
What is the benefit of preserving the superior segment in anatomical thoracoscopic resections of the lower lobe?
Highlight box
Key findings
• Preserved superior segment may not be beneficially effective in the preservation of postoperative computed tomography (CT)-measured lung volume and function after common basal segmentectomy compared with lower lobectomy.
What is known and what is new?
• There have been several studies on the effect of segmentectomy on lung function and volume compared with lobectomy. However, the topic remains controversial.
• Preserved superior segment may not be beneficially effective in the preservation of postoperative CT-measured lung volume and function after common basal segmentectomy compared with lower lobectomy
What is the implication, and what should change now?
• Based on the findings, preserving the superior segment to preserve lung volume and function is not supported by actual data
• Considering preservation of the superior segment, the fact that this does not appear to result in better postoperative CT-measured lung volume and function compared with lower lobectomy should be taken into account.
Introduction
Early surgical treatment of early lung cancer is increasing owing to advances in diagnostic technology (1). There has been ongoing research on sublobar resection, which is known to provide an advantage in future resection, during the past 20 years (2,3). Moreover, although several studies on the effect of segmentectomy on lung function preservation compared with lobectomy have been conducted, the topic remains controversial (2,4-6). Furthermore, there is ongoing research regarding whether lung volume after segmentectomy could be actually more preserved compared with that after lobectomy (7). Herein, we focused on the preserved superior segment (S6) after common basal segmentectomy. Although it was a common basal segmentectomy that is relatively more commonly applied among various types of segmentectomy, the preserved superior segment is too small to preserve the parenchyme. Therefore, it was not included in the segmentectomy category according to recent randomized control trials (8-10). In other words, the beneficial effect of preserved superior segment after common basal segmentectomy remains unknown (11). Changes in pre- and post-operative lung volume were measured by three-dimensional (3D) computed tomography (CT) reconstruction, and changes in lung function were assessed using the pulmonary function test (PFT). We aimed to evaluate the effect of preserved superior segment on CT-measured lung volume and function. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-791/rc).
Methods
Patients
We retrospectively examined the data of 2,920 patients who underwent lobectomy or segmentectomy at Asan Medical Center, Seoul, South Korea, between January 2004 and April 2020. Among 671 segmentectomies and 2,249 lobectomies for clinical stage IA lung cancer, 48 patients and 661 patients who underwent thoracoscopic common basal segmentectomy and thoracoscopic lower lobe lobectomy, respectively, were evaluated. After propensity score matching [1:2, matched variables: age, sex, comorbidity, smoking history, preoperative forced expiratory volume in 1 second (FEV1), clinical T stage, histology, and tumor location], 48 patients allocated to the common basal segmentectomy group (segmentectomy group) and 96 to the lower lobe lobectomy group (lobectomy group) were included in the study (Figure S1). Lung volume was assessed using a 3D CT-based volumetric method.
The primary endpoints were changes in CT-measured lung volume and lung function. The secondary endpoints were surgery-related complications.
Operative procedures
At our institution, lobectomy was the standard treatment of lung cancer. Segmentectomy was performed only in patients whose lung function and performance status were insufficient to withstand lobectomy or those with comorbidity or needed additional lung resection due to multiple lesions. The intersegmental plane was checked through the inflation-deflation line. At the surgeon’s discretion, mostly, intersegmental plane division was made by stapling. In addition, energy device was used only for a limited number of cases. Generally, systematic lymph node dissection was mainly performed at our institution rather than nodal sampling or selective lymph node dissection. After segmentectomy, if malignant cells existed at the cut margin or there was doubt that the safety margin would be sufficiently secured, lobectomy was performed. If the rotation of the preserved segment or lobe was anticipated, fixation was performed using a stapling device or suture. All operations included in the study group were performed using video-assisted thoracoscopic surgery
Lung image reconstruction
CT data were used for reconstructing 3D images using the Synapse Vincent software (Fujifilm Corp, Tokyo, Japan). The most recent CT data obtained closest to the operation day was used. Furthermore, reconstruction was performed using data from the series with the lowest slice thickness available among the CT image set. In some cases, series with a larger thickness were used for keeping input data uniform for pre- and post-surgery reconstruction.
Follow-up strategy
The postoperative follow-up strategy differed among surgeons. However, the first follow-up CT scan was performed 3 or 6 months postoperatively. Subsequently, follow-up was performed at 6-month intervals until the fifth year and subsequently conducted annually. Although CT scan was performed in almost all patients, PFT was not routinely performed. Some physicians performed PFT every one to two years in asymptomatic patients, whereas others performed PFT only when patients were symptomatic.
Definition of variables
Changes in lung function and CT-measured volume pre- and postoperatively were expressed as percentages using the following formula: [(postoperative value – preoperative value)/preoperative value] × 100 (%)
The predicted postoperative lung volume and FEV1 was calculated using the following formula: [preoperative value × (postoperative preserved functional segment/19)] (12).
Statistical analysis
The baseline characteristics of each group were analyzed using the independent t-test for continuous variables and chi-squared and Fisher’s exact test for categorical variables. Continuous variables are presented as means and standard deviations. Categorical variables are presented as frequencies and percentages. The normality of individual parameter distributions was assessed using the Shapiro-Wilk test. The propensity scores were calculated by the logistic regression models, including the following variables: age, sex, comorbidity, smoking history, preoperative FEV1, clinical T stage, histology, and tumor location. Propensity score matching revealed similarly distributed propensity scores between the segmentectomy and lobectomy groups, indicating that differences in the covariates between both groups were minimized. We matched propensity scores individually using optimal methods. Regarding comparison between the matched groups, Student’s t-test or the Wilcoxon rank-sum test was used for comparing continuous variables depending on the normality of distribution.
All statistical calculations were performed using R Statistical Software (version 4.2.2; The R Foundation for Statistical Computing, Vanderbilt University, Nashville, TN, USA) using the MatchIt package (version 4.5.0; Ho et al. 2011), ggplot2 package (version 3.4.0; Wickham 2016), and moonBook package (version 0.3.1; Moon 2015). A P value <0.05 was considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of the Asan Medical Center (No. 2022-1345), and individual consent for this retrospective analysis was waived.
Results
Operative results
Patient characteristics are presented in Table 1. The segmentectomy group exhibited a statistically significantly worse preoperative FEV1 (P=0.009), and more comorbidity (P=0.046) than in the lobectomy group. After propensity score matching, demographic data were well matched between the lobectomy and segmentectomy groups (Table 2). The overall operation results are summarized in Table 3. Both groups showed similar operative results, length of stay (7.0±3.6 versus 6.6±3.8 days, P=0.50), and postoperative complication rate (14.6% versus 8.3%, P=0.42). Specifically, persistent air leakage (>7 days) occurred in 7 patients (7.3%), and pneumonia occurred in 5 patients (5.2%) in the lobectomy group. Persistent air leakage occurred in 2 patients (4.2%), and pneumonia occurred in 1 patient (2.1%) in the segmentectomy group. There were no 30- and 90-day mortalities.
Table 1
| Characteristics | Lower lobe lobectomy (n=661) | Common basal segmentectomy (n=48) | P value |
|---|---|---|---|
| Age, years | 61.1±9.9 | 63.7±9.0 | 0.08 |
| Sex, male | 315 (47.7) | 20 (41.7) | 0.51 |
| Never smoker | 390 (59.0) | 28 (58.3) | >0.99 |
| Comorbidity | 0.046 | ||
| 0–1 | 402 (60.9) | 23 (47.9) | |
| 2 | 210 (31.8) | 16 (33.3) | |
| ≥3 | 49 (7.5) | 9 (18.8) | |
| Preoperative PFT values | |||
| FEV1, % | 91.4±14.0 | 85.9±15.6 | 0.009 |
| Cell type | 0.41 | ||
| Adenocarcinoma | 568 (85.9) | 39 (81.2) | |
| Squamous cell carcinoma | 60 (9.1) | 5 (10.4) | |
| Others | 33 (5.0) | 4 (8.4) | |
| Resected location | 0.50 | ||
| Right lower lobe | 373 (56.4) | 30 (62.5) | |
| Left lower lobe | 288 (43.6) | 18 (37.5) |
Values are presented as n (%) or mean ± standard deviation. PFT, pulmonary function test; FEV1, forced expiratory volume in 1 second.
Table 2
| Characteristics | Lower lobe lobectomy (n=96) | Common basal segmentectomy (n=48) | P value | SMD |
|---|---|---|---|---|
| Age, years | 63.9±9.2 | 63.7±9.0 | 0.89 | 0.025 |
| Sex, male | 39 (40.6) | 20 (41.7) | >0.99 | 0.021 |
| Never smoker | 58 (60.4) | 28 (58.3) | 0.95 | 0.042 |
| Comorbidity | 0.90 | 0.100 | ||
| 0–1 | 49 (51.0) | 23 (47.9) | ||
| 2 | 33 (34.4) | 16 (33.3) | ||
| ≥3 | 14 (14.6) | 9 (18.8) | ||
| Preoperative PFT values | ||||
| FEV1, % | 87.1±14.2 | 85.9±15.6 | 0.64 | 0.082 |
| Cell type | >0.99 | 0.034 | ||
| Adenocarcinoma | 77 (80.2) | 39 (81.2) | ||
| Squamous cell carcinoma | 10 (10.4) | 5 (10.4) | ||
| Others | 9 (9.4) | 4 (8.4) | ||
| Resected location | >0.99 | <0.001 | ||
| Right lower lobe | 60 (62.5) | 30 (62.5) | ||
| Left lower lobe | 36 (37.5) | 18 (37.5) |
Values are presented as n (%) or mean ± standard deviation. SMD, standardized mean difference; PFT, pulmonary function test; FEV1, forced expiratory volume in 1 second.
Table 3
| Characteristics | Lower lobe lobectomy (n=96) | Common basal segmentectomy (n=48) | P value |
|---|---|---|---|
| Length of stay, days | 7.0±3.6 | 6.6±3.8 | 0.50 |
| Complications | 14 (14.6) | 4 (8.3) | 0.42 |
| Persistent air leak (>7 days) | 7 (7.3) | 2 (4.2) | |
| Atelectasis/pneumonia | 5 (5.2) | 1 (2.1) | |
| POAF | 1 (1.0) | 1 (2.1) | |
| High chest tube output | 1 (1.0) | 0 | |
| 30-day mortality | 0 | 0 | |
| 90-day mortality | 0 | 0 |
Values are presented as n (%) or mean ± standard deviation. POAF, postoperative atrial fibrillation.
Changes in CT-measured lung volume
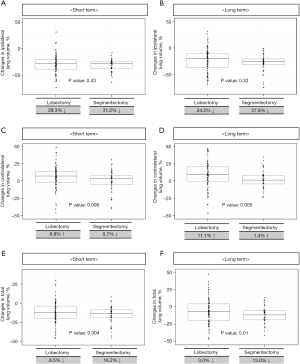
In the matched population, there were no significant differences in preoperative lung volume between the segmentectomy and lobectomy groups (4,183.8±1,114.9 versus 3,850.7±1,132.1 mL; P=0.10). Postoperative CT-measured lung volume changes are illustrated in Figure 1. At the immediate postoperative median follow-up period (6.4 months), there was no statistically significant difference in ipsilateral lung volume reduction between the lobectomy and segmentectomy groups postoperatively (P=0.43) (Figure 1A). In contrast, the contralateral lung volume increased by 8.9% in the lobectomy group but decreased by 0.7% in the segmentectomy group (P=0.006) (Figure 1C). Consequently, the total lung volume also decreased to a greater extent in the segmentectomy group compared with the lobectomy group (−16.2% versus −6.5%; P=0.004; Figures 1E,2). Similarly, the reduction in the percentage of total lung volume in the segmentectomy group remained larger than that in the lobectomy group (−13.0% versus −3.0%, P=0.01) at the last median follow-up period (43.1 months) (Figures 1F,2). When the predicted postoperative ipsilateral lung volume was assessed, the measured lung volume was smaller than the predicted lung volume at both the immediate and last follow-up periods. Moreover, the difference between the measured lung volume and expected lung volume was greater in the segmentectomy group than that in the lobectomy group (Figure S2).

Changes in lung function
In the matched population, there was no statistically significant difference in preoperative lung function between the segmentectomy and lobectomy groups (85.9%±15.6% versus 87.1%±14.2%, respectively; P=0.64) (Table 4). Similarly, there was no significant difference in lung function between both groups postoperatively (77.6%±15.5% versus 76.8%±16.0%; P=0.81). The segmentectomy and lobectomy groups exhibited similar reductions in postoperative FEV1 (−9.9% versus −11.5%; P=0.63) (Figure 3). Both groups achieved larger FEV1 values compared with the predicted postoperative FEV1. Although the difference was not statistically significant, the lobectomy group showed a larger FEV1 value than expected (Figure S3). Similarly, changes in diffusing capacity for carbon monoxide (DLCO) were not statistically significant (Table 4).
Table 4
| Characteristics | Lower lobe lobectomy (n=96) | Common basal segmentectomy (n=48) | P value |
|---|---|---|---|
| Preoperative PFT | |||
| FVC, L | 3.1±0.7 | 3.2±0.7 | 0.17 |
| FEV1% | 87.1±14.2 | 85.9±15.6 | 0.64 |
| FEV1, L | 2.2±0.5 | 2.3±0.5 | 0.87 |
| DLCO†, % | 85.8±15.1 | 80.4±16.0 | 0.07 |
| Postoperative PFT | |||
| FVC‡, L | 2.7±0.7 | 2.9±0.7 | 0.17 |
| Difference§, % | −13.7±15.4 | −12.5±13.3 | 0.73 |
| FEV1%‡ | 76.8±16.0 | 77.6±15.5 | 0.81 |
| Difference§, % | −11.5±13.7 | −9.9±12.7 | 0.63 |
| FEV1‡, L | 1.93±0.53 | 2.09±0.43 | 0.16 |
| Difference§, % | −12.7±13.6 | −12.0±12.2 | 0.84 |
| DLCO†, mL | 12.3±3.6 | 11.9±3.4 | 0.64 |
| Difference§, % | −21.2±15.17 | −19.9±13.3 | 0.77 |
Values are presented as mean ± standard deviation. †, for DLCO, 127 patients were analyzed before surgery (lobectomy group; n=86, segmentectomy group; n=41) and 44 patients after surgery (lobectomy group; n=27, segmentectomy group; n=17). ‡, for FEV1 and FVC, analysis was performed in 129 patients who underwent follow-up pulmonary function test (lobectomy group; n=95, segmentectomy group; n=34). §, [(postoperative value − preoperative value)/preoperative value] × 100%. PFT, pulmonary function test; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide.
Discussion
Since the late 2000s, sublobar resection, including segmentectomy or wedge resection, has garnered attention as lung parenchymal preserving surgery (13). Since then, comparisons with lobectomy continued and are still ongoing. Compared with lobectomy, segmentectomy was considered more likely to preserve lung volume because the amount of tissue removed was smaller. Although studies on changes in lung volume and function after pulmonary resection have continued, there is still no consensus (2,3,7,9). In this study, the preserved superior segment after common basal segmentectomy reduced CT-measured total lung volume by limiting the lung expansion of the ipsilateral and contralateral lung compared with lower lobe lobectomy. Moreover, no statistically significant benefit for pulmonary function was identified.
Sengul et al. reported that total lung volume loss was 5.57% after lower lobectomy (ipsilateral volume: −25.58%; contralateral volume: 12.03%) (14). Lung volume of the segments preserved following segmentectomy showed a significant decrease compared with its preoperative value. Furthermore, both ipsilateral unaffected lobe and contralateral lung volume increases were greater in the lobectomy group than in the segmentectomy group (7). This result is in agreement with our findings (Figure 1). In our study, more specifically, we focused only on the preserved superior segment and checked the results twice—during the short- and long-term follow-ups.
In our study, CT-measured lung volume was more reduced in the segmentectomy group than in the lobectomy group. This result was unexpected. However, the compensatory response after pulmonary resection is still not fully understood (5,14,15). Although it was a lobectomy-level study, the study by Sengul et al. suggested reasons for changes in pre- and post-operative lung volume according to the type of lobectomy. The reason that the volume changes in the ipsilateral lobe remaining after upper lobectomy was relatively small is that the diameter of the superior thorax was smaller than that of the inferior thorax. Additionally, the superior mediastinum was less active (14). In other words, the extent of expansion was affected by the characteristics of the space remaining after lung resection. Accordingly, it can be surmised that the superior segment remaining after common basal segmentectomy may interfere with the free expansion of the other ipsilateral lobe and contralateral lung. Moreover, in a previous study, physical strain following pulmonary resection, which is imposed in accordance to the extent of lung resection and acts on extending the remaining lung, was the key stimulating factor for compensatory lung response among the different factors reported (5,16). Based on these studies, we suggested that the preserved superior segment could affect lung stretching, a major factor for the compensatory expansion response. Therefore, the common basal segmentectomy group showed less compensatory lung expansion than did the lower lobectomy group, meaning that there was no benefit in terms of lung volume preservation, as shown in our study.
Bongiolatti et al. confirmed the reproducibility of the study in that there was no statistically significant difference in FEV1 between the common basal segmentectomy and the lobectomy groups (11). However, unlike our study, there was a statistically significant difference between forced vital capacity and DLCO, which we suggest it due to two differences. First, the fact that Bongiolatti et al. did not perform matching unlike our study, which may have affected the results. However, more importantly, the other reason was that there was a difference in the period of testing. In the study by Bongiolatti et al., lung function was evaluated one month after surgery. However, in our study, lung function was evaluated 6 months after surgery. Combining the results of the two studies, there might be a difference in lung function between the two groups immediately after surgery. However, this gradually recovered without differences after 6 months.
Furthermore, the changes in pre- and post-operative CT-measured lung volume and function in our study were also inconsistent. There is no clear consensus on this. However, some studies suggest the growth hypothesis theory for lung function postoperatively, unlike what was previously thought. The reason why changes in CT-measured lung volume and function showed different results could be explained by the growth hypothesis of adult lung presented in experimental evidence (17,18). Hsia reported that the compensatory expansion of the remaining lungs after lobectomy could be associated with an increase in vital lung tissue rather than as a result of hyperinflation of the remaining alveolar tissues (19). Dane et al. suggested that perfusion-related stimuli might affect lung growth (18). In other words, pulmonary function may be more related to the regeneration of lung tissue by an unknown mechanism that requires further study rather than simple volume change due to the hyperinflation of alveolar tissue (5,15). Therefore, compared with the predicted postoperative FEV1 with measured FEV1, it can be considered that the preserved superior segment also affects the regeneration of vital lung tissue. Furthermore, postoperative lung function may not be correlated because it might be affected by underlying conditions such as emphysematous disease or interstitial lung disease.
Similar to other reports, we found no statistically significant differences in early complications between the lobectomy and segmentectomy groups in our study (9,20,21).
This study has several limitations. First, we analyzed a relatively small cohort at a single institution. Moreover, this was a retrospective and non-randomized study. To overcome these limitations, we performed propensity score matching. Second, all patients had a postoperative follow-up CT, but not all underwent a PFT. Therefore, only data of 34 patients undergoing segmentectomy were included in the PFT analysis. A follow-up study is required because this could be the reason for the lack of statistical significance. Third, there was a difference between the groups regarding preoperative CT-measured lung volume. However, because this difference was not statistically significant, it did not affect the main findings of this article. Fourth, since the clinical relationship between lung volume and function is still unclear, further studies are required. Fifth, studies on pulmonary function at the segmental level have not been conducted. The 3D reconstruction program used in this study had a limitation in that it was subjective to identify an accurate segment in a patient with postoperative change. Therefore, it is necessary to develop a more accurate reconstruction technique.
Conclusions
In conclusion, a preserved superior segment may not be beneficially effective in the preservation of postoperative lung volume and function after common basal segmentectomy compared with lower lobectomy. Therefore, common basal segmentectomy should be carefully considered in patients with clinical stage IA lung cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-791/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-791/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-791/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-791/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of the Asan Medical Center (No. 2022-1345), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Muraoka Y, Yoshida Y, Nakagawa K, et al. Maximum standardized uptake value of the primary tumor does not improve candidate selection for sublobar resection. J Thorac Cardiovasc Surg 2022;163:1656-1665.e3. [Crossref] [PubMed]
- Tane S, Nishio W, Nishioka Y, et al. Evaluation of the Residual Lung Function After Thoracoscopic Segmentectomy Compared With Lobectomy. Ann Thorac Surg 2019;108:1543-50. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Kushibe K, Takahama M, Tojo T, et al. Assessment of pulmonary function after lobectomy for lung cancer--upper lobectomy might have the same effect as lung volume reduction surgery. Eur J Cardiothorac Surg 2006;29:886-90. [Crossref] [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. Compensation of pulmonary function after upper lobectomy versus lower lobectomy. J Thorac Cardiovasc Surg 2011;142:762-7. [Crossref] [PubMed]
- Yoshimoto K, Nomori H, Mori T, et al. Postoperative change in pulmonary function of the ipsilateral preserved lung after segmentectomy versus lobectomy. Eur J Cardiothorac Surg 2010;37:36-9. [Crossref] [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. Computed tomography-defined functional lung volume after segmentectomy versus lobectomy. Eur J Cardiothorac Surg 2010;37:1433-7. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Bae SY, Lee H, Na KJ, et al. Computed tomography volumetric analysis for predicting postoperative lung function for segmentectomy. Interact Cardiovasc Thorac Surg 2022;35:ivac195. [Crossref] [PubMed]
- Bongiolatti S, Salvicchi A, Mugnaini G, et al. Does thoracoscopic basal pyramid segmentectomy really offer functional advantages in comparison with thoracoscopic lower lobectomy? Interdiscip Cardiovasc Thorac Surg 2023;36:ivad018. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Sengul AT, Sahin B, Celenk C, et al. Postoperative lung volume change depending on the resected lobe. Thorac Cardiovasc Surg 2013;61:131-7. [Crossref] [PubMed]
- Ali MK, Mountain CF, Ewer MS, et al. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980;77:337-42. [Crossref] [PubMed]
- Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med 2004;170:319-43. [Crossref] [PubMed]
- Butler JP, Loring SH, Patz S, et al. Evidence for adult lung growth in humans. N Engl J Med 2012;367:244-7. [Crossref] [PubMed]
- Dane DM, Yilmaz C, Gyawali D, et al. Perfusion-related stimuli for compensatory lung growth following pneumonectomy. J Appl Physiol (1985) 2016;121:312-23. [Crossref] [PubMed]
- Hsia CC. Comparative analysis of the mechanical signals in lung development and compensatory growth. Cell Tissue Res 2017;367:687-705. [Crossref] [PubMed]
- Bédat B, Abdelnour-Berchtold E, Krueger T, et al. Clinical outcome and risk factors for complications after pulmonary segmentectomy by video-assisted thoracoscopic surgery: results of an initial experience. J Thorac Dis 2018;10:5023-9. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]



