiltration on chest X-ray were among the significant independent risk factors of acute respiratory failure, which possibly indicated that these patients may suffer from severe acute tuberculous pneumonia. Several reports showed that acute tuberculous pneumonia and miliary tuberculosis are the significant causes of acute respiratory failure in pulmonary tuberculosis (10,30).
Our study also confirmed the findings of previous studies that delayed initiation of anti-tuberculosis agents was an independent factor of respiratory failure and worse clinical outcomes (5,19,20,32). Therefore, patients suspected of acute tuberculous pneumonia need to be considered for development of acute respiratory failure and anti-tuberculosis agents must not be delayed in such patients. However, it is difficult to clinically and radiologically differentiate between severe bacterial pneumonia and acute tuberculous pneumonia in particular patients. If the clinical condition of patients begins to deteriorate despite appropriate antibiotic treatment, anti-tuberculosis agents should be started promptly and Mycobacterium tuberculosis should be identified (26).
From previous studies, the incidence of active pulmonary tuberculosis complicated by acute respiratory failure was approximately 1.5% to 5% and the mortality rate was significantly high in a range of 30% to 100% (4,8,10,12). Our study also demonstrated the incidence of acute respiratory failure in 50% of hospitalized patients with pulmonary tuberculosis, which resulted in a mortality rate of around 54%. The major cause of death in our cohort was ventilator associated pneumonia. The incidence of acute respiratory failure in our study was significantly high, which may not represent general clinical practice. In the context of tertiary care, our hospital is the only hospital in this area with isolation rooms for mechanical ventilator dependent patients with tuberculosis. Therefore, we admitted several referral patients with active pulmonary tuberculosis complicated by acute respiratory failure from surrounding hospitals. This may not determine exactly the true incidence of pulmonary tuberculosis with acute respiratory failure in the general population.
According to mortality risk factors of TBMV patients, male gender, and consolidation on chest X-ray were the significant independent mortality risk factors. This present study has confirmed a previous study that consolidation on chest X-ray was an independent factor for mortality in active pulmonary tuberculosis with acute respiratory failure (4).
From our findings, the TBMV group and non-survivor group had significantly lower levels of serum albumin, which probably indicated a greater severity of illness. Zahar et al. (7) also found that a serum albumin level of less than 2 gm/dL correlated to mortality in active pulmonary tuberculosis with acute respiratory failure.
Albumin is a natural protein in plasma that is synthesized exclusively by the liver and possesses numerous properties. This pertains to such properties as holding the intravascular blood volume by its colloidal osmotic effect, acting as a protein carrier of medication and endogenous substances, and promoting immunologic activities. During stress, albumin redistributes from the liver for antioxidant functions and escapes from the intravascular space by capillary leakage. This results in a low serum albumin level. A higher severity of stress correlates with a lower serum albumin level. Therefore, a low serum albumin level indicated the severity of illness and correlated with mortality in the critically ill setting (33).
The time of symptoms onset to treatment, number of organ failures, sepsis, large amounts of acid fast bacilli from sputum staining, and APACHE II >20 were independently associated to mortality in previous studies (4,7,10,11), but not found in our study. Diabetes mellitus was a strong indicator for treatment failure and mortality in general pulmonary tuberculosis patients (34-37). No studies in acute respiratory failure confirmed this finding.
However, this retrospective data collection may have limitations on appropriateness and completeness of variables. This limitation may be corrected by a prospective study to determine the risk factors that may discriminate these patients. Since this study was a single center observational study, the results of our study cannot be applied to different places and contexts of healthcare institutes. A multicenter database registration may diminish this limitation.
Conclusions
The incidence of pulmonary tuberculosis with acute respiratory failure in our 10-year retrospective review was essentially high. Short duration of illness, fever and dyspnea, underlying disease of AIDS, amount of acid fast bacilli on sputum smear, multiple lobe infiltration on chest X-ray, and delayed initiation of anti-tuberculosis agents were the independent risk factors to develop acute respiratory failure requiring mechanical ventilation. Male gender and consolidation on chest X-ray were significant mortality risk factor in TBMV patients. Low serum albumin was an independent risk factor in both development of acute respiratory failure and mortality in our cohort. From the evidence, the patients in our study possibly suffered from acute tuberculous pneumonia rather than the usual reactivated tuberculous pulmonary infection. Therefore, the patients who developed acute tuberculous pneumonia should be considered for close monitoring for development of acute respiratory failure and rapid initiation of anti-tuberculous agents to prevent morbidity and mortality.
Acknowledgements
The authors are grateful to the International Affairs Department, Faculty of Medicine, Prince of Songkla University for the language correction services.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Ethic Committee (EC) at Faculty of Medicine, Prince of Songkla University (EC number: 51-039-14-4-3). A waiver of consent was approved and the investigators assigned a confidentiality term.
References
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Chen CH, Shu KH, Ho HC, et al. A nationwide population-based study of the risk of tuberculosis in different solid organ transplantations in Taiwan. Transplant Proc 2014;46:1032-5. [Crossref] [PubMed]
- Chen CH, Wu MJ, Lin CH, et al. Comparison of tuberculosis infection rates in a national database of renal transplant patients with data from a single center in Taiwan. Transplant Proc 2014;46:588-91. [Crossref] [PubMed]
- Lee PL, Jerng JS, Chang YL, et al. Patient mortality of active pulmonary tuberculosis requiring mechanical ventilation. Eur Respir J 2003;22:141-7. [Crossref] [PubMed]
- Lin CH, Lin CJ, Kuo YW, et al. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis 2014;14:5. [Crossref] [PubMed]
- Marks SM, Taylor Z, Burrows NR, et al. Hospitalization of homeless persons with tuberculosis in the United States. Am J Public Health 2000;90:435-8. [Crossref] [PubMed]
- Zahar JR, Azoulay E, Klement E, et al. Delayed treatment contributes to mortality in ICU patients with severe active pulmonary tuberculosis and acute respiratory failure. Intensive Care Med 2001;27:513-20. [Crossref] [PubMed]
- Aggarwal D, Mohapatra PR. Pulmonary tuberculosis with acute respiratory failure: yet to be conquered. Eur Respir J 2008;32:1667. [Crossref] [PubMed]
- Karapetsa M, Makris D, Mpaka M, et al. Acute respiratory failure from pulmonary tuberculosis due to head trauma immunosupression. Anaesth Intensive Care 2012;40:361-2. [PubMed]
- Kim YJ, Pack KM, Jeong E, et al. Pulmonary tuberculosis with acute respiratory failure. Eur Respir J 2008;32:1625-30. [Crossref] [PubMed]
- Ryu YJ, Koh WJ, Kang EH, et al. Prognostic factors in pulmonary tuberculosis requiring mechanical ventilation for acute respiratory failure. Respirology 2007;12:406-11. [Crossref] [PubMed]
- Taylor Z, Marks SM, Ríos Burrows NM, et al. Causes and costs of hospitalization of tuberculosis patients in the United States. Int J Tuberc Lung Dis 2000;4:931-9. [PubMed]
- Shneerson JM. Respiratory failure in tuberculosis: a modern perspective. Clin Med (Lond) 2004;4:72-6. [Crossref] [PubMed]
- Calligaro GL, Theron G, Khalfey H, et al. Burden of tuberculosis in intensive care units in Cape Town, South Africa, and assessment of the accuracy and effect on patient outcomes of the Xpert MTB/RIF test on tracheal aspirate samples for diagnosis of pulmonary tuberculosis: a prospective burden of disease study with a nested randomised controlled trial. Lancet Respir Med 2015;3:621-30. [Crossref] [PubMed]
- Chakhaia T, Magee MJ, Kempker RR, et al. High utility of contact investigation for latent and active tuberculosis case detection among the contacts: a retrospective cohort study in Tbilisi, Georgia, 2010-2011. PLoS One 2014;9:e111773. [Crossref] [PubMed]
- Singhal R, Myneedu VP. Microscopy as a diagnostic tool in pulmonary tuberculosis. Int J Mycobacteriol 2015;4:1-6. [Crossref] [PubMed]
- Bhalla AS, Goyal A, Guleria R, et al. Chest tuberculosis: Radiological review and imaging recommendations. Indian J Radiol Imaging 2015;25:213-25. [Crossref] [PubMed]
- World Health Organization. Global tuberculosis report 2015. Available online: http://www.who.int/tb/publications/global_report/en/
- Sharma SK, Mohan A, Banga A, et al. Predictors of development and outcome in patients with acute respiratory distress syndrome due to tuberculosis. Int J Tuberc Lung Dis 2006;10:429-35. [PubMed]
- Lin SM, Wang TY, Liu WT, et al. Predictive factors for mortality among non-HIV-infected patients with pulmonary tuberculosis and respiratory failure. Int J Tuberc Lung Dis 2009;13:335-40. [PubMed]
- Lin TM, Chao SL, Luan HW, et al. An analytical study on the mortality and prevalence rates of pulmonary tuberculosis in the aboriginal area in Taiwan. Taiwan Yi Xue Hui Za Zhi 1981;80:359-68. [PubMed]
- Stead WW, Kerby GR, Schlueter DP, et al. The clinical spectrum of primary tuberculosis in adults. Confusion with reinfection in the pathogenesis of chronic tuberculosis. Ann Intern Med 1968;68:731-45. [Crossref] [PubMed]
- Barnes PF, Verdegem TD, Vachon LA, et al. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest 1988;94:316-20. [Crossref] [PubMed]
- Van den Brande P, Dockx S, Valck B, et al. Pulmonary tuberculosis in the adult in a low prevalence area: is the radiological presentation changing? Int J Tuberc Lung Dis 1998;2:904-8. [PubMed]
- Van den Brande P, Gyselen A, Demedts M. Roentgenographic manifestations of pulmonary tuberculosis in the adult: frequency of atypical findings. Acta Clin Belg 1988;43:58-61. [Crossref] [PubMed]
- Hagan G, Nathani N. Clinical review: tuberculosis on the intensive care unit. Crit Care 2013;17:240. [Crossref] [PubMed]
- Puri MM, Kumar S, Prakash B, et al. Tuberculosis pneumonia as a primary cause of respiratory failure--report of two cases. Indian J Tuberc 2010;57:41-7. [PubMed]
- Septimus EJ, Awe RJ, Greenberg SD, et al. Acute tuberculous pneumonia. Chest 1977;71:774-5. [Crossref] [PubMed]
- Calix AA, Ziskind MM, Leonard AJ, et al. Acute tuberculous pneumonia in the Negro. Am Rev Tuberc 1953;68:382-92. [PubMed]
- Kim CW, Kim SH, Lee SN, et al. Risk factors related with mortality in patient with pulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2012;73:38-47. [Crossref] [PubMed]
- Jacob JT, Mehta AK, Leonard MK. Acute forms of tuberculosis in adults. Am J Med 2009;122:12-7. [Crossref] [PubMed]
- Lisha PV, James PT, Ravindran C. Morbidity and mortality at five years after initiating Category I treatment among patients with new sputum smear positive pulmonary tuberculosis. Indian J Tuberc 2012;59:83-91. [PubMed]
- Vincent JL, Russell JA, Jacob M, et al. Albumin administration in the acutely ill: what is new and where next? Crit Care 2014;18:231. [Crossref] [PubMed]
- Filla E, Comenale Pinto D. Mortality and causes of death in patients with diabetes and pulmonary tuberculosis. Minerva Med 1966;57:3808-14. [PubMed]
- Fossati C. Associated diabetes and pulmonary tuberculosis: occurrence, development, mortality and causes of death; statistical study of Arab-Libyan patients in Cyrene and covering the 10 past years. Diabete 1969.261-7. [PubMed]
- Gil-Santana L, Almeida-Junior JL, Oliveira CA, et al. Diabetes Is Associated with Worse Clinical Presentation in Tuberculosis Patients from Brazil: A Retrospective Cohort Study. PLoS One 2016;11:e0146876. [Crossref] [PubMed]
- Magee MJ, Foote M, Maggio DM, et al. Diabetes mellitus and risk of all-cause mortality among patients with tuberculosis in the state of Georgia, 2009-2012. Ann Epidemiol 2014;24:369-75. [Crossref] [PubMed]


