
PM2.5 induces lung inflammation and fibrosis via airway smooth muscle cell expression of the Wnt5a/JNK pathway
Highlight box
Key findings
• The increase in wingless-related integration site 5a (Wnt5a) expression in chronic obstructive pulmonary disease (COPD) patients is positively correlated with the level of particulate matter 2.5 (PM2.5) exposure. In addition, PM2.5 can increase the production of Wnt5a, which leads to the phosphorylation of c-Jun N-terminal kinase (JNK), increases nuclear factor kappa-light-chain-enhancer of activated B cells phosphorylation, and subsequently promotes inflammatory cytokine production and the accumulation of collagen in airway smooth muscle cells (ASMCs).
What is known and what is new?
• The Wnt5a/JNK pathway may be effectively activated, promoting pulmonary fibrosis and inflammation in COPD.
• PM2.5 stimulation of ASMCs induces pulmonary inflammatory factor expression and collagen deposition during COPD via the Wnt5a/JNK pathway.
What is the implication, and what should change now?
• These findings indicate that modulating the Wnt5a/JNK pathway could be a promising therapeutic strategy for PM2.5-induced COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterized by persistent respiratory symptoms and airflow obstruction due to airway and alveolar abnormalities. COPD is one of the chief causes of morbidity and mortality worldwide and poses an increasing social and economic burdens. Airway smooth muscle cells (ASMCs) are crucial components of airway contraction, can produce several cytokines and proteases in response to a range of inflammatory stimuli, and may be involved in airway inflammation and remodeling in COPD (1). Therefore, ASMCs are indispensable in the pathogenesis of lung inflammation and fibrosis in COPD (2). It is necessary to clarify the mechanism by which ASMCs affect COPD.
Particulate matter 2.5 (PM2.5) exposure is associated with an increased risk of COPD and is negatively correlated with the symptoms and lung function of COPD patients, as shown by many epidemiological studies (3,4). According to previous studies, PM2.5 not only influences the proliferation of ASMCs (5) but also induces inflammatory mediator secretion by human bronchial epithelial cells (HBECs) through the expression of wingless-related integration site 5a (Wnt5a) (6).
Wnt5a, which is a member of the noncanonical Wnt glycoprotein family, can trigger different kinds of downstream pathways to stimulate cell polarity, migration and proliferation. Generally, Wnt5a expression is increased in the lungs of COPD animal models. Furthermore, in a cigarette smoke extract (CSE)-induced COPD model, Wnt5a upregulation is accompanied by inflammation (7). Specifically, Wnt5a is the most highly expressed protein in its cytokine family in ASMCs. ASMC-derived Wnt5a can promote lung fibrosis (8). These studies suggest that ASMC-derived Wnt5a may lead to airway inflammation and fibrosis in COPD.
Jun N-terminal kinase (JNK) is a serine/threonine protein kinase that promotes the production of inflammatory factors, and the Wnt5a/JNK/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway plays an important role (9). Recently, the JNK/NF-κB pathway was shown to mediate the progression of pulmonary fibrosis and inflammation in mice (10). Therefore, we hypothesized that ASMC expression of Wnt5a/JNK/NF-κB pathway factors may be involved in lung inflammation and fibrosis caused by PM2.5.
Thus, in this study, the function of Wnt5a in the progression of pulmonary inflammation and fibrosis was further assessed by using ASMCs and an experimental model of COPD. We present this article in accordance with the MDAR and ARRIVE reporting checklists (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-780/rc).
Methods
Clinical specimens and patients
The study was approved by the Medical Ethics Committee of Guangzhou Chest Hospital (No. 202026). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants were required to sign an informed consent form. Based on our preliminary study (11), participants were screened from rural counties and urban districts of Guangdong Province, which have different representative PM2.5 exposure levels. The subjects were divided into low levels of PM2.5 exposure (less than 75 µg/m3) in the rural counties of Shaoguan, Heyuan and Zhanjiang and high levels of PM2.5 exposure (higher than 75 µg/m3) in the urban districts of Guangzhou. A postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) of less than 70% can be diagnosed as COPD. The exclusion criteria for patient enrollment were as follows: (I) smoking history of at least five packs of cigarettes each year; (II) a history of occupational exposure; or (III) other chronic pulmonary diseases (asthma, pulmonary cancer, tuberculosis or interstitial pulmonary disease). The participants were between 40 and 60 years old. Samples of serum and bronchoalveolar lavage fluid (BALF) were collected from the participants. Seventy-seven individuals participated in this study, which included 32 healthy controls (n=15, low levels of PM2.5 exposure; n=17, high levels of PM2.5 exposure) and 45 COPD patients (n=22, low levels of PM2.5 exposure; n=23, high levels of PM2.5 exposure).
Cell culture
SMCM (ScienCell, CA, USA) was used to culture ASMCs (American Type Culture Collection, Manassas, VA, USA), which were pretreated with BOX5 (Merck Millipore, Burlington, MA, USA) or SP600125 (Sigma-Aldrich, Louis, USA) for 1 h, transfected with control small interfering RNA (siRNA) or Wnt5a siRNA (Santa Cruz, CA, USA) for 48 hours, and subsequently exposed to PM2.5 in medium for 24 hours.
PM2.5 preparation
PM2.5 was produced using aerodynamic impactors with a glass fiber filter, quartz filter, or Teflon membrane, depending on the specific application. Gravimetric analysis was used to assess the physicochemical characteristics of PM2.5. The mean concentrations of polynuclear aromatic hydrocarbons (PAHs) and n-alkanes in PM2.5 were 108.453 and 18,670.883 µg/g, respectively, with a final PAH recovery of 44.62%; the mean concentrations of these dimethyl sulfoxide (DMSO) extracts were 48.392 and 164.675 µg/g, respectively. The collection and analysis of PM2.5 samples were based on our previous research (5).
Animal experiments
C57BL/6 (6–8 weeks) mice were obtained from the Laboratory Animal Center of Guangzhou Medical University, which approved the animal experiments. The animal experiments were performed according to the Chinese Association for Laboratory Animal Science Policy and were approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University (No. 202093) (5). Four random groups of mice were created: 20 µL of phosphate buffer saline (PBS), 100 µg/20 µL of PM2.5, 100 µg/20 µL + 0.5 µg/mL 10 µL of PM2.5+BOX5 and 0.5 µg/mL 10 µL of BOX5 were continuously administered by a tracheal drip two times per week for 1 month. Finally, BALF, plasma, serum and lung tissue samples were collected for further experiments after the lung function test had been performed.
Lung function analysis
When the animal model was completed, 1.25% avertin (2,2,2-tribromoethanol) was used to anesthetize the mice by intraperitoneal injection. A whole-body manometer was used (Buxco-Force Pulmonary Maneuvers) after tracheotomy, and then Boyle’s law maneuvers were performed to test lung function, including the peak inspiratory flow (PIF) rate and peak expiratory flow (PEF) rate.
Histology
Based on the manufacturer’s instructions for the SABC-POD (Mouse/Rabbit IgG) Kit (SA1020, BOSTER, China), sections of small airways and lung tissue were evaluated histologically as previously described. The area of the small airways was assessed using a confocal microscope at a magnification of 200×.
Airway remodeling analysis
As described in our previous report (5), Image-Pro Plus 6.0 (IPP6.0) software was used to analyze lung tissue sections, and wall thickness in the small airway was described as the total area of the small airway wall (WAt) after standardization of the basement membrane circumference (Pbm) to determined airway remodeling as follows: WAt/Pbm (µm2/µm).
Quantitative reverse transcription polymerase chain reaction
According to the manufacturer’s instructions, total RNA was extracted from ASMCs using a Universal RNA Extraction Kit (B0004D, EZB, Shanghai, China). Reverse transcription of total RNA into complementary DNA (cDNA) was performed with the PrimeScriptTM RT Reagent Kit (RR031A, TaKaRa, Beijing, China). The housekeeping control gene was glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Table 1 shows the primer sequences.
Table 1
| Genes | Forward (5'–3') | Reverse (5'–3') |
|---|---|---|
| GAPDH | TGACCTCAACTACATGGTCTACA | CTTCCCATTCTCGGCCTTG |
| α-SMA | CTGAAGAGCATCCGACACTG | AGAGGCATAGAGGGACAGCA |
| Collagen I | GTCCTCCTGGTTCTCCTGGT | GACCGTTGAGTCCGTCTTTG |
| Collagen III | GCGAGCGGCTGAGTTTTATG | GCAGCTCAGAGTAGCACCAT |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; α-SMA, alpha smooth muscle actin.
Western blot analysis
We performed Western blotting and protein extraction in accordance with the manufacturer’s protocols. The membranes were incubated with antibodies [alpha smooth muscle actin (α-SMA), collagen I, collagen III, Wnt5a, p-JNK, NF-κB p65, p-NF-κB p65, β-actin and GAPDH, 1:1,000, Santa Cruz, CA, USA]. Chemiluminescence was used to detect the antibodies with peroxidase-conjugated anti-rabbit or anti-mouse antibodies. Results were expressed relative to the control (control, set as 1.0).
Enzyme-linked immunosorbent assay (ELISA)
In accordance with the manufacturer’s instructions, ELISA kits were used to assess the levels of Wnt5a (ELK5544, Elkbiotech, China), tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8) and IL-6 (R&D Systems, Minneapolis, MN, USA) in serum and cell supernatants. A standard curve was prepared using a standard solution, and the results were calculated.
Statistical analysis
Experimental data were analyzed using SPSS 25.0 statistical software and are presented as the means ± standard deviation (SD). Statistical evaluation of continuous data was performed by analysis of variance (ANOVA) or Student’s t-test for comparisons between the two groups. We considered statistical significance at P values <0.05.
Results
Increased Wnt5a expression in COPD patients was positively correlated with the levels of PM2.5 exposure
Wnt5a expression in the BALF of COPD patients exposed to various levels of PM2.5 was evaluated by ELISA. As shown in Figure 1, in contrast to that in the healthy control group, Wnt5a production was markedly enhanced in the BALF of COPD patients with mild or moderate disease (stage I or II) in counties with low levels of PM2.5 exposure. In districts with high levels of PM2.5 exposure, Wnt5a levels also displayed the increased trend in the BALF of stage I or II COPD patients. Moreover, Wnt5a expression was higher in the BALF of COPD patients exposed to high levels of PM2.5 than in patients exposed to low levels of PM2.5, which suggested that the increase in Wnt5a expression in COPD patients was positively correlated with the levels of PM2.5 exposure.

PM2.5 exposure promoted lung inflammation and fibrosis in mice
Based on the structural changes in alveoli, lung function and survival rates of mice, PM2.5 (100 µg/20 µL) was administered to animals according to the results of our previous animal study (5). The confirm the reliability of the model, alterations in lung histology were investigated by hematoxylin and eosin (H&E) staining, and the serum levels of IL-6, IL-8 and TNF-α were investigated by ELISA. The production of α-SMA, collagen I and collagen III was examined by Western blotting. As shown in Figure 2A,2B, compared with the control group, the PM2.5 group exhibited a marked increase in inflammatory cells and inflammatory mediators, which indicated that PM2.5 activated inflammation in mice. Moreover, in the PM2.5 group, airway wall thickness was increased (Figure 2C), and lung function tests showed that the PIF and PEF were reduced (Figure 2D), and the production of α-SMA, collagen I and collagen III was increased (Figure 2E). Furthermore, the quantitative real time polymerase chain reaction (qRT-PCR) and Western blot results showed the same trend, which indicated that PM2.5 promoted pulmonary fibrosis in mice (Figure 2F).

Wnt5a/JNK/NF-κB activation contributed to PM2.5-mediated lung inflammation and fibrosis in mice
Based on the PM2.5-induced model, to further examine the function of Wnt5a, BOX5, an antagonistic peptide derived from Wnt5a, was used to suppress the endogenous signaling of Wnt5a and clarify the mechanism by which PM2.5 triggers lung inflammation and fibrosis in mice. As shown in Figure 3A,3B, compared with that in the control group, the production of Wnt5a, p-JNK and p-NF-κB was markedly enhanced, and the ratios of p-JNK/JNK and p-NF-κB/NF-κB were enhanced in the PM2.5 group. However, in contrast to that in the PM2.5 group, Wnt5a production was markedly decreased in the BOX5 + PM2.5 group. In addition, the ratios of p-JNK/JNK and p-NF-κB/NF-κB were decreased in the BOX5 + PM2.5 group, indicating that BOX5 suppressed the activation of the JNK/NF-κB pathway caused by PM2.5.

In contrast to that in the PM2.5 group, the production of α-SMA, collagen I and collagen III was alleviated, as shown by Western blotting (Figure 3C), ELISA showed that the release of IL-6, IL-8 and TNF-α was reduced (Figure 3D), and airway wall thickness was decreased in the BOX5 + PM2.5 group, as shown by H&E staining (Figure 3E). These results indicated that the suppression of Wnt5a attenuated lung inflammation and fibrosis induced by PM2.5 in mice.
There was a positive correlation between Wnt5a and JNK/NF-κB in PM2.5-exposed ASMCs
Based on our previous results, A 24 hours exposure to PM2.5 (3 µg/mL) promoted ASMC proliferation (5), and this concentration of PM2.5 was selected for follow-up experiments. We next investigated the relationship among Wnt5a, JNK and the NF-κB signaling pathway during this process. Wnt5a siRNA was used to block Wnt5a expression. As expected, Wnt5a siRNA significantly suppressed Wnt5a expression in ASMCs treated with or without PM2.5, and control siRNA did not block PM2.5-induced Wnt5a production (Figure 4A), which demonstrated the successful blockade of Wnt5a expression. In addition, PM2.5 treatment markedly increased the ratios of p-JNK/JNK and p-NF-κB/NF-κB, while the ratios of p-JNK/JNK and p-NF-κB/NF-κB were reduced in the PM2.5 + Wnt5a siRNA group (Figure 4B). Based on our preliminary research results, we chose 100 µM BOX5 to further verify the specificity of Wnt5a siRNA. As shown in Figure 4C, BOX5 mitigated the PM2.5-induced increase in Wnt5a expression, which was consistent with the Wnt5a siRNA results. In contrast to those in the PM2.5 group, the ratios of p-JNK/JNK and p-NF-κB/NF-κB were reduced in the BOX5 + PM2.5 group (Figure 4D), which indicated that inhibiting Wnt5a expression alleviated PM2.5-induced JNK/NF-κB activation.
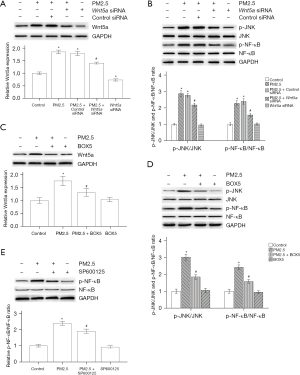
Wnt5a is the upstream regulator of the JNK/NF-κB pathway. To further investigate the interactions between JNK and NF-κB, we examined the p-NF-κB/NF-κB ratio in PM2.5-exposed ASMCs treated with the JNK inhibitor SP600125. The results showed that SP600125 significantly reduced the p-NF-κB/NF-κB ratio (Figure 4E), suggesting that the JNK inhibitor suppressed PM2.5-mediated NF-κB phosphorylation.
PM2.5 enhanced the inflammatory response and fibrosis production via the Wnt5a/JNK pathway in ASMCs
Subsequently, we assessed the function of the Wnt5a/JNK/NF-κB pathway in PM2.5-mediated inflammation and fibrosis. ASMCs were transfected with Wnt5a siRNA, BOX5 or SP600125. As shown in Figure 5A, the secretion of IL-6, IL-8 and TNF-α was upregulated in PM2.5-exposed ASMCs, similar to that in the animal model. Wnt5a siRNA inhibited the increase in IL-6, IL-8 and TNF-α in PM2.5-exposed ASMCs; however, control siRNA treatment did not decrease these levels. Moreover, the PM2.5-induced increases in IL-6, IL-8 and TNF-α were decreased in the BOX5 + PM2.5 group and the SP600125 + PM2.5 group (Figure 5B), which indicated that suppressing the Wnt5a/JNK pathway blocked the PM2.5-induced inflammatory response. Furthermore, Wnt5a siRNA abrogated PM2.5-mediated upregulation of α-SMA, collagen I and collagen III, and control siRNA treatment also did not decrease these effects (Figure 5C). BOX5 and SP600125 attenuated these effects (Figure 5D). These results indicate that suppressing the Wnt5a/JNK pathway prevents PM2.5-induced pulmonary fibrosis.

Discussion
With the advances in industrialization and urbanization, the COPD burden associated with PM2.5 exposure has increased in the past two decades (12), and the increase in COPD prevalence is accompanied by an increase in PM2.5 exposure (13). High PM2.5 exposure levels correlate with acute exacerbation hazards, as well as decreased lung function and mortality in COPD patients. In the current study, we showed that Wnt5a expression was increased in the BALF of COPD patients compared with that of individuals without COPD. This finding is consistent with the results of Baarsma et al. (14). Moreover, our study showed that BALF levels of Wnt5a in the COPD group exposed to high levels of PM2.5 was higher than that in the low-level group, which suggested that the increase in Wnt5a in the BALF of COPD patients was positively correlated with the concentration of PM2.5 exposure and the inflammatory response was more severe in the COPD group exposed to higher PM2.5 levels. Furthermore, PM2.5 exposure activated the Wnt5a/JNK/NF-κB pathway in ASMCs and mouse lung tissue. An increase in Wnt5a phosphorylated JNK and NF-κB, stimulated IL-6, IL-8 and TNF-α production, and enhanced the production of collagen I, collagen III and α-SMA in ASMCs. Therefore, targeting the Wnt5a/JNK/NF-κB pathway may be an effective strategy for treating PM2.5-associated lung inflammation and fibrosis in COPD.
The critical pathological characteristics of COPD are inflammation and airway remodeling, which are caused by the aberrant function of ASMCs to a certain extent. The accumulation of ASMCs worsened disease severity in COPD patients. ASMCs participate in the inflammatory process in COPD by synthesizing and secreting inflammatory cytokines in response to external injury, including IL-6, IL-8 and TNF-α (15). Our previous studies demonstrated that PM2.5 exposure promoted not only the release of inflammatory mediators by HBECs (6) but also the proliferation of ASMCs (5), which promoted airway remodeling. Fibronectin and the deposition of collagen I, III, and V are the chief markers of fibrosis and contribute to airway remodeling. Consistent with previous studies, the current study further showed that PM2.5 promoted the release of IL-6, IL-8 and TNF-α and upregulated the production of collagen I, collagen III and α-SMA in mice and ASMCs, which proved that ASMCs played a crucial role in PM2.5-associated lung inflammation and fibrosis.
Wnt5a preferentially activates the transcription factor JNK to stimulate cell polarity and motility (16). Increased production of Wnt5a was observed in experimental and human COPD; functionally, the inhibition of Wnt5a reduced the destruction of lung tissue and improved the function and airspace enlargement of the lung in cigarette smoke (CS)-induced COPD (14). ASMCs are one of the cellular sources of Wnt5a, which is the most highly expressed member of its family in ASMCs. Increasing evidence indicates that ASMC-derived Wnt5a exacerbates lung fibrosis, which is accompanied by poor clinical conditions. In our study, Wnt5a was upregulated in PM2.5-exposed mice and ASMCs. Combined with previous results, our study established a connection between Wnt5a and PM2.5-mediated lung inflammation and fibrosis in ASMCs.
The Wnt5a/JNK pathway may be effectively activated, promoting pulmonary fibrosis (17) and inflammation in COPD (18). JNK is a stress-activated protein kinase that is involved in fibrosis and inflammation in the lung. Once JNK is activated, its isoforms phosphorylate substrates, and transcription factors modulate fibrotic genes and inflammatory factor expression (19), and the Wnt5a/JNK/NF-κB axis plays a vital role (9). NF-κB is an important proinflammatory factor that can induce inflammation in response to external injury. In the lungs of patients with idiopathic pulmonary fibrosis (IPF), smooth muscle cells exhibit increased levels of activated JNK, which are accompanied by fibrosis. A phase 2 clinical experimental study showed that inhibiting JNK reduced pulmonary fibrosis in patients (20). As previously reported, the activation of NF-κB (21) and inflammatory factor secretion mediated by PM2.5 (22) could be blocked by a JNK inhibitor, and Wnt5a suppression abrogated the PM2.5-mediated increase in inflammatory cytokines in HBECs. We further demonstrated that PM2.5 increased the levels of phosphorylated JNK and NF-κB, resulting in inflammatory factor secretion and collagen production by ASMCs, which was contrary to the application effect of BOX5 (a specific Wnt5a antagonist) or Wnt5a siRNA. These results indicate that ASMC-derived Wnt5a is primarily involved in lung inflammation and fibrosis mediated by PM2.5 in COPD.
Additionally, since NF-κB and JNK are located downstream of Wnt5a, both factors are regulated by Wnt5a. We further verified the interactions between JNK and NF-κB. The JNK inhibitor SP600125 was used to suppress the expression of JNK. Consistent with previous studies, our study showed that SP600125 reduced PM2.5-mediated NF-κB phosphorylation and inhibited inflammation and fibrosis in PM2.5-exposed ASMCs. These data illustrate that JNK is upstream of the NF-κB pathway and has an indispensable effect on PM2.5-induced outcomes. Compared with that in the Wnt5a suppression group, the degree of NF-κB phosphorylation remained high in the presence of SP60025. Our results indicated that Wnt5a activated transcription factors downstream of JNK/NF-κB and could partly participate in crosstalk with additional pathways in PM2.5-exposed ASMCs.
However, the limitations of this study cannot be ignored. First, there were not enough cases in this study, and COPD patients (stage I or II) were not specifically differentiated. Living environments are influenced by many uncontrollable factors, such as climate and time spent outdoors. Therefore, a larger sample size may be needed to demonstrate the association of PM2.5 concentration with Wnt5a expression in COPD. Second, we should use an aerosol method, which is the normal way an individual is exposed to PM2.5. However, PM2.5 was injected into the trachea of animals in this study due to limitations in the amount of PM2.5 and laboratory conditions. We attempted to ensure that PM2.5 was uniformly injected into each trachea during the modeling process, and the results of the two models were similar. Moreover, in future experiments, we will add another COPD models to observe the effects of PM2.5 on lung function and inflammation. Third, it would be ideal to use primary human bronchial smooth muscle cells for experiments, but due to technical limitations, we used a human bronchial smooth muscle cell line, and we cannot exclude the possibility of differences.
Conclusions
In conclusion, our results prove that the increase in Wnt5a expression in COPD patients is positively correlated with the level of PM2.5 exposure. In addition, PM2.5 can increase the production of Wnt5a, which leads to the phosphorylation of JNK, increases NF-κB phosphorylation, and subsequently promotes inflammatory cytokine production and the accumulation of collagen in ASMCs. Overall, the Wnt5a/JNK pathway has an indispensable role in controlling the increase in lung inflammation and fibrosis in PM2.5-associated COPD. Suppressing the Wnt5a/JNK pathway provides new insights into potential COPD therapeutics targeting the Wnt5a/JNK pathway.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR and ARRIVE reporting checklists. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-780/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-780/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-780/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-780/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Guangzhou Chest Hospital (No. 202026). All participants were required to sign an informed consent form. The animal experiments were performed according to the Chinese Association for Laboratory Animal Science Policy and were approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University (No. 202093).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- O'Leary L, Sevinç K, Papazoglou IM, et al. Airway smooth muscle inflammation is regulated by microRNA-145 in COPD. FEBS Lett 2016;590:1324-34. [Crossref] [PubMed]
- Yan F, Gao H, Zhao H, et al. Roles of airway smooth muscle dysfunction in chronic obstructive pulmonary disease. J Transl Med 2018;16:262. [Crossref] [PubMed]
- Li N, Ma J, Ji K, et al. Association of PM2.5 and PM10 with Acute Exacerbation of Chronic Obstructive Pulmonary Disease at lag0 to lag7: A Systematic Review and Meta-Analysis. COPD 2022;19:243-54. [Crossref] [PubMed]
- Guo X, Lin Y, Lin Y, et al. PM2.5 induces pulmonary microvascular injury in COPD via METTL16-mediated m6A modification. Environ Pollut 2022;303:119115. [Crossref] [PubMed]
- Zou W, Wang X, Sun R, et al. PM2.5 Induces Airway Remodeling in Chronic Obstructive Pulmonary Diseases via the Wnt5a/β-Catenin Pathway. Int J Chron Obstruct Pulmon Dis 2021;16:3285-95. [Crossref] [PubMed]
- Zou W, Wang X, Hong W, et al. PM2.5 Induces the Expression of Inflammatory Cytokines via the Wnt5a/Ror2 Pathway in Human Bronchial Epithelial Cells. Int J Chron Obstruct Pulmon Dis 2020;15:2653-62. [Crossref] [PubMed]
- Wang Z, Zhao J, Wang T, et al. Fine-particulate matter aggravates cigarette smoke extract-induced airway inflammation via Wnt5a-ERK pathway in COPD. Int J Chron Obstruct Pulmon Dis 2019;14:979-94. [Crossref] [PubMed]
- Carmo-Fernandes A, Puschkarow M, Peters K, et al. The Pathogenic Role of Smooth Muscle Cell-Derived Wnt5a in a Murine Model of Lung Fibrosis. Pharmaceuticals (Basel) 2021;14:755. [Crossref] [PubMed]
- Yu T, Dong D, Guan J, et al. Alprostadil attenuates LPS-induced cardiomyocyte injury by inhibiting the Wnt5a/JNK/NF-κB pathway. Herz 2020;45:130-8. [Crossref] [PubMed]
- Xiong Y, Cui X, Zhou Y, et al. Dehydrocostus lactone inhibits BLM-induced pulmonary fibrosis and inflammation in mice via the JNK and p38 MAPK-mediated NF-kappaB signaling pathways. Int Immunopharmacol 2021;98:107780. [Crossref] [PubMed]
- Liu S, Zhou Y, Liu S, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax 2017;72:788-95. [Crossref] [PubMed]
- Yang X, Zhang T, Zhang Y, et al. Global burden of COPD attributable to ambient PM2.5 in 204 countries and territories, 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Sci Total Environ 2021;796:148819. [Crossref] [PubMed]
- Lee KY, Ho SC, Sun WL, et al. Lnc-IL7R alleviates PM(2.5)-mediated cellular senescence and apoptosis through EZH2 recruitment in chronic obstructive pulmonary disease. Cell Biol Toxicol 2022;38:1097-120. [Crossref] [PubMed]
- Baarsma HA, Skronska-Wasek W, Mutze K, et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J Exp Med 2017;214:143-63. [Crossref] [PubMed]
- Cheng Y, Wang N, Zhao L, et al. Knockdown of NOVA1 inhibits inflammation and migration of asthmatic airway smooth muscle cells to regulate PTEN/Akt pathway by targeting PTBP1. Mol Immunol 2021;138:31-7. [Crossref] [PubMed]
- Reggio A, Rosina M, Palma A, et al. Adipogenesis of skeletal muscle fibro/adipogenic progenitors is affected by the WNT5a/GSK3/beta-catenin axis. Cell Death Differ 2020;27:2921-41. [Crossref] [PubMed]
- Liu T, Gonzalez De Los Santos F, Hirsch M, et al. Noncanonical Wnt Signaling Promotes Myofibroblast Differentiation in Pulmonary Fibrosis. Am J Respir Cell Mol Biol 2021;65:489-99. [Crossref] [PubMed]
- Zhu Z, Yin S, Wu K, et al. Downregulation of Sfrp5 in insulin resistant rats promotes macrophage-mediated pulmonary inflammation through activation of Wnt5a/JNK1 signaling. Biochem Biophys Res Commun 2018;505:498-504. [Crossref] [PubMed]
- van der Velden JL, Alcorn JF, Chapman DG, et al. Airway epithelial specific deletion of Jun-N-terminal kinase 1 attenuates pulmonary fibrosis in two independent mouse models. PLoS One 2020;15:e0226904. [Crossref] [PubMed]
- Popmihajlov Z, Sutherland DJ, Horan GS, et al. CC-90001, a c-Jun N-terminal kinase (JNK) inhibitor, in patients with pulmonary fibrosis: design of a phase 2, randomised, placebo-controlled trial. BMJ Open Respir Res 2022;9:e001060. [Crossref] [PubMed]
- Zou W, Ye D, Liu S, et al. GSK-3beta Inhibitors Attenuate the PM2.5-Induced Inflammatory Response in Bronchial Epithelial Cells. Int J Chron Obstruct Pulmon Dis 2021;16:2845-56. [Crossref] [PubMed]
- Chen L, Yuan X, Zou L, et al. Effects of 1,25-Dihydroxyvitamin D3 on the Prevention of Chronic Obstructive Pulmonary Disease (COPD) in Rats Exposed to Air Pollutant Particles Less than 2.5 Micrometers in Diameter (PM2.5). Med Sci Monit 2018;24:356-62. [Crossref] [PubMed]


