
Adjacent angle size correlates with diagnostic sensitivity of endobronchial ultrasonography with a guide sheath
Highlight box
Key findings
• In adjacent lesions, the diagnostic yields differed significantly depending on the adjacent angle.
• The adjacent angle between lesions diagnosed using transbronchial biopsy using endobronchial ultrasonography with a guide sheath (EBUS-GS-TBB) was large.
• The diagnostic yield varied greatly depending on the adjacent angle.
What is known and what is new?
• An association has been reported between the diagnostic yields of EBUS-GS-TBB and simple within or adjacent endobronchial ultrasonography findings.
• In adjacent lesions, the adjacent angle was significantly larger in lesions diagnosed using EBUS-GS-TBB than in lesions that remained undiagnosed (P=0.003), and the diagnostic yields differed significantly depending on the adjacent angle.
What is the implication, and what should change now?
• The operator should select a branch of the bronchus or turn the bronchoscope up or down to detect a greater adjacent angle and consider additional bronchoscopic procedures for lesions with small adjacent angles.
Introduction
Transbronchial biopsy (TBB) is an essential procedure for a definitive lung cancer diagnosis. TBB using endobronchial ultrasonography (EBUS) reportedly improves the diagnostic yield of peripheral lesions than does TBB under fluoroscopic guidance (1-5). TBB using EBUS with a guide sheath (EBUS-GS-TBB) enables repeat biopsies at the same site by implanting a guide sheath (GS) in the lesion; therefore, it is widely used for inspection (6).
Factors that influence the diagnostic yields of EBUS-GS-TBB include lesion size, presence of the computed tomography (CT) bronchus sign, and EBUS findings (7-10). Among these, EBUS findings significantly contribute to diagnostic yields, which are conventionally classified into three categories according to the location of the probe as follows: within, adjacent to, and outside. According to previous reports, within provides the highest diagnostic yield (68–92.1%), followed by adjacent to (42–61%) and outside (4%) (11,12). Based on the EBUS findings, the operators select the bronchial branch to perform a biopsy and decide on the biopsy method and number of biopsies.
However, in clinical settings, the three classifications of EBUS findings are insufficient to predict whether a diagnosis is possible (11,12). Notably, EBUS findings differ from case to case. Even with the same EBUS finding within cases, the size of a lesion detected outside of the probe is quite different, and similarly, the finding of how large the angle of the lesion is detected outside of the probe is very different in each of the adjacent cases. However, few studies have examined the relationship between the details of EBUS findings and diagnostic yields.
Therefore, in this study, we aimed to investigate factors affecting the diagnostic yield of lung cancer in EBUS-GS biopsies, including more detailed EBUS findings. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-755/rc).
Methods
Study design and participants
In this single-center, retrospective, hospital-based cohort study, the following patients were consecutively enrolled: (I) patients who underwent EBUS-GS-TBB between January 2020 and April 2021 at Wakayama Medical University Hospital; (II) patients whose final diagnosis was primary lung cancer. Patients whose diagnosis of primary lung cancer could not be confirmed using EBUS-GS-TBB and who were diagnosed using other methods (e.g., CT-guided biopsy, video-assisted thoracoscopy, and surgery) were also included. The cutoff for follow-up on whether a diagnosis was made was December 31, 2022. In our clinical practice, EBUS-GS-TBB was performed regardless of the lesion size except for lesions that could be directly visualized by bronchial lumen observation. The primary endpoint was examination of factors affecting the diagnostic yield of primary lung cancer by EBUS-GS-TBB. The secondary endpoint was a subgroup analysis of factors affecting the diagnostic yield of primary lung cancer, separately for within and adjacent lesions.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board of Wakayama Medical University (approval No. 3413). Informed consent for the use of electronic patient records was obtained through an opt-out strategy owing to the retrospective nature of the study.
TBB using EBUS-GS
EBUS-GS-TBB was performed by at least two pulmonologists under sedation with pentazocine and midazolam. EBUS-GS-TBB was performed for lesions that could not be biopsied under direct visualization by observation of the bronchial lumen. An endoscopic ultrasound system (EU-ME1; Olympus, Tokyo, Japan) with a built-in 20-MHz mechanical radial-type probe (UM-S20-17S or UM-S20-20R; Olympus) was used. We used a flexible fiberoptic bronchoscope with a 2.0-mm diameter working channel (BF-Q290; Olympus) and a GS with an external diameter of 1.95 mm (K201; Olympus); or a flexible fiberoptic bronchoscope with a 3.0-mm diameter working channel (BF-1TQ290; Olympus) and a GS with an external diameter of 2.55 mm (K203; Olympus). Biopsies were performed as previously reported (11,13).
Definitions and assessments
The size of the lesion, presence or absence of the CT bronchus sign, and position of the lesion were determined using CT images with a slice of ≤5 mm; CT was performed before bronchoscopy. The lesion size was defined as the long diameter of the CT slice where the lesion could be maximally delineated. The presence of the CT bronchus sign was defined as the presence of accessible branches of the bronchus inside the lesion margin (14,15). Lesions located in the outer 2/3 and those located in the inner 1/3 of the thorax were defined as peripheral and central lesions, respectively (16,17). A positive diagnosis using EBUS-GS-TBB was defined as a pathologically malignant finding in the tissue specimen. Even in cases where additional procedures, such as EBUS-guided transbronchial needle aspiration (TBNA) and conventional forceps biopsy, were performed, the pathology of each specimen was confirmed separately, and a positive diagnosis by EBUS-GS-TBB was defined as a malignant finding in the EBUS-GS-TBB tissue specimen. Particularly, “within” was defined as when the ultrasound probe reached inside the lesion and 360° around the ultrasound probe was covered by the lesion. By contrast, “adjacent to” was defined as when the probe reached the lesion margin and was in contact with the lesion. For lesions where EBUS findings were within, we defined the shortest diameter as the shortest distance from the probe to the shadow of the tumor margin (Figure 1A). For lesions where EBUS findings were adjacent, we defined the adjacent angle as the angle formed by the midpoint of the probe and the two points where the edge of the probe and the shadow of the tumor intersected (Figure 1B). In a patient with pure ground glass nodule, we measured the adjacent angle of the blizzard range. We measured the angles in 10° increments. We used EBUS images taken prior to biopsy to determine the shortest diameter and adjacent angle.

Statistical analysis
Continuous variables are presented as medians and interquartile ranges and were analyzed using the Wilcoxon rank-sum test. Categorical variables are presented as numbers and percentages and were compared using the Chi-square or Fisher’s exact tests. If more than one factor was statistically significant in univariable analysis, multivariable analysis with logistic regression models was performed on those factors. Cut-off values of the adjacent angle for diagnosis by EBUS-GS-TBB were estimated using a receiver operating characteristic (ROC) curve. A two-sided P<0.05 was considered statistically significant. All analyses were performed using JMP Pro 16 software (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
In total, 239 lesions were assessed using EBUS-GS-TBB. Thirty-one cases with a diagnosis other than lung cancer and 29 unknown cases were excluded. The median observation period from bronchoscopy to the end of follow-up in unknown cases was 856 days (range, 659–1,058 days). In total, 179 lesions whose final diagnosis was primary lung cancer were included in the analysis. Among them, 140 lesions (78.2%) were diagnosed and 39 (21.8%) were not diagnosed using EBUS-GS-TBB (Figure 2).
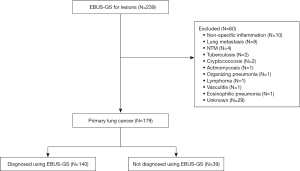
Table 1 shows the characteristics of lesions. The median patient age was 73 years, and most patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 (90%). The median lesion size was 36 mm, and 121 lesions (68%) showed the CT bronchus sign. The lesion structure was as follows: 171 lesions (96%) were solid nodules, 7 (4%) were part-solid nodules, and 1 (1%) was a pure ground glass nodule. The locations of the lesions were as follows: 82 lesions (46%) were located in the upper lobe, 18 (10%) in the middle lobe or lingula, 79 (44%) in the lower lobe, and 89 (50%) in the periphery. The median number of biopsies performed was ten. EBUS findings in this study were 119 within lesions (66%) and 60 adjacent lesions (34%).
Table 1
| Characteristics of lesions | Values (n=179) |
|---|---|
| Age (years) | 73 [22–93] |
| Sex | |
| Male | 128 [72] |
| Female | 51 [28] |
| Smoking status | |
| Never | 38 [21] |
| Former/current | 141 [79] |
| Performance status | |
| 0 | 66 [37] |
| 1 | 95 [53] |
| 2 | 15 [8] |
| 3 | 2 [1] |
| 4 | 1 [1] |
| Stage | |
| I | 38 [21] |
| II | 26 [15] |
| III | 34 [19] |
| IV | 81 [45] |
| Lesion size (mm) | 36 [10–93] |
| Lesion structure | |
| Solid nodule | 171 [96] |
| Part-solid | 7 [4] |
| Pure GGN | 1 [1] |
| CT bronchus sign | |
| Positive | 121 [68] |
| Negative | 58 [32] |
| Location of lesion | |
| Left upper lobe/right upper lobe | 82 [46] |
| Left lingula/right middle lobe | 18 [10] |
| Left lower lobe/right lower lobe | 79 [44] |
| Location of lesion | |
| Peripheral | 89 [50] |
| Central | 90 [50] |
| Numbers of biopsy | 10 [3–25] |
| EBUS findings | |
| Within | 119 [66] |
| Adjacent to | 60 [34] |
Data are presented as median [range] or n [%]. GGN, ground glass nodule; CT, computed tomography; EBUS, endobronchial ultrasonography.
Factors affecting the diagnostic yield of primary lung cancer in EBUS-GS-TBB
Table 2 shows factors associated with the diagnostic yield of TBB using EBUS-GS. The diagnostic yield for all lesions was 78.2%, whereas the yields for within and adjacent lesions were 91.6% and 51.7%, respectively. In the univariable analysis, positive CT bronchus sign lesions had a significantly higher diagnostic yield than did negative CT bronchus sign lesions [odds ratio (OR): 2.55, 95% confidence interval (CI): 1.23–5.28, P=0.011]. In addition, upper lobe lesions had a significantly higher diagnostic yield than did other lesions, and within lesions had a significantly higher diagnostic yield than did adjacent lesions (OR: 0.44, 95% CI: 0.21–0.95, P=0.031; OR: 10.20, 95% CI: 4.48–23.20, P<0.001, respectively). In the multivariable analysis, within lesions had a significantly higher diagnostic yield than did adjacent lesions and was the only factor associated with diagnostic yield (OR: 8.56, 95% CI: 3.70–19.83, P<0.001).
Table 2
| Characteristics | Positive diagnosis (N=140) | Negative diagnosis (N=39) | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Age (years) | 72.5 [22–93] | 73 [51–91] | – | 0.713 | – | – | |
| Sex | 1.43 (0.62–3.27) | 0.390 | – | – | |||
| Male | 98 [70] | 30 [77] | |||||
| Female | 42 [30] | 9 [23] | |||||
| Smoking status | 0.95 (0.39–2.27) | 0.901 | – | – | |||
| Never | 30 [21] | 8 [21] | |||||
| Former/current | 110 [79] | 31 [79] | |||||
| Performance status | 2.39 (0.52–10.86) | 0.216 | – | – | |||
| 0–1 | 124 [89] | 37 [95] | |||||
| 2–4 | 16 [11] | 2 [5] | |||||
| Stage | 1.53 (0.74–3.15) | 0.253 | – | – | |||
| I–II | 47 [34] | 17 [44] | |||||
| III–IV | 93 [66] | 22 [56] | |||||
| Lesion size (mm) | 36.5 [12–93] | 35 [10–83] | – | 0.312 | – | – | |
| CT bronchus sign | 2.55 (1.23–5.28) | 0.011 | |||||
| Positive | 86 [61] | 15 [38] | 1.90 (0.84–4.33) | 0.126 | |||
| Negative | 54 [39] | 24 [62] | Reference | – | |||
| Location of lesion | 0.44 (0.21–0.95) | 0.031 | |||||
| Left upper lobe/right upper lobe | 70 [50] | 12 [31] | 0.53 (0.14–0.81) | 0.144 | |||
| Others | 70 [50] | 27 [69] | Reference | – | |||
| Location of lesion | 1.85 (0.89–3.81) | 0.094 | – | – | |||
| Peripheral | 65 [46] | 24 [62] | |||||
| Central | 75 [54] | 15 [38] | |||||
| Numbers of biopsy | 10 [3–25] | 10 [3–20] | – | 0.240 | – | – | |
| EBUS findings | 10.20 (4.48–23.20) | <0.001 | |||||
| Within | 109 [78] | 10 [26] | 8.56 (3.70–19.83) | <0.001 | |||
| Adjacent to | 31 [22] | 29 [74] | Reference | – | |||
Data are presented as median [range] or n [%] if not otherwise specified. EBUS, endobronchial ultrasonography; GS, guide sheath; TBB, transbronchial biopsy; OR, odds ratio; CI, confidence interval; CT, computed tomography.
Analysis focusing on detailed findings of EBUS
Table 3 shows factors associated with the diagnostic yield of EBUS-GS-TBB for the 119 within lesions. No significant factors were associated with the diagnostic yield. The median shortest diameter was numerically longer in lesions diagnosed using EBUS-GS-TBB than in lesions not diagnosed; however, the difference was not significant (P=0.092).
Table 3
| Characteristics | Positive diagnosis (N=109) | Negative diagnosis (N=10) | Univariable analysis | |
|---|---|---|---|---|
| OR (95% CI) | P value | |||
| Age (years) | 72 [22–92] | 75 [66–91] | – | 0.283 |
| Lesion size (mm) | 39 [13–93] | 52.5 [30–83] | – | 0.215 |
| CT bronchus sign | 2.92 (0.78–10.99) | 0.109 | ||
| Positive | 72 [66] | 4 [40] | ||
| Negative | 37 [34] | 6 [60] | ||
| Location of lesion | 0.38 (0.09–1.53) | 0.155 | ||
| Left upper lobe/right upper lobe | 58 [53] | 3 [30] | ||
| Others | 51 [47] | 7 [70] | ||
| Location of lesion | 0.57 (0.14–2.30) | 0.413 | ||
| Peripheral | 47 [43] | 3 [30] | ||
| Central | 62 [57] | 7 [70] | ||
| Numbers of biopsy | 10 [3–25] | 6.5 [3–17] | – | 0.079 |
| Short diameter in within lesion (mm) | 5 [2–18] | 4.5 [2–8] | – | 0.092 |
Data are presented as median [range] or n [%] if not otherwise specified. OR, odds ratio; CI, confidence interval; CT, computed tomography.
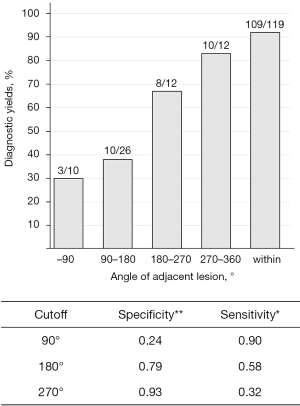
Table 4 shows the factors associated with the diagnostic yield of EBUS-GS-TBB for the 60 adjacent lesions. The adjacent angle was significantly larger in lesions diagnosed using EBUS-GS-TBB than in lesions that remained undiagnosed (P=0.003). Using the ROC curve to estimate the cut-off values of the adjacent angle for diagnosis by EBUS-GS-TBB, the most appropriate cut-off angle was 180° and the area under the curve was 0.724. The diagnostic yield of EBUS-GS-TBB was 75.0% for lesions ≥180° and 36.1% for those <180°. When grouped by every 90°, the diagnostic yields were 30% (3/10), 38% (10/26), 67% (8/12), and 83% (10/12) for <90°, ≥90° to <180°, ≥180° to <270°, and ≥270°, respectively (Figure 3).
Table 4
| Characteristics | Positive diagnosis (N=31) | Negative diagnosis (N=29) | Univariable analysis | |
|---|---|---|---|---|
| OR (95% CI) | P value | |||
| Age (years) | 73 [38–93] | 73 [51–87] | – | 0.509 |
| Lesion size (mm) | 25 [12–73] | 31 [10–60] | – | 0.242 |
| CT bronchus sign | 1.35 (0.48–3.78) | 0.570 | ||
| Positive | 14 [45] | 11 [38] | ||
| Negative | 17 [55] | 18 [62] | ||
| Location of lesion | 0.71 (0.24–2.07) | 0.533 | ||
| Left upper lobe/right upper lobe | 12 [39] | 9 [31] | ||
| Others | 19 [61] | 20 [69] | ||
| Location of lesion | 1.90 (0.64–5.60) | 0.242 | ||
| Peripheral | 18 [58] | 21 [72] | ||
| Central | 13 [42] | 8 [28] | ||
| Numbers of biopsy | 10 [5–15] | 10 [5–20] | – | 0.806 |
| Angle of adjacent lesion (°) | 180 [70–330] | 120 [30–300] | – | 0.003 |
Data are presented as median [range] or n [%] if not otherwise specified. OR, odds ratio; CI, confidence interval; CT, computed tomography.
Discussion
In this study, we investigated the factors affecting the diagnostic yield of primary lung cancer by EBUS-GS-TBB, including detailed EBUS findings. The conventional classifications of EBUS findings (within and adjacent) were useful for diagnosis, and detailed EBUS findings could be an important predictive factor of a definitive diagnosis. In particular, the adjacent angle was very different in each of the adjacent cases, having a large impact on the diagnostic yield depending on the angle.
The percentages of patients with within and adjacent lesions were comparable to those in previous reports (within lesions accounted for 57–86% and adjacent lesions accounted for 14–43%) (7,18,19). Additionally, these EBUS findings were the only independent predictive factors for positive results in the present study. Several previous reports have also shown a significant difference in the diagnostic yield between within lesions (78.7–83%) and adjacent lesions (52–61%) (8,11,12). Consequently, considering the findings from previously reported studies and our study, we confirmed that patients presenting with either finding were common, and the diagnostic yield was significantly different between within and adjacent lesions. However, as detailed EBUS findings, such as adjacent angle and the shortest diameter, vary from case to case, we believe that it would be useful to generate more detailed EBUS findings during EBUS-GS-TBB to identify patients who would benefit from additional testing to improve the diagnostic yield.
In our study, the diagnostic yield of adjacent lesions varied considerably depending on whether the adjacent angle was above or below 180°. A recent report showed that the addition of TBNA using a GS resulted in a significantly higher diagnostic yield than in the non-addition group in cases where the lesion was not confirmed by EBUS (20,21). Considering these results, further investigation on modalities, such as TBNA, using a GS may be a worthwhile pursuit to improve the diagnostic yield, especially in lesions with an adjacent angle <180°.
There was no significant difference within a short diameter between diagnosable and non-diagnosable lesions using EBUS-GS-TBB. This result may be because within lesions were easily diagnosable, making it difficult to find a significant difference. According to the ROC curve, the most appropriate cut-off for a short diameter was 9 mm. In our study, the diagnostic yield of EBUS-GS-TBB was 100% for lesions ≥9 mm and 88.2% for lesions <9 mm. From these results, we suggested that the classification of within lesions by more detailed EBUS findings is only slightly significant in clinical practice.
This study had some limitations. First, it was a retrospective study resulting in a lack of uniformity in operators, number of biopsies, and processes. No protocols for follow-up or additional diagnostic procedures for patients with initial negative EBUS-GS-TBB results were set, and further testing was left to the discretion of the individual clinician. Second, as the sample size of patients was small from a single-center cohort and not based on a particular statistical design, our data are not robust. Third, the use of a combined navigation system has recently been shown to improve the diagnostic yield of EBUS-GS biopsies; however, we did not use it (22-26).
Conclusions
This study demonstrated that the diagnostic yield of adjacent lesions varied greatly depending on the adjacent angle. Even if the EBUS findings are adjacent, the operator should select a branch of the bronchus or turn the bronchoscope up or down to detect a greater adjacent angle. Further studies should be conducted to determine whether additional biopsies or procedures should be performed according to the adjacent angle.
Acknowledgments
We thank the support staff at Wakayama Medical University.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-755/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-755/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-755/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-755/coif). D.F. has received grants and personal fees from AstraZeneca KK and Boehringer Ingelheim Japan Inc. and personal fees from Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., MSD KK, Eli Lilly Japan KK, Kyowa Kirin, and Novartis Pharma KK outside of the submitted work. S.T. has received personal fees from AstraZeneca KK, Boehringer Ingelheim Japan Inc., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan KK, Novartis Pharma KK, Ono Pharmaceutical Co. Ltd., Pfizer R&D Japan G.K., and Taiho Pharmaceutical Co. Ltd. outside of the submitted work. H.A. has received grants and personal fees from Chugai Pharmaceutical Co. Ltd., Amgen Inc., and MSD KK and personal fees from Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Amgen Inc., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb, Eli Lilly Japan KK, MSD KK, Nippon Kayaku. Co. Ltd., Novartis Pharma KK, Ono Pharmaceutical Co. Ltd., Pfizer Inc., Takeda Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd. outside of the submitted work. H.A. has participated on the data safety monitoring board or advisory board in Amgen Inc., Janssen Pharmaceutical KK, Sandoz. H.A. also holds a leadership/fiduciary role in the World Conference on Lung Cancer Patient Advocate Committee. Y.K. has received grants and personal fees from Takeda Pharmaceutical Co. Ltd., Tosoh Corporation, AstraZeneca KK, Boehringer Ingelheim Japan Inc., Amgen Inc., Chugai Pharmaceutical Co. Ltd., Zeon Corporation, and Daiichi Sankyo Co., Ltd. and consulting fees from Tosoh Corporation; Y.K. has also received personal fees from Amgen Inc., Boehringer Ingelheim Japan Inc., Guardant Health, Chugai Pharmaceutical Co. Ltd., Tosoh Corporation, and Takeda Pharmaceutical Co. Ltd. outside of the submitted work. N.Y. has received grants and personal fees from Boehringer Ingelheim Japan Inc., Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Shionogi & Co., Ltd., Eli Lilly Japan KK, Daiichi Sankyo Co., TSUMURA & CO., Nippon Kayaku. Co. Ltd., Asahikasei-pharma, AstraZeneca KK, Janssen Pharmaceutical KK, Sanofi, AMGEN, Novartis Pharma KK, Astellas, MSD KK, Esai, Bristol-Myers Squibb Co. Ltd., Abbvie, and Tosoh Corporation; N.Y. has also received personal fees from MSD KK, AstraZeneca KK, AMGEN, Ono Pharmaceutical Co. Ltd., Otsuka, Guardant Health Japan, TSUMURA & CO., Kyowa Kirin, Kyorin, GlaxoSmithKline KK, Sanofi, Daiichi Sankyo Co., Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan KK, Nippon Kayaku, Boehringer Ingelheim Japan Inc., Novartis Pharma KK, Pfizer R&D Japan G.K., Bristol-Myers Squibb Co. Ltd., Miyarisan, Merk biopharma, and Janssen Pharmaceutical KK outside of the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Wakayama Medical University (approval No. 3413). Informed consent for the use of electronic patient records was obtained through an opt-out strategy owing to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Paone G, Nicastri E, Lucantoni G, et al. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest 2005;128:3551-7. [Crossref] [PubMed]
- Sánchez-Font A, Giralt L, Vollmer I, et al. Endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions. A controlled study with fluoroscopy. Arch Bronconeumol 2014;50:166-71. [Crossref] [PubMed]
- Tanner NT, Yarmus L, Chen A, et al. Standard Bronchoscopy With Fluoroscopy vs Thin Bronchoscopy and Radial Endobronchial Ultrasound for Biopsy of Pulmonary Lesions: A Multicenter, Prospective, Randomized Trial. Chest 2018;154:1035-43. [Crossref] [PubMed]
- Roth K, Eagan TM, Andreassen AH, et al. A randomised trial of endobronchial ultrasound guided sampling in peripheral lung lesions. Lung Cancer 2011;74:219-25. [Crossref] [PubMed]
- Minami D, Takigawa N, Morichika D, et al. Endobronchial ultrasound-guided transbronchial biopsy with or without a guide sheath for diagnosis of lung cancer. Respir Investig 2015;53:93-7. [Crossref] [PubMed]
- Eom JS, Mok JH, Kim I, et al. Radial probe endobronchial ultrasound using a guide sheath for peripheral lung lesions in beginners. BMC Pulm Med 2018;18:137. [Crossref] [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]
- Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017;22:443-53. [Crossref] [PubMed]
- Ikezawa Y, Sukoh N, Shinagawa N, et al. Endobronchial ultrasonography with a guide sheath for pure or mixed ground-glass opacity lesions. Respiration 2014;88:137-43. [Crossref] [PubMed]
- Minezawa T, Okamura T, Yatsuya H, et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging 2015;15:21. [Crossref] [PubMed]
- Zhang L, Wu H, Wang G. Endobronchial ultrasonography using a guide sheath technique for diagnosis of peripheral pulmonary lesions. Endosc Ultrasound 2017;6:292-9. [Crossref] [PubMed]
- Yamada N, Yamazaki K, Kurimoto N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007;132:603-8. [Crossref] [PubMed]
- Souma T, Minezawa T, Yatsuya H, et al. Risk Factors of Infectious Complications After Endobronchial Ultrasound-Guided Transbronchial Biopsy. Chest 2020;158:797-807. [Crossref] [PubMed]
- Naidich DP, Sussman R, Kutcher WL, et al. Solitary pulmonary nodules. CT-bronchoscopic correlation. Chest 1988;93:595-8. [Crossref] [PubMed]
- Gaeta M, Pandolfo I, Volta S, et al. Bronchus sign on CT in peripheral carcinoma of the lung: value in predicting results of transbronchial biopsy. AJR Am J Roentgenol 1991;157:1181-5. [Crossref] [PubMed]
- Sakurai H, Asamura H, Watanabe S, et al. Clinicopathologic features of peripheral squamous cell carcinoma of the lung. Ann Thorac Surg 2004;78:222-7. [Crossref] [PubMed]
- Bandoh S, Fujita J, Ueda Y, et al. Expression of carcinoembryonic antigen in peripheral- or central-located small cell lung cancer: its clinical significance. Jpn J Clin Oncol 2001;31:305-10. [Crossref] [PubMed]
- Chavez C, Sasada S, Izumo T, et al. Endobronchial ultrasound with a guide sheath for small malignant pulmonary nodules: a retrospective comparison between central and peripheral locations. J Thorac Dis 2015;7:596-602. [Crossref] [PubMed]
- Okachi S, Imai N, Imaizumi K, et al. Factors Affecting the Diagnostic Yield of Transbronchial Biopsy Using Endobronchial Ultrasonography with a Guide Sheath in Peripheral Lung Cancer. Intern Med 2016;55:1705-12. [Crossref] [PubMed]
- Hayama M, Izumo T, Chavez C, et al. Additional transbronchial needle aspiration through a guide sheath for peripheral pulmonary lesions that cannot be detected by radial EBUS. Clin Respir J 2017;11:757-64. [Crossref] [PubMed]
- Arimura K, Sekine Y, Hiroshima K, et al. The efficacy of transbronchial needle aspiration with endobronchial ultrasonography using a guide sheath for peripheral pulmonary lesions suspected to be lung cancer. Respir Investig 2017;55:365-71. [Crossref] [PubMed]
- Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014;88:430-40. [Crossref] [PubMed]
- Khandhar SJ, Bowling MR, Flandes J, et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med 2017;17:59. [Crossref] [PubMed]
- Ishiwata T, Gregor A, Inage T, et al. Advances in interventional diagnostic bronchoscopy for peripheral pulmonary lesions. Expert Rev Respir Med 2019;13:885-97. [Crossref] [PubMed]
- Matsumoto Y, Izumo T, Sasada S, et al. Diagnostic utility of endobronchial ultrasound with a guide sheath under the computed tomography workstation (ziostation) for small peripheral pulmonary lesions. Clin Respir J 2017;11:185-92. [Crossref] [PubMed]
- Ikezawa Y, Shinagawa N, Sukoh N, et al. Usefulness of Endobronchial Ultrasonography With a Guide Sheath and Virtual Bronchoscopic Navigation for Ground-Glass Opacity Lesions. Ann Thorac Surg 2017;103:470-5. [Crossref] [PubMed]


