
Clinical implications of wheezing in patients with chronic obstructive pulmonary disease
Highlight box
Key findings
• The presence of wheezing in chronic obstructive pulmonary disease (COPD) patients is associated with more severe symptoms, lower lung function, and a higher risk of exacerbation, irrespective of whether it is an asthma-COPD overlap (ACO) or non-ACO phenotype.
What is known and what is new?
• Wheezing, potentially indicating airway hyper-responsiveness, was considered as a trait associated with the ACO phenotype.
• Wheezing is an independent predictor of the risk of exacerbation in patients with COPD irrespective of both the ACO phenotype and the severity of airflow limitation, and the risk of exacerbation was higher in COPD patients with more frequent wheezing.
What is the implication, and what should change now?
• In COPD patients, wheezing may serve as a valuable marker for predicting the development of exacerbations, rather than being indicative of an ACO phenotype. A proactive pharmacological treatment should be considered in COPD patients with wheezing.
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition characterized by chronic respiratory symptoms and persistent airflow limitation due to abnormalities of the airways and/or alveoli (1). There are several COPD phenotypes, including chronic bronchitis, emphysema, asthma-COPD overlap (ACO), and frequent exacerbator, and these phenotypes exhibit different prognosis, such as exacerbations, response to treatment, and disease progression (2,3). Since COPD causes significant morbidity and mortality (4,5), phenotype assessment is important to predict prognosis and guide appropriate management.
Wheezing may arise from any mechanisms that causes the narrowing of the airway caliber, and asthma and COPD are common clinical conditions exhibiting wheezing (6). Some previous studies used the presence of wheezing as a characteristic of ACO in COPD patients (7,8), because wheezing might reflect airway hyper-responsiveness (9). Wheezing is present not only in patients with ACO but also in those without ACO among individuals with COPD. However, studies on the prevalence of wheezing in the ACO and non-ACO groups, as well as the clinical characteristics of wheezing patients in each group, are rare. A previous study conducted in Taiwan investigated the prevalence and clinical characteristics of COPD patients with wheezing (10). They reported that 38% of COPD patients had a wheezing phenotype, and the wheezing group was associated with worse symptoms, diminished pulmonary function, and more exacerbations. However, the wheezing phenotype of COPD was not evaluated in terms of the association with ACO as a risk factor for worse outcomes.
In this study, we investigated the prevalence of self-reported wheezing in ACO and non-ACO patients, respectively. Furthermore, we aimed to identify clinical characteristics such as the severity of symptoms, lung function, and frequency of exacerbation in patients with wheezing compared to those without wheezing in the ACO and non-ACO groups. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1031/rc).
Methods
Study population
The Korean COPD Subgroup Study (KOCOSS) cohort is an ongoing, prospective, and multicenter cohort study that enrolled COPD patients from 54 medical centers in South Korea. The inclusion criteria were South Korean patients aged ≥40 years and with post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70% of the normal predicted value. Enrolled patients underwent follow-up evaluations every six months after the initial assessment. All information was collected using the case report form that a doctor or trained nurse filled out. To assess the clinical characteristics of ACO and non-ACO patients with wheezing, we extracted the data from the KOCOSS cohort, which was registered between January 2012 and December 2018. This study excluded patients with a smoking history of less than 10 pack-years, and with missing values in eosinophil count, history of wheezing, and 1-year follow up exacerbation history. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of each participating hospital, and all patients provided written informed consent. The names of the approving ethics committees are mentioned in Appendix 1. The Institutional Review Board of the Kyungpook National University Hospital approved the study protocol (IRB No. 2012-01-001).
Clinical data
We obtained the baseline characteristics, including age, sex, smoking history, exposure to biomass fuel (firewood or briquette), and body mass index (BMI). Moreover, we collected the modified Medical Research Council (mMRC) dyspnea score (11), COPD Assessment Test (CAT) score (12), COPD-specific version of St. George’s Respiratory Questionnaire (SGRQ-C) score (13), and 6-minute walk distance test score. In addition, a history of exacerbations in the previous year was obtained. Exacerbation was defined as the acute worsening of respiratory symptoms that requires additional therapy (14). Moderate exacerbation was defined as an exacerbation that necessitated the outpatient administration of antibiotics or systemic corticosteroids. Severe exacerbation was defined as an exacerbation that required hospitalization or an emergency room visit. In addition, results of pulmonary function tests and blood eosinophil count were collected. Inhalers used in baseline were classified as follows: long-acting beta2-agonist (LABA), long-acting muscarinic antagonist (LAMA), LABA/LAMA, inhaled corticosteroid (ICS)/LABA, and LABA/LAMA/ICS (triple therapy). Information about comorbidities and chest radiography findings were also obtained. According to the radiologist’s interpretations, the chest radiography determined the following results: tuberculosis destroyed lung, old tuberculosis, emphysema, and bronchiectasis. In addition, the development of moderate and severe exacerbations during a 1-year follow-up was analyzed.
Definition of wheezing and ACO
In this study, the presence of wheezing was determined through the patient’s answer to the SGRQ-C. For the question “I have attacks of wheezing”, patients who answered “most days a week”, “several days a week”, “a few days a month”, and “only with chest infections” were classified as the wheezing group. Those who answered “not at all” were classified as the non-wheezing group. ACO was defined according to the updated Spanish criteria (15). This study only included COPD patients aged ≥40 years with a post-bronchodilator FEV1/FVC <0.7 and a smoking history of at least 10 pack-years. ACO was determined in case of very positive results on a bronchodilator test (FEV1 ≥15% and ≥400 mL) and/or eosinophil in blood ≥300 cells/µL. We classified patients into four groups according to whether they were ACO patients or had wheezing: ACO with wheezing, ACO without wheezing, non-ACO with wheezing, and non-ACO without wheezing.
Statistical analysis
We used SPSS version 25.0 for Windows (IBM Corporation, Armonk, NY, USA) for statistical analysis. For categorical variables, we used the chi-squared or Fisher’s exact test to compare the groups, and data were shown as numbers with percentages. For continuous variables, a one-way analysis of variance was performed to compare the groups, and data were presented as mean with standard deviation. P values <0.05 were regarded as statistically significant. In addition, multiple logistic regression analyses were conducted to evaluate the association between the presence of wheezing and the risk of COPD exacerbation, and Hosmer-Lemeshow test was performed to evaluate goodness-of-fit for logistic regression models.
Results
Study subjects
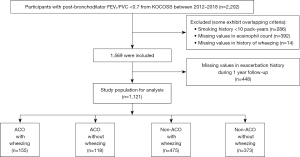
A total of 2,202 patients with spirometry-defined COPD were enrolled in the KOCOSS cohort from January 2012 to December 2018 (Figure 1). Patients with a smoking history <10 pack-years (n=286) and those with missing data for eosinophil count (n=392) or presence of wheezing (n=14) were excluded. After then, 448 patients were excluded due to missing value in exacerbation history during 1 year follow-up, and 1,121 patients were included in the study for analysis. Of the total enrolled patients, 273 (24.4%) were ACO, and 630 (56.2%) had wheezing: wheezing was present in 155 (56.8%) out of 273 ACO patients and in 475 (56.0%) out of 848 non-ACO patients. The numbers of the four groups according to whether the patients had ACO or wheezing were as follows: ACO with wheezing [155 (13.8%)], ACO without wheezing [118 (10.5%)], non-ACO with wheezing [475 (42.4%)], and non-ACO without wheezing [373 (33.3%)]. Figure 2 shows the distribution of patients according to the frequency of wheezing in ACO and non-ACO groups. There was no difference in the distribution of patients according to the frequency of wheezing between the two groups.

Baseline characteristics
Age, sex, smoking history, and biomass exposure did not differ among the four groups (Table 1). CAT score and mMRC grade were higher in patients with wheezing than those without wheezing in both ACO and non-ACO groups. There was no difference in blood eosinophil count between patients with wheezing and those without wheezing in both ACO and non-ACO groups. Among the four groups, the proportion of patients who experienced moderate to severe exacerbation in the previous year was the highest in ACO patients with wheezing, followed by non-ACO patients with wheezing. In terms of pulmonary function test parameters, post-bronchodilator FEV1 and FEV1/FVC were significantly lower in patients with wheezing than those without wheezing both in ACO and non-ACO patients. When compared to patients with wheezing in both ACO and non-ACO groups, the use of the ICS containing regimen was less common in non-ACO patients without wheezing. An ICS-containing regimen was more commonly utilized in the group that had experienced moderate to severe exacerbations in the previous year compared to the group that had not (54% vs. 32%, P<0.001). Additionally, there was no significant difference in comorbidities between the four groups. Regarding chest radiographic findings, emphysema was most commonly detected in ACO patients with wheezing, followed by non-ACO patients with wheezing (Table 2).
Table 1
| Characteristics | Total (n=1,121) | ACO (n=273) | Non-ACO (n=848) | P value | |||
|---|---|---|---|---|---|---|---|
| With wheezing (n=155) | Without wheezing (n=118) | With wheezing (n=475) | Without wheezing (n=373) | ||||
| Age, years | 68.9±7.6 | 68.3±7.8 | 68.8±7.2 | 68.6±7.8 | 69.4±7.3 | 0.324 | |
| Male sex | 1,092 (97.4) | 153 (98.7) | 118 (100.0) | 462 (97.3) | 359 (96.2) | 0.094 | |
| Smoking | |||||||
| Current smoker | 331 (29.5) | 53 (34.2) | 35 (29.7) | 146 (30.7) | 97 (26.0) | 0.243 | |
| Pack-years | 45.1±23.3 | 46.8±24.3 | 47.4±25.1 | 46.7±23.8 | 43.1±21.7 | 0.178 | |
| Biomass exposure | 837 (74.7) | 116 (74.8) | 84 (71.2) | 364 (76.6) | 273 (73.2) | 0.616 | |
| Body mass index, kg/m2 | 22.9±3.4 | 23.0±3.2 | 22.8±3.6 | 23.1±3.4 | 22.7±3.3 | 0.468 | |
| CAT score | 14.8±7.9 | 17.3±7.4 | 11.8±7.2 | 17.0±7.9 | 11.9±7.0 | <0.001 | |
| CAT ≥10 | 804 (71.7) | 136 (87.7) | 69 (58.5) | 390 (82.1) | 209 (56.0) | <0.001 | |
| mMRC grade | 1.3±0.9 | 1.5±0.9 | 1.2±0.9 | 1.5±0.9 | 1.1±0.8 | <0.001 | |
| ≥2 | 401 (35.8) | 63 (40.6) | 31 (26.3) | 211 (44.4) | 96 (25.7) | <0.001 | |
| SGRQ-C | |||||||
| Symptom | 43.1±21.0 | 56.1±19.4 | 29.6±14.6 | 52.6±19.0 | 29.8±14.7 | <0.001 | |
| Activity | 42.9±27.7 | 51.9±28.9 | 31.1±26.7 | 50.3±27.4 | 33.0±23.5 | <0.001 | |
| Impact | 24.2±22.5 | 33.2±23.8 | 16.7±17.4 | 30.5±23.8 | 15.0±16.9 | <0.001 | |
| Total | 33.3±21.3 | 42.9±21.9 | 24.2±17.5 | 40.6±21.5 | 23.1±15.8 | <0.001 | |
| 6MWD, m | 378.1±116.5 | 370.2±118.1 | 383.0±114.6 | 370.0±109.2 | 391.3±125.4 | 0.105 | |
| Blood eosinophil count (cells/μL) | 236.2±270.8 | 534.7±393.9 | 533.8±426.1 | 140.5±74.1 | 139.8±78.7 | <0.001 | |
| Eosinophil ≥300 cells/µL | 259 (23.1) | 147 (94.8) | 112 (94.9) | 0 | 0 | <0.001 | |
| 100≤ eosinophil <300 cells/µL | 566 (50.5) | 7 (4.5) | 5 (4.2) | 318 (66.9) | 236 (63.3) | <0.001 | |
| Eosinophil <100 cells/µL | 296 (26.4) | 1 (0.6) | 1 (0.8) | 157 (33.1) | 137 (36.7) | <0.001 | |
| Moderate to severe exacerbation in the previous year | 221 (19.7) | 39 (25.2) | 17 (14.4) | 108 (22.7) | 57 (15.3) | 0.009 | |
| Pulmonary function test | |||||||
| postBD FVC (L) | 3.4±0.8 | 3.3±0.8 | 3.5±0.7 | 3.3±0.8 | 3.4±0.8 | 0.019 | |
| postBD FVC (% predicted) | 81.3±16.2 | 78.2±16.8 | 82.3±15.7 | 80.6±16.2 | 83.1±16.0 | 0.008 | |
| postBD FEV1 (L) | 1.7±0.6 | 1.6±0.6 | 1.8±0.5 | 1.6±0.6 | 1.8±0.6 | <0.001 | |
| postBD FEV1 (% predicted) | 58.2±18.2 | 54.2±18.6 | 61.4±18.0 | 56.1±17.9 | 61.6±17.7 | <0.001 | |
| ≥80% pred | 137 (12.2) | 16 (10.3) | 18 (15.3) | 45 (9.5) | 58 (15.5) | 0.033 | |
| 50% pred ≤ FEV1 <80% pred | 618 (55.1) | 73 (47.1) | 68 (57.6) | 257 (54.1) | 220 (59.0) | 0.079 | |
| 30% pred ≤ FEV1 <50% pred | 308 (27.5) | 53 (34.2) | 29 (24.6) | 143 (30.1) | 83 (22.3) | 0.013 | |
| <30% pred | 58 (5.2) | 13 (8.4) | 3 (2.5) | 30 (6.3) | 12 (3.2) | 0.028 | |
| postBD FEV1/FVC (%) | 50.5±11.7 | 48.9±11.6 | 52.8±11.6 | 49.1±11.8 | 52.3±11.4 | <0.001 | |
| DLCO (% predicted) | 63.0±20.4 | 63.8±22.3 | 63.6±19.1 | 62.3±20.5 | 63.5±19.9 | 0.798 | |
| Baseline inhaler use | |||||||
| LABA | 122 (10.9) | 18 (11.6) | 13 (11.0) | 51 (10.7) | 40 (10.7) | 0.991 | |
| LAMA | 302 (26.9) | 48 (31.0) | 29 (24.6) | 124 (26.1) | 101 (27.1) | 0.618 | |
| LABA + LAMA | 171 (15.3) | 13 (8.4) | 23 (19.5) | 64 (13.5) | 71 (19.0) | 0.006 | |
| ICS + LABA | 132 (11.8) | 20 (12.9) | 16 (13.6) | 63 (13.3) | 33 (8.8) | 0.200 | |
| ICS + LABA + LAMA | 267 (23.8) | 42 (27.1) | 21 (17.8) | 134 (28.2) | 70 (18.8) | 0.004 | |
| ICS containing treatment | 409 (36.5) | 63 (40.6) | 40 (33.9) | 201 (42.3) | 105 (28.2) | <0.001 | |
| Comorbidities | |||||||
| Myocardial infarction | 45 (4.0) | 5 (3.2) | 5 (4.2) | 22 (4.6) | 13 (3.5) | 0.800 | |
| Heart failure | 38 (3.4) | 7 (4.5) | 4 (3.4) | 16 (3.4) | 11 (2.9) | 0.844 | |
| Diabetes mellitus | 220 (19.6) | 27 (17.4) | 22 (18.6) | 97 (20.4) | 74 (19.8) | 0.861 | |
| Hypertension | 410 (36.6) | 54 (34.8) | 47 (39.8) | 189 (39.8) | 120 (32.2) | 0.113 | |
| Previous pulmonary tuberculosis | 261 (23.3) | 40 (25.8) | 23 (19.5) | 108 (22.7) | 90 (24.1) | 0.629 | |
| Allergic rhinitis | 90 (8.0) | 15 (9.7) | 10 (8.5) | 45 (9.5) | 20 (5.4) | 0.127 | |
Data are presented as mean ± standard deviation or n (%). COPD, chronic obstructive pulmonary disease; ACO, asthma-COPD overlap; CAT, COPD assessment test; mMRC, modified Medical Research Council; SGRQ-C, COPD-specific version of St. George’s Respiratory Questionnaire; 6MWD, 6-minute walk distance; postBD, post bronchodilator; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid.
Table 2
| Variables | Total (n=886) | ACO (n=219) | Non-ACO (n=667) | P value | |||
|---|---|---|---|---|---|---|---|
| With wheezing (n=120) | Without wheezing (n=99) | With wheezing (n=374) | Without wheezing (n=293) | ||||
| Tuberculosis destroyed lung | 42 (4.7) | 3 (2.5) | 5 (5.1) | 16 (4.3) | 18 (6.1) | 0.422 | |
| Old tuberculosis | 109 (12.3) | 17 (14.2) | 14 (14.1) | 46 (12.3) | 32 (10.9) | 0.749 | |
| Emphysema | 304 (34.3) | 48 (40.0) | 35 (35.4) | 139 (37.2) | 82 (28.0) | 0.040 | |
| Bronchiectasis | 56 (6.3) | 10 (8.3) | 9 (9.1) | 20 (5.3) | 17 (5.8) | 0.418 | |
Data are presented as n (%). COPD, chronic obstructive pulmonary disease; ACO, asthma-COPD overlap.
Table S1 shows a comparison of baseline characteristics when enrolled patients were divided into the wheezing and non-wheezing group. Individuals in the wheezing group showed elevated CAT score, mMRC grade, and SGRQ-C score, as well as reduced post-bronchodilator FVC, FEV1, and FEV1/FVC than the non-wheezing group. The blood eosinophil count showed no statistically significant difference between the groups with and without wheezing. The use of the ICS containing regimen and the presence of emphysema in chest radiography were more common in the wheezing group compared with the non-wheezing group.
Wheezing and risk of exacerbations during 1-year follow-up
During the 1-year follow-up, moderate to severe exacerbation occurred more frequently in patients with wheezing than those without wheezing in both ACO and non-ACO groups (Table 3), and proportion of patients who experienced moderate to severe exacerbation was the highest in the ACO patients with wheezing. Additionally, the non-ACO patients with wheezing experienced moderate to severe exacerbation more frequently than the ACO patients without wheezing.
Table 3
| Variables | Total (n=1,121) | ACO (n=273) | Non-ACO (n=848) | P value | |||
|---|---|---|---|---|---|---|---|
| With wheezing (n=155) | Without wheezing (n=118) | With wheezing (n=475) | Without wheezing (n=373) | ||||
| Moderate to severe exacerbation | 467 (41.7) | 92 (59.4) | 38 (32.2) | 226 (47.6) | 111 (29.8) | <0.001 | |
| Severe exacerbation | 127 (11.3) | 25 (16.1) | 10 (8.5) | 69 (14.5) | 23 (6.2) | <0.001 | |
| Frequency of moderate exacerbation | 0.94±1.80 | 1.61±2.22 | 0.62±1.22 | 1.11±2.05 | 0.55±1.22 | <0.001 | |
| Frequency of severe exacerbation | 0.19±0.71 | 0.30±0.97 | 0.22±1.11 | 0.23±0.63 | 0.09±0.45 | <0.001 | |
Data are presented as mean ± standard deviation or n (%). COPD, chronic obstructive pulmonary disease; ACO, asthma-COPD overlap.
We performed multiple logistic regression analyses to evaluate the association between presence of wheezing and the risk of exacerbations during 1-year follow-up in COPD patients (Table 4). In model 1, we adjusted age, sex, smoking pack-year, and post-bronchodilator FEV1 (% predicted). In model 2, we further adjusted ACO and exacerbation history during the previous year in addition to model 1. COPD patients with wheezing were associated with a higher risk of moderate to severe exacerbation [adjusted odds ratio (OR) 2.074, 95% confidence interval (CI): 1.592–2.702 in model 1; adjusted OR 2.005, 95% CI: 1.529–2.630 in model 2]. In addition, the risk of severe exacerbation was also higher in COPD patients with wheezing (adjusted OR 1.950, 95% CI: 1.260–3.017 in model 1; adjusted OR 1.804, 95% CI: 1.160–2.806 in model 2).
Table 4
| Variables | Moderate to severe exacerbation | Severe exacerbation | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Wheezing | |||||||
| Crude | 2.339 | 1.826–2.997 | <0.001 | 2.434 | 1.606–3.689 | <0.001 | |
| Model 1 | 2.074 | 1.592–2.702 | <0.001 | 1.950 | 1.260–3.017 | 0.003 | |
| Model 2 | 2.005 | 1.529–2.630 | <0.001 | 1.804 | 1.160–2.806 | 0.009 | |
| Frequency of wheezing | |||||||
| Model 1 | |||||||
| No wheezing | 1 (ref.) | 1 (ref.) | |||||
| A | 2.595 | 1.583–4.253 | <0.001 | 2.657 | 1.363–5.179 | 0.004 | |
| B | 2.236 | 1.498–3.337 | <0.001 | 1.970 | 1.093–3.553 | 0.024 | |
| C | 1.818 | 1.198–2.759 | 0.005 | 1.747 | 0.915–3.333 | 0.091 | |
| D | 2.014 | 1.443–2.810 | <0.001 | 1.868 | 1.100–3.173 | 0.021 | |
| Model 2 | |||||||
| No wheezing | 1 (ref.) | 1 (ref.) | |||||
| A | 2.350 | 1.418–3.895 | 0.001 | 2.338 | 1.191–4.588 | 0.014 | |
| B | 2.169 | 1.440–3.266 | <0.001 | 1.827 | 1.009–3.308 | 0.047 | |
| C | 1.709 | 1.116–2.616 | 0.014 | 1.562 | 0.807–3.022 | 0.186 | |
| D | 1.974 | 1.403–2.777 | <0.001 | 1.728 | 1.011–2.953 | 0.045 | |
Model 1: adjusted for age, sex, smoking pack-year, and post bronchodilator FEV1 (% predicted). Model 2: adjusted for age, sex, smoking pack-year, post bronchodilator FEV1 (% predicted), ACO, and exacerbation history during the previous year. According to the answer to “I have attacks of wheezing”, patients were classified as follows: A, most days a week; B, several days a week; C, a few days a month; D, only with chest infections. Patients without wheezing were the reference group. COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval; FEV1, forced expiratory volume in 1 second; ACO, asthma-COPD overlap.
Additionally, we evaluated the risk of exacerbation according to the frequency of wheezing. Regardless of the frequency of wheezing, individuals with wheezing had a greater risk of moderate to severe exacerbation compared to those without wheezing. Patients who experienced wheezing on most days of the week had the highest OR for moderate to severe and severe exacerbations, while those who had wheezing on several days of the week had the second highest OR.
Discussion
In the present study, the proportion of patients with self-reported wheezing in both the ACO and non-ACO groups was similar, about 56%. In both ACO and non-ACO groups, patients with wheezing had more severe symptoms, lower lung function, and a higher risk of exacerbation compared to those without wheezing. Association between blood eosinophil count and wheezing was not observed in both the ACO and non-ACO groups. During the 1-year follow-up, exacerbations were most frequent in ACO patients with wheezing among the four groups, followed by non-ACO patients with wheezing. In addition, self-reported wheezing was an independent predictor of the risk of exacerbation in patients with COPD irrespective of both the ACO phenotype and the severity of airflow limitation, and the risk of exacerbation was higher in COPD patients with more frequent wheezing.
In previous studies, the prevalence of wheezing in patients with COPD ranged from 18% to 60% (7,10,16,17). The variation in the degree of airflow limitation among the included patients may contribute to the variable prevalence observed across studies. Prevalence of wheezing was higher in studies including COPD patients who had more severe airflow limitations. Furthermore, the disparity in the prevalence of wheezing could be attributed to the distinct approaches, such as chart review and questionnaire, employed in each study to identify wheezing. Our study comprised COPD patients with mild to very severe airflow limitation, and presence of wheezing was determined using a questionnaire; the prevalence of wheezing was approximately 56%.
This study demonstrated that presence of wheezing in patients with COPD was associated with more severe symptoms, poorer lung function, and a higher risk of exacerbation in both ACO and non-ACO patients. A previous study using data from the Korean national survey investigated the heterogeneity of ACO (7). They included subjects who were ≥40 years and had prebronchodilator FEV1/FVC <0.7 and FEV1 ≥50% of predicted value in the study and divided four groups based on their smoking history and the presence of wheezing. They reported that COPD patients with wheezing had lower lung function, poorer quality of life, and higher hospitalized rates compared with those without wheezing, regardless of smoking history. These results are consistent with our findings, as our study revealed that patients with wheezing had lower lung function in both the ACO and non-ACO groups. This implies that the presence of wheezing is not indicative of an ACO phenotype, but rather signifies more prominent airflow obstruction. This correlation could potentially be associated with heightened symptoms and an increased risk of exacerbations. For individuals facing challenges in performing lung function tests, the presence of wheezing could potentially signal a higher degree of airflow obstruction.
Several previous studies reported that ACO patients exhibited more severe symptoms, worse lung function, and increased exacerbation risk than non-ACO patients (18-20). However, in this study, there was no difference in the severity of symptoms and lung function between the ACO and non-ACO patients in both wheezing and non-wheezing groups, respectively. Various diagnostic criteria exist for ACO, and a prior study suggests that the prevalence and clinical characteristics of ACO vary depending on the criteria used for diagnosis (21). Therefore, it is thought that the discrepancy between the results of previous studies and ours might be related to different diagnostic criteria of ACO. During the 1-year follow-up, the proportion of patients who experienced moderate to severe exacerbation was the highest in the ACO patients with wheezing, followed by non-ACO with wheezing, ACO without wheezing, and non-ACO without wheezing. This result was partially consistent with previous studies which demonstrated that ACO patients had a greater propensity for experiencing exacerbations than non-ACO patients. Furthermore, this study indicated that COPD patients with wheezing had a higher risk of exacerbation irrespective of the presence of ACO.
A previous study examining risk factors for inconsistencies between the risk of exacerbation and the severity of airflow limitation in COPD patients found that the presence of wheezing was an independent risk factor for a high risk of exacerbation in those with mild airflow limitation (22). This result suggests that the presence of wheezing is associated with an increased risk of exacerbation, even in COPD patients with less severe airflow limitation, and is consistent with our findings. In our study, the presence of wheezing was independently associated with exacerbation even after adjusting confounding variables such as age, sex, smoking amount, FEV1, ACO, and exacerbation history during the previous year. Moreover, COPD patients who experienced wheezing more frequently had an increased risk of exacerbation. This implies that wheezing is a significant trait that can predict exacerbation in COPD patients. Therefore, a proactive pharmacological treatment strategy should be considered to prevent exacerbation in COPD patients with wheezing.
Previous studies examining the findings of chest computed tomography (CT) in patients with COPD reported that the presence of wheezing was associated with airway wall thickening (23,24). However, studies investigating the relationship between the presence of wheezing and radiological emphysema are limited. Although chest radiography has limitations in determining the existence of emphysema, our results showed more frequent emphysema in patients with wheezing compared to those without wheezing, which might be related to the loss of elastic recoil due to the reduction of elastic tissue in emphysema. Further studies using chest CT will be required to confirm the relationship between emphysema and the presence of wheezing. Moreover, our study revealed that the presence of wheezing did not correlate with eosinophil count, regardless of patients being classified into either the ACO or non-ACO group. While eosinophilic inflammation can also manifest in ACO patients, it is plausible that chronic non-eosinophilic inflammation or the decline in lung elasticity like emphysema exert a more predominant impact on driving wheezing episodes within the ACO or non-ACO patient population.
The present study has several limitations. First, since the KOCOSS cohort mainly comprises patients treated in a tertiary hospital, it may not accurately reflect the general COPD population. Second, the one-year follow-up period may be short to assess the association between the presence of wheezing and exacerbation risk. In addition, the relatively large number of patients excluded from the study due to missing exacerbation data may be a limitation in evaluating the exacerbation risk. Later, it will be necessary to assess the long-term follow-up results. Third, since the presence of wheezing was assessed using a questionnaire rather than a physical examination by a physician, it is possible that it differs slightly from the actual presence and frequency of wheezing. Fourth, our study determined the definition of ACO using marked positive bronchodilator response or elevated blood eosinophil count according to the updated Spanish criteria, but the information about the diagnosis of current asthma was not included, which might have led to an underestimation of ACO. Finally, assessing the effectiveness of ICS in preventing exacerbations based on the presence of wheezing was hindered by the inherent bias associated with an observational study.
Conclusions
In conclusion, the presence of self-reported wheezing in COPD patients was associated with severe symptoms, poor lung function, and a high risk of exacerbation in both the ACO and non-ACO groups. Moreover, the presence of wheezing independently predicted the risk of exacerbation in COPD patients, and the exacerbation risk was higher in COPD patients who experienced wheezing more frequently. Wheezing, as an indicator of more pronounced airflow restriction and a predictor of exacerbation development, could be considered a severe phenotype of COPD rather than a characteristic of an ACO phenotype.
Acknowledgments
The authors would like to thank Enago (www.enago.co.kr) for the English language review.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1031/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1031/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1031/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1031/coif). K.S.J. serves as an unpaid editorial board member of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of each participating hospital, and all patients provided written informed consent. The names of the approving ethics committees are mentioned in Appendix 1. The Institutional Review Board of the Kyungpook National University Hospital approved the study protocol (IRB No. 2012-01-001).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Celli B, Fabbri L, Criner G, et al. Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. Am J Respir Crit Care Med 2022;206:1317-25. [Crossref] [PubMed]
- Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur Respir J 2013;41:1252-6. [Crossref] [PubMed]
- Corlateanu A, Mendez Y, Wang Y, et al. Chronic obstructive pulmonary disease and phenotypes: a state-of-the-art. Pulmonology 2020;26:95-100. [Crossref] [PubMed]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [Crossref] [PubMed]
- Meghji J, Mortimer K, Agusti A, et al. Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet 2021;397:928-40. [Crossref] [PubMed]
- Meslier N, Charbonneau G, Racineux JL. Wheezes. Eur Respir J 1995;8:1942-8. [Crossref] [PubMed]
- Kim MH, Rhee CK, Kim K, et al. Heterogeneity of asthma and COPD overlap. Int J Chron Obstruct Pulmon Dis 2018;13:1251-60. [Crossref] [PubMed]
- Kim J, Kim YS, Kim K, et al. Socioeconomic impact of asthma, chronic obstructive pulmonary disease and asthma-COPD overlap syndrome. J Thorac Dis 2017;9:1547-56. [Crossref] [PubMed]
- Burney PG, Chinn S, Britton JR, et al. What symptoms predict the bronchial response to histamine? Evaluation in a community survey of the bronchial symptoms questionnaire (1984) of the International Union Against Tuberculosis and Lung Disease. Int J Epidemiol 1989;18:165-73. [Crossref] [PubMed]
- Huang WC, Tsai YH, Wei YF, et al. Wheezing, a significant clinical phenotype of COPD: experience from the Taiwan Obstructive Lung Disease Study. Int J Chron Obstruct Pulmon Dis 2015;10:2121-6. [Crossref] [PubMed]
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93:580-6. [Crossref] [PubMed]
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648-54. [Crossref] [PubMed]
- Meguro M, Barley EA, Spencer S, et al. Development and Validation of an Improved, COPD-Specific Version of the St. George Respiratory Questionnaire. Chest 2007;132:456-63. [Crossref] [PubMed]
- Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786-96. [Crossref] [PubMed]
- Plaza V, Álvarez F, Calle M, et al. Consensus on the Asthma-COPD Overlap Syndrome (ACOS) Between the Spanish COPD Guidelines (GesEPOC) and the Spanish Guidelines on the Management of Asthma (GEMA). Arch Bronconeumol 2017;53:443-9. [Crossref] [PubMed]
- Kulich K, Keininger DL, Tiplady B, et al. Symptoms and impact of COPD assessed by an electronic diary in patients with moderate-to-severe COPD: psychometric results from the SHINE study. Int J Chron Obstruct Pulmon Dis 2015;10:79-94. [Crossref] [PubMed]
- Jankrift N, Kellerer C, Magnussen H, et al. The role of clinical signs and spirometry in the diagnosis of obstructive airway diseases: a systematic analysis adapted to general practice settings. J Thorac Dis 2021;13:3369-82. [Crossref] [PubMed]
- Menezes AMB, Montes de Oca M, Pérez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 2014;145:297-304. [Crossref] [PubMed]
- Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med 2013;107:1053-60. [Crossref] [PubMed]
- Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res 2011;12:127. [Crossref] [PubMed]
- Lim JU, Kim DK, Lee MG, et al. Clinical Characteristics and Changes of Clinical Features in Patients with Asthma-COPD Overlap in Korea according to Different Diagnostic Criteria. Tuberc Respir Dis (Seoul) 2020;83:S34-45. [Crossref] [PubMed]
- Huang WC, Wu MF, Chen HC, et al. Characteristics and risk factors for inconsistency between the risk of exacerbations and the severity of airflow limitation in COPD based on GOLD 2017: A retrospective, cross-sectional study. PLoS One 2018;13:e0193880. [Crossref] [PubMed]
- Dijkstra AE, Postma DS, ten Hacken N, et al. Low-dose CT measurements of airway dimensions and emphysema associated with airflow limitation in heavy smokers: a cross sectional study. Respir Res 2013;14:11. [Crossref] [PubMed]
- Kitaguchi Y, Fujimoto K, Kubo K, et al. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir Med 2006;100:1742-52. [Crossref] [PubMed]


