
Comparison of video assisted thoracoscopic surgery and thoracotomy for treatment of pleural infection stage II and III: a literature review
Introduction
Pleural infection is associated with significant mortality and morbidity worldwide, with a steadily increasing incidence (1,2). Classically, pleural infection has been staged based on pathophysiological characteristics: an initial exudative phase, a fibrinopurulent phase and a final organizing phase (3) as used by the American Thoracic Society. Recent clinical guidelines [e.g., British Thoracic Society 2010 (4)] divide the disease into three stages based on the results of pleural fluid aspiration: (I) a simple parapneumonic effusion, stage (II) complicated parapneumonic effusion, and (III) pleural empyema (4,5). Considering the heterogeneous nature of pleural infection over time, treatment varies according to stage of disease (6). The aim of treatment is removal of infection, prevention of chronic and recurrent disease in the pleural space, and avoidance of restrictive lung disease by achieving full lung expansion (7).
During the first stage of pleural infection, antibiotics and chest tube drainage have been shown to constitute effective treatment and are recommended in current guidelines (4,8). Stages II and III require invasive intervention with initial treatment consisting of surgical or ultrasound-guided drainage (4,8,9). Major surgical intervention is reserved primarily for cases in which initial medical treatment fails and the pleural collection persists, although some data exists to support its use as an alternative to initial ultrasound-guided drainage (10,11). Decortication by thoracotomy has been the preferred surgical approach until recently; however, many studies have indicated that debridement by video-assisted thoracoscopic surgery (VATS) is an adequate method for treating empyema (12,13). In addition, VATS is established as a standard approach for other thoracic procedures, such as lobectomy in early-stage lung cancer, where favorable outcomes including reduced postoperative pain, postoperative morbidities, complications, and mortality have been demonstrated in a randomized trial (14). However, in empyema surgery, VATS can be technically more challenging and has a high perioperative conversion rate to open thoracotomy (13). Thus, the indications for VATS as opposed to thoracotomy remain controversial, and direct evidence in the literature is not clear (13,15,16).
The aim of this study was therefore to investigate which surgical approach (VATS or thoracotomy) provided the best patient outcome in the treatment of pleural infection in stage II (complicated parapneumonic effusion) and stage III (pleural empyema) disease assessing clinically important outcomes. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-928/rc).
Methods
Literature search
The search strategy summary is shown in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | March 14th, 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | ((“Empyema”[MeSH Terms] OR “Empyema”[All Fields] OR “empyemas”[All Fields]) AND (“thoracic surgery, video assisted”[MeSH Terms] OR (“thoracic”[All Fields] AND “surgery”[All Fields] AND “video assisted”[All Fields]) OR “video-assisted thoracic surgery”[All Fields] OR (“video”[All Fields] AND “assisted”[All Fields] AND “thoracoscopic”[All Fields] AND “surgery”[All Fields]) OR “video assisted thoracoscopic surgery”[All Fields]) AND (“thoracotomy”[MeSH Terms] OR “thoracotomy”[All Fields] OR “thoracotomies”[All Fields]) AND (“adult”[MeSH Terms] OR “adult”[All Fields] OR “adults”[All Fields] OR “adult s”[All Fields])) |
| Timeframe | Published on or before March 14th, 2023 |
| Inclusion and exclusion criteria | Inclusion: eligible studies included observational and randomized studies published in English that compared VATS and thoracotomy in the treatment of pleural empyema of all etiologies. Study characteristics included the requirement for participants >16 years of age on the day of hospital admission who were diagnosed with stage II or III pleural infection |
| Exclusion: all case reports were excluded regardless of relevance, and an assessment of quality was applied to all studies | |
| Selection process | All papers identified through the literature search were screened for study inclusion by reviewing titles and abstracts (if available). Five articles were subsequently acquired from included references. A total of 154 articles were screened, of which 18 full articles were retrieved. Screening and assessment of study eligibility, performed using the predefined criteria described in detail above, was performed by two reviewers (Steen K and Sørensen J), as well as data extraction and analysis |
| Additional considerations | Each study was assessed for quality using the Quality Assessment Tool for Observational Cohort and Cross-sectional studies from the National Heart, Lung, and Blood Institute, USA. Quality assessment was conducted in relation to relevance and clarity of the research question, specification and definition of the study population, and the validity, reliability, and consistency of outcome measures. Studies were required to assess a number of exposures in relation to outcomes, and key potential confounding variables were to be measured and statistically adjusted for their influence on the association between exposure and outcome |
VATS, video-assisted thoracic surgery.
A literature search of the PubMed database was conducted using the following search strategy: ((“Empyema”[MeSH Terms] OR “Empyema”[All Fields] OR “empyemas”[All Fields]) AND (“thoracic surgery, video assisted”[MeSH Terms] OR (“thoracic”[All Fields] AND “surgery”[All Fields] AND “video assisted”[All Fields]) OR “video-assisted thoracic surgery”[All Fields] OR (“video”[All Fields] AND “assisted”[All Fields] AND “thoracoscopic”[All Fields] AND “surgery”[All Fields]) OR “video assisted thoracoscopic surgery”[All Fields]) AND (“thoracotomy”[MeSH Terms] OR “thoracotomy”[All Fields] OR “thoracotomies”[All Fields]) AND (“adult”[MeSH Terms] OR “adult”[All Fields] OR “adults”[All Fields] OR “adult s”[All Fields])). Original articles were selected based on titles and abstracts relevant to the topic. In addition, relevant articles were identified by reviewing references in key publications. The concluding search was conducted on March 14th, 2023, with no restrictions on the date of publication.
Eligibility criteria
Eligible studies included observational and randomized studies published in English that compared VATS and thoracotomy in the treatment of pleural empyema of all etiologies. Study characteristics included the requirement for participants >16 years of age on the day of hospital admission who were diagnosed with stage II or III pleural infection. All case reports were excluded regardless of relevance, and an assessment of quality was applied to all studies.
Screening and assessment of study eligibility
All papers identified through the literature search were screened for study inclusion by reviewing titles and abstracts (if available). Five articles were subsequently acquired from included references. A total of 154 articles were screened, of which 18 full articles were retrieved. Screening and assessment of study eligibility, performed using the predefined criteria described in detail above, was performed by two reviewers (Steen K and Sørensen J), as well as data extraction and analysis.
Data extraction
The following information was extracted from the included studies: authors, journal name, publication year, country of origin, study design, number of patients, mean age, sex distribution, surgical procedure performed, stage of empyema, and treatment response, including patient outcome in terms of operative time, duration of chest drainage, hospital stay before and after surgery, postoperative pain, patient satisfaction, mortality rate, and conversion rate.
Quality assessment
Each study was assessed for quality using the Quality Assessment Tool for Observational Cohort and Cross-sectional studies (17) from the National Heart, Lung, and Blood Institute, USA.
Quality assessment was conducted in relation to relevance and clarity of the research question, specification and definition of the study population, and the validity, reliability, and consistency of outcome measures. Studies were required to assess a number of exposures in relation to outcomes, and key potential confounding variables were to be measured and statistically adjusted for their influence on the association between exposure and outcome.
Patients and public involvement
No patients were involved due to the nature of the paper.
Results
Included studies
Nine studies with a total of 2,121 patients were included. The screening process for inclusion and exclusion is illustrated in Figure 1. Table 2 provides an overview of the included studies. All selected studies were non-randomized retrospective studies published between 1996 and 2020. The mean age ranged from 37 to 57 (±12.9) years, with a significant overrepresentation of men (51.4% to 94.4%). Two studies were conducted in Asia (12,22), two in the Americas (15,18), and the remainder in Europe.
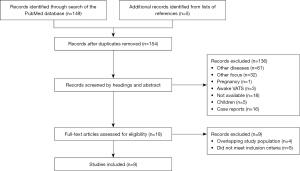
Table 2
| First author, year, reference | Country of origin and time period | Study design |
Procedure performed | No. of patients, n | Age of patients (years), mean ± SD/mean/median [IQR] | Males, n [%] |
Pleural infection stage (pathophysiological) |
|---|---|---|---|---|---|---|---|
| Chan et al., 2007, (12) | China (Hong Kong), January 2001 to December 2005 | Retrospective cohort study | TH; VATS | 36; 41 | 48.6±16.0; 46.1±14.7 |
34 [94.4]; 33 [90.5] |
Stage II + III |
| Podbielski et al., 2000, (15) | USA, June 1994 to April 1997 | Retrospective cohort study | TH; VATS | 14; 16 | 50.9; 51.6 | 12 [85.71]; 9 [56.25] |
Stage II + III |
| Angelillo Mackinlay et al., 1996, (18) | Argentina, 1985 to 1994 | Retrospective cohort study | TH; VATS | 33; 31 | 51.1; 48.9 | 20 [60.6]; 23 [74.19] |
Stage II; postpneumonic |
| Marks et al., 2012, (1) | UK, 1999 to 2010 | Retrospective cohort study | TH; VATS | 277; 116 | 53† | 298 [74]† | Stage II + III |
| Cardillo et al., 2009, (19) | Italy, January 1996 to December 2006 | Retrospective cohort study | TH; VATS | 123; 185 | 57±12.9; 55.8±10.6 |
79 [64.2]; 95 [51.4] |
Stage II + III postpneumonia; empyema |
| Waller et al., 2001, (11) | UK, an unspecified 3-year period | Retrospective cohort study | TH; VATS | 12; 36 | 43.5±4.1; 45±4.1 |
10 [83.3]; 24 [66.67] |
Stage III‡ |
| Lardinois et al., 2005, (20) | Switzerland, 1992 to 2002 | Prospective cohort study | TH; VATS | 150; 178 | 55 [18–90]† | 227 [69]† | Stage II + III |
| Shahin et al., 2010, (21) | UK, August 2005 to October 2008 | Retrospective cohort study | Stage II debridement: TH, VATS; stage III decortication: TH, VATS | 1, 8; 20, 32 | 53†; 52† | 20 [69]†, 38 [73]† |
Stage II + III postpneumonic |
| Majeed et al., 2020, (22) | Pakistan, January 2006 to March 2018 | Retrospective cohort study | TH; VATS | 650; 162 | 37† | 537 [66.1]† | Stage II + III |
†, the study did not distinguish outcomes between VATS and thoracotomy group; ‡, the study used clinical based staging of pleural infection. SD, standard deviation; IQR, interquartile range; TH, thoracotomy (open surgical approach); VATS, video-assisted thoracic surgery.
Causes of pleural infection
The included studies differed in etiology of pleural infection. Several studies only included patients diagnosed with postpneumonic pleural infection (18,19,21), with one study excluding human immunodeficiency virus (HIV)-positive patients (19). Another study (12) included all causes of pleural infection, except malignancy. The remaining studies (1,11,12,15,20,22) did not exclude patients based on etiology. Two studies included a minority of patients with fungal pleural infection [0.7% (22) and 2.2% (1) respectively].
Staging system
One of the included studies staged pleural infection based on clinical features (11), whereas all remaining studies staged using the American Thoracic Society staging system (1,12,15,18-22).
Surgical approach
A comparison between the surgical treatment of empyema with thoracotomy and VATS is presented in Table 3. The study size ranged from 12 to 650 patients treated with thoracotomy and 16 to 185 with VATS. Operative time was higher for thoracotomy compared with VATS in four of the included studies (11,12,15,19).
Table 3
| First author, year, reference | Procedure | OP time (minutes), mean ± SD | Post OP chest tube time (days), mean ± SD | Post OP hospital stay (days), mean ± SD/median | Pre OP hospital stay (days), mean ± SD | Mortality (%) | Patient satisfaction† | Post OP pain‡ | Conversion to TH, n [%] |
|---|---|---|---|---|---|---|---|---|---|
| Chan et al., 2007, (12) | TH; VATS | 228±84; 150±57.6 (P<0.001) | 8.5±4.4; 7.9±5.7 (P=0.609) | 21±14.2; 16±6.5 (P=0.052) |
N/A | 0; 0 | VATS superior (P=0.006) | VATS with less pain (P=0.04) | 0 |
| Podbielski et al., 2000, (15) |
TH; VATS | 125±71.7; 76.2±30.7 | 8.3±4.6; 4.7±2.8 (P=0.01) | 10.2±7.2; 17.6±16.8 (P=0.26) |
16±10.2; 11.4±6.5 | N/A | N/A | N/A | 2 [7] |
| Angelillo Mackinlay et al., 1996, (18) |
TH; VATS | 123±25.8; 119±32.5 (P: NS) |
6.1±2.3; 4.3±1.5 (P=0.02) | 11.6±9.1; 6.7±3.0 (P=0.007) |
17.5±13.7; 11.4±8.2 | 3; 3.2 | VATS superior | N/A | 3 [10] |
| Marks et al., 2012, (1) | TH; VATS | N/A | N/A | 7; 5 (P<0.0001) |
N/A | ca. 5.7; ca. 5.7 |
N/A | N/A | 17 [14.66] |
| Cardillo et al., 2009, (19) | TH; VATS | 79.7±6.8; 70±7.4 (P<0.0001) | 3.9±4.3; 2.8±2.4 (P=0.004) | N/A | N/A | 3.2; 0 | VATS superior | VATS with less pain (P<0.0001) | 11 [5.9]; all stage III |
| Waller et al., 2001, (11) | TH, VATS; conversion | 109±5.5, 78.8±6.5 (P=0.001); 119.6±13.5 | N/A | 8.4±0.7, 5.5±0.6 (P=0.004); 8.5±1.3 |
28.7±7.1; 19.1±3.4; 24.0±2.0 |
0; 4.8; 6.7 | N/A | N/A | 15 [42] |
| Lardinois et al., 2005, (20) |
TH; VATS | N/A | N/A | N/A | N/A | 3; 4 | N/A | N/A | 79 [44] |
| Shahin et al., 2010, (21) | Decortication: TH; VATS | N/A | N/A | 8; 5 | N/A | 0; 0 | N/A | N/A | 6 [19] |
| Majeed et al., 2020, (22) | TH; VATS | N/A | N/A | 70.8% <5 days; 85.1% <5 days |
N/A | 1.3; 0 | N/A | N/A | 22 [13.5] |
†, satisfaction with surgical outcome, including cosmesis and acceptance (using an individual score from 1–10, the best being 10); ‡, measured on postoperative days 1 and 6 or 7 (using an individual pain score from 1–10, the worst pain scored as 10). VATS, video-assisted thoracic surgery; OP, operative; SD, standard deviation; TH, thoracotomy (open surgical approach); N/A, not applicable; NS, nonsignificant.
Intraoperative conversions from VATS to open surgery
The conversion rate from VATS to thoracotomy varied from 0% to 44% [mean: 17.3%, 95% confidence interval (CI): 7.8% to 26.9%] as presented in Figure 2. A total of 805 patients underwent VATS, and of these 155 were converted to thoracotomy, of which 135 (87%) were stage III (pleural empyema). Studies with high conversion rates demonstrated gram-negative microorganisms (P<0.01) and delayed referral (P<0.0001) were statistically significant predictors of conversion to thoracotomy at stage II (complicated parapneumonic effusion) (11,20).
Postoperative complications
The postoperative chest tube period was significantly higher with thoracotomy in three studies (15,18,19), and postoperative hospital stay was significantly higher with thoracotomy in five studies (1,11,18,21,22). In studies reporting patient satisfaction, VATS was found to be superior to thoracotomy (12,18,19), with reduced postoperative pain (12,19).
Mortality
Mortality rates in thoracotomy ranged from 0–6.7% (mean mortality of 2.0%, 95% CI: 0.7% to 3.4%), and in VATS were 0–5.7% (mean mortality of 2.2%, 95% CI: 0.6% to 3.8%).
Discussion
Within this systematic review, all nine studies demonstrated VATS and thoracotomy to be effective in treatment of stage II and III pleural infection (1,11,12,15,18-22). In studies that reported outcomes for mixed populations of stage II and III treated with VATS or thoracotomy, several outcomes suggested advantages of VATS over thoracotomy, including reduced operative time, fewer days with tube drainage, and shorter hospital stay. In addition, decreased postoperative pain and greater patient satisfaction with both wound appearance and the procedure were shown with VATS. There were no significant differences in mortality rates between the two procedures. These findings suggest that treatment of pleural infection with VATS appears to be as adequate as thoracotomy in a significant proportion of patients, with the advantages of a less invasive approach (11,18,21).
Considering that VATS is less invasive than thoracotomy, the introduction of VATS as first-line therapy may result in less treatment delay and shorter pre- and postoperative duration of medical treatment. The reduction in length of hospital stays with VATS compared with open surgery was demonstrated in most of the reviewed papers (1,11,18,19,21,22). The only paper that identified a longer average hospital stay (15) included patients considered too vulnerable for open surgery in the VATS cohort. Thus, the coexisting morbidities often resulted in prolonged postoperative hospital stays, even after removal of the chest tube. We postulate that the difference in this study was due to inclusion of such patients as Schweigert et al. have made the connection in their retrospective study of 335 surgically treated parapneumonic empyema patients, where preexisting comorbidity was shown to have a determining influence on adverse outcome (23).
The overall reduction in hospitalization time in VATS compared with thoracotomy-treated patients has been demonstrated for both pleural infection stages II and III (1,19).
Chan et al. (12) found that 83.3% of their thoracotomy-treated patients and 68.3% in the VATS group were stage III. All procedures were successful, and there were no significant differences in complications between groups, suggesting both treatments can yield equally effective results in terms of radiographic and functional outcomes in stage III pleural infection. These results were further supported by Shahin et al. (21), where 97% of VATS debridements were successful in the chronic organized phase. In addition, successful outcomes were achieved in 62% of patients with stage III treated with VATS, where shorter postoperative hospital stays and fewer postoperative complications were observed in the VATS group. Striffeler et al. (6) stated that VATS is an effective procedure for the treatment of stage II pleural infection, being superior to thoracotomy in terms of postoperative pain and function. However, in contrast to the previously presented results, it was suggested that VATS is not well-suited for the treatment of late-stage empyema and failure to acknowledge the limitations of VATS can result in poor outcomes in terms of lung function.
Lardinois et al. (20) found that the interval between the onset of pleural effusion symptoms and surgery plays a significant role in the development of chronic empyema and in predicting treatment failure with VATS. The included stage III patients had more than a 3-week history of disease and CT images showing thickened pleura with lung restriction, making them inaccessible to treatment by VATS. The likelihood of conversion to thoracotomy for empyema in a presumed fibropurulent phase assessed by VATS was increased when there were more than 2 weeks between onset of symptoms and surgery. The interval between symptoms and surgery was shown to be responsible for VATS accessibility in treatment of pleural infection at both stages II and III. Delay between diagnosis and treatment, leading to stage progression and further organization, has also been found to be a major hurdle in the treatment of pleural empyema (15,24).
In contrast, Waller et al. (11) found no significant difference in the delay from either start of symptoms or hospital admission to surgery between the VATS and thoracotomy group. Conversely, a significantly shorter operative time was found in VATS with prolonged delays to surgery. Open decortication may be beneficial in developing countries with financial and logistical constraints. This is likely due to constraints that lead to late referrals and thereby late presentation. Early-stage empyema can be treated with VATS, resulting in significantly lower mortality compared with thoracotomy, but of the 42 patients with chronic empyema treated with VATS by Majeed et al. (22), 52% required intraoperative conversion to utility thoracotomy. In total, 156 of the 805 VATS surgeries were converted to thoracotomy. 87% of the conversions involved patients who had stage III pleural infection. Failure of VATS was attributable to inadequate accessibility and visualization of the cavity (11,15,19-22). In cases of inadequacy between remaining pleural cavity and lung volume, as seen in late-stage pleural infection, an open procedure such as thoracoplasty may play a role. Majeed et al. utilized the procedure in 43 cases of bronchopleural fistulas and simultaneous collapse consolidation (25). Generally, the procedure is seldom used, but is a potential option in selected cases, especially in empyema after lung resections and other etiologies of bronchopleural fistulas (26).
Conversion rates, from VATS to thoracotomy, were shown to be as high as 44% in the nine eligible studies, implying that VATS has some limitations in the treatment of chronic empyema. Other than late referral (P<0.0001), studies with high conversion rates attributed the increase to postpneumonic etiology and presence of gram-negative organisms (20). In contrast, no conversions were reported by Chan et al. (12) despite treatment of stage III pleural infection with VATS, suggesting that VATS may also be an effective treatment for more chronic stages of empyema. In the latter study, the procedure was performed by the same experienced surgeon in all cases of VATS. Although diverse, the results indicate that VATS could be considered first choice in the treatment of both stage II and stage III pleural infection when performed by experienced surgeons with prompt conversion to thoracotomy should the surgeon deem necessary (13).
In all studies, the performing surgeons decided the surgical approach. Furthermore, the indications for using either of the two approaches were not standardized when comparing studies. This non-randomized design introduces a strong selection bias, considering patients who underwent VATS may have had a lower stage or less complicated empyema (10,12). Ultimately, the choice of surgical approach depended on the surgeon’s individual preference, abilities, skills, local conditions, and experience (e.g., anesthesia, ward) which may account for discrepancies in outcomes (10,12). According to recent guidelines (27), the efficacy of surgical methods (decortication, drainage or debridement) for pleural infections cannot be determined based on available evidence.
Comparison of outcomes across the eight studies revealed several commonalities with remaining distinguishing factors. The study populations were a mixture of stage II and III pleural infection with either exclusively pneumonic causes (18,19,21,22) or a varying mixture of etiologies for pleural infection (1,11,12,15,20,22) and the treatment strategy with VATS and thoracotomy varied from debridement to decortication. VATS and thoracotomy rates were not equally distributed in the eight studies, with VATS predominating as the surgical intervention for stage II pleural infection and thoracotomy predominating for stage III pleural infections. This should be considered a confounding factor, reducing the comparison between the two techniques. The staging system used varied between papers, with the majority being based on pathophysiological characteristics (1,12,15,18-22), and the one being based on clinical features (11). The two staging systems do not correspond directly, why a comparison of outcome of staging based surgical approach can potentially be misleading. To make a fair comparison of the surgical interventions examined, a consensus in applied staging system is needed, as classifications differ significantly between papers of both surgical and nonsurgical nature.
The possibility of chronology bias can be profound, as the studies were all retrospective in design and the study population recruitment time frames varied widely among the studies. For example, the reduced operation time noted in the studies (11,12,15,19) could be affected by medical advancement over time, such as improved surgeon experience or better preoperative radiographic imaging. Consequently, the possible variations between surgeons and individual surgeons’ performance, surgical plan, experience, and technical maneuvers could lead to performance bias (28). In addition, the retrospective design adds further recall bias to the examined studies.
As included studies were characterized by severe selection and possibly chronology and recall bias, future trials comparing VATS and thoracotomy in the treatment of pleural infection stage II and III are necessary to confirm our findings of VATS superiority. Included studies varied in staging method, surgical method (debridement with or without decortication) and thoracotomy technique, why a more precise algorithmic systematization of management strategy should also be included to further minimize selection bias. The predefined criteria should also include comorbidities and referral time with consideration to prior interventions (duration of initial chest tube treatment and antibiotic failure). Accordingly, future studies should be prospective in design with objective decision-making criteria or shared datasets.
Since the publication of the nine included studies, British Thoracic Society has released new guidelines for managing pleural infection (27) and The European Respiratory Society and European Society of Thoracic Surgeons (29) have published their statement on management of pleural infection. A significant update to the publications compared to preexisting guidelines, is a recommendation of VATS as the optimal surgical approach for managing pleural infection.
Conclusions
We found that both VATS and thoracotomy are viable options for the treatment of patients with stage II and III pleural infection. However, VATS has potential advantages in terms of decreased operation time, fewer days with tube drainage, shorter postoperative hospital stay, reduced postoperative pain, increased patient satisfaction with the procedure, and wound appearance. VATS has its limitations in the treatment of patients with stage III pleural infection, where delayed surgical referral has been shown to increase the risk of intraoperative conversion to thoracotomy. The data to date implies that debridement by VATS should be proposed as soon as possible in stage II pleural infection and considered in cases of stage III pleural infection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-928/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-928/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-928/coif). R.H.P. serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2022 to September 2024. R.H.P. has received a speaker’s fee from Medtronic, AstraZeneca, Ambu and Medela and on the advisory board for AstraZeneca, Bristol-Myers Squibb, Roche and Merck Sharp & Dohme (MSD). T.D.C. has been on the speaker bureaus for AstraZeneca, Bristol-Myers and Novartis and has been in an Advisory Board for AstraZeneca and Sanofi. NMR has received consulting fee from Rocket Medical UK and Cook Medical USA. C.B.L. has received payment for lectures at educational events/symposia/courses organised by AstraZeneca and royalties as author of book chapters/book editor from Munksgaard (publisher). Unpaid, he is ERS TUS training program committee member, ERS Monograph Deputy Chief Editor and a Board Member, Organization of Danish Medical Societies. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Given the nature of the study as a systematic review, no experiments were conducted on either humans or animals in the process of conducting this paper.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marks DJ, Fisk MD, Koo CY, et al. Thoracic empyema: a 12-year study from a UK tertiary cardiothoracic referral centre. PLoS One 2012;7:e30074. [Crossref] [PubMed]
- Arnold DT, Hamilton FW, Morris TT, et al. Epidemiology of pleural empyema in English hospitals and the impact of influenza. Eur Respir J 2021;57:2003546. [Crossref] [PubMed]
- Armbruster K, Schultz HH, Christensen TD, et al. Empyema pleurae / Parapneumonisk effusion. Dansk Lungemedicins Selskab. 2021. Available online: https://lungemedicin.dk/empyema-pleurae-parapneumonisk-effusion/
- Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii41-53. [Crossref] [PubMed]
- Bedawi EO, Hassan M, McCracken D, et al. Pleural infection: a closer look at the etiopathogenesis, microbiology and role of antibiotics. Expert Rev Respir Med 2019;13:337-47. [Crossref] [PubMed]
- Striffeler H, Gugger M. Video-assisted thoracoscopic surgery for fibrinopurulent pleural empyema in 67 patients. Ann Thorac Surg 1998;65:319-23. [Crossref] [PubMed]
- Wurnig PN, Wittmer V, Pridun NS, et al. Video-assisted thoracic surgery for pleural empyema. Ann Thorac Surg 2006;81:309-13. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Christensen TD, Bach P, Neckelmann K, et al. Vejledning til behandling af pleuraempyem. Dansk Thoraxkirurgisk Selskab. 2017. Available online: https://thoraxkirurgi.dk/nbv/pleuraempyem/
- Scarci M, Abah U, Solli P, et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur J Cardiothorac Surg 2015;48:642-53. [Crossref] [PubMed]
- Waller DA, Rengarajan A. Thoracoscopic decortication: a role for video-assisted surgery in chronic postpneumonic pleural empyema. Ann Thorac Surg 2001;71:1813-6. [Crossref] [PubMed]
- Chan DT, Sihoe AD, Chan S, et al. Surgical treatment for empyema thoracis: is video-assisted thoracic surgery "better" than thoracotomy? Ann Thorac Surg 2007;84:225-31. [Crossref] [PubMed]
- Chung JH, Lee SH, Kim KT, et al. Optimal timing of thoracoscopic drainage and decortication for empyema. Ann Thorac Surg 2014;97:224-9. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Podbielski FJ, Maniar HS, Rodriguez HE, et al. Surgical strategy of complex empyema thoracis. JSLS 2000;4:287-90.
- Lawrence DR, Ohri SK, Moxon RE, et al. Thoracoscopic debridement of empyema thoracis. Ann Thorac Surg 1997;64:1448-50. [Crossref] [PubMed]
- Study Quality Assessment Tools, NHLBI. National Heart, Lung, and Blood Institute (NHLBI). Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Angelillo Mackinlay TA, Lyons GA, Chimondeguy DJ, et al. VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema. Ann Thorac Surg 1996;61:1626-30. [Crossref] [PubMed]
- Cardillo G, Carleo F, Carbone L, et al. Chronic postpneumonic pleural empyema: comparative merits of thoracoscopic versus open decortication. Eur J Cardiothorac Surg 2009;36:914-8. [Crossref] [PubMed]
- Lardinois D, Gock M, Pezzetta E, et al. Delayed referral and gram-negative organisms increase the conversion thoracotomy rate in patients undergoing video-assisted thoracoscopic surgery for empyema. Ann Thorac Surg 2005;79:1851-6. [Crossref] [PubMed]
- Shahin Y, Duffy J, Beggs D, et al. Surgical management of primary empyema of the pleural cavity: outcome of 81 patients. Interact Cardiovasc Thorac Surg 2010;10:565-7. [Crossref] [PubMed]
- Majeed FA, Zafar U, Chatha SS, et al. Decortication as an Option for Empyema Thoracis. J Coll Physicians Surg Pak 2020;30:313-7. [Crossref] [PubMed]
- Schweigert M, Solymosi N, Dubecz A, et al. Surgery for parapneumonic pleural empyema--What influence does the rising prevalence of multimorbidity and advanced age has on the current outcome? Surgeon 2016;14:69-75. [Crossref] [PubMed]
- Ricciardi S, Giovanniello D, Carleo F, et al. Which Surgery for Stage II-III Empyema Patients? Observational Single-Center Cohort Study of 719 Consecutive Patients. J Clin Med 2022;12:136. [Crossref] [PubMed]
- Majeed FA, Chatha SS, Zafar U, et al. VATS thoracoscopic decortication for empyema thoracic: A retrospective experience and analysis of 162 cases. J Pak Med Assoc 2021;71:502-4. [Crossref] [PubMed]
- Shinohara S, Chikaishi Y, Kuwata T, et al. Benefits of using omental pedicle flap over muscle flap for closure of open window thoracotomy. J Thorac Dis 2016;8:1697-703. [Crossref] [PubMed]
- Roberts ME, Rahman NM, Maskell NA, et al. British Thoracic Society Guideline for pleural disease. Thorax 2023;78:s1-42. [Crossref] [PubMed]
- Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg 2010;126:619-25. [Crossref] [PubMed]
- Bedawi EO, Ricciardi S, Hassan M, et al. ERS/ESTS statement on the management of pleural infection in adults. Eur Respir J 2023;61:2201062. [Crossref] [PubMed]



