
Health inequities across the lung cancer care continuum in ten marginalized populations: a narrative review
Introduction
Lung cancer is the third most common cancer in the United States, impacting over 220,000 each year (1). However, lung cancer remains the leading cause of cancer-related death, comprising of more deaths than breast, colorectal, and prostate cancer combined (1). The 5-year survival rate is 22.9% compared to breast which is 90.6%, further demonstrating the severity of this disease (2,3). Although there has been an expansion in treatment options and guidelines, only slight improvements have occurred in prevention, detection, and overall survival. Unfortunately, people of color and other marginalized populations experience variable treatment quality and access across critical points of care, leading to worse outcomes when compared to Non-Hispanic Whites (NHW) and non-marginalized populations. These disparities significantly impact outcomes, including higher rates of advanced/metastatic disease; and lower rates of clinical trial participation, receipt of precision medicines, and overall survival (4-10). Furthermore, marginalized populations have been targeted by tobacco companies, leading to higher rates of smoking and an increased risk of developing lung cancer within these groups. However, lung cancer screening guidelines do not take into account factors such as race, ethnicity, socioeconomic status (SES), or sex-based differences in smoking behaviors or lung cancer risks, which results in systemic barriers to optimizing screening for higher-risk marginalized patients (11). Previous articles described disparities across several populations, but limited data are available within one source that describes disparities across critical populations targeted by tobacco manufacturing companies. Therefore, this review provides a comprehensive discussion of lung cancer disparities for several populations across the continuum of lung cancer, detection, diagnosis, supportive care survival, and social/structural determinants of health. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-727/rc).
Methods
We conducted a narrative literature review and interviewed a panel of experts to further understand health disparities across the lung cancer continuum of care in the following ten populations: race/ethnicity [African Americans/Blacks (AA/B), American Indian/Alaska Native (AI/AN), Hispanic/Latinx (HL), Asian American and Pacific Islander (AAPI)], low income, rural, LGBTQIA+, women, military (veterans and active duty), and small cell lung cancer (SCLC) (Figure 1). This review will focus on the narrative literature review and a future manuscript will provide additional qualitative results from the interviews. For the narrative review, five databases (PubMed, the Cochrane Library, EMBASE, Web of Knowledge, and EBSCO Discovery Service) were queried from February 2022–June 2022 using search terms agreed upon by the authors (Table 1). We also used data from Center for Disease Control and Prevention/United States (CDC/US) Cancer Statistics; National Institute of Health (NIH)/National Cancer Institute data for incidence, mortality, survival, prevalence, and risk of death/dying; organizational data from Lungevity and American Lung Association; and social determinant of health data from the Health Equity Tracker. The inclusion criteria were (I) peer-reviewed academic journals or data reports published in English between the years 2000 and 2022; (II) research that focused on disparities across the lung cancer continuum; (III) research highlighting social and structural barriers to lung cancer health care access; and (IV) research that mentioned at least one of the 10 populations of interest. A total of 95 articles and 24 reports were used for this narrative review. A narrative synthesis approach was used to analyze and summarize data from the reviews (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 1 February 2022, 1 June 2022 |
| Databases and other sources searched | PubMed, the Cochrane Library of Systematic Reviews, EMBASE, Web of Knowledge, and EBSCO Discovery Service; CDC/US Cancer Statistics; NIH/National Cancer Institute data for incidence, mortality, survival, prevalence, and risk of death/dying; Lungevity, American Lung Association; Health Equity Tracker |
| Search terms used | Disease states [Lung cancer, small cell lung cancer, non-small cell lung cancer (NSCLC)]; continuum of care (treatment, biomarker testing, screening, clinical trial, smoking, risk factors, screening, diagnosis, survival, palliative care); equity (racial, socio-economic, population) and inequities; disparities; social determinants of health |
| Timeframe | 2000 to 2022 |
| Inclusion criteria | Inclusion: (I) peer-reviewed academic journals or data reports published in English between the years 2000 and 2022; (II) research that focused on disparities across the lung cancer continuum; (III) research highlighting social and structural barriers to lung cancer health care access; and (IV) research that mentioned at least one of the 10 populations of interest |
| Selection process | Articles were selected based on the inclusion criteria and were led by Alongi, Carlson, and Redding |
CDC/US, Center for Disease Control and Prevention/United States; NIH, National Institute of Health.
Results
AA/B
AA/B have the highest lung cancer incidence rate (55.8 per 100,000 people per year) among other racial/ethnic minority groups (Table 2) (1). Within AA/B communities, lung cancer screening rates are predicted to be lower because AA/B who smoke are less likely to be eligible for screening due to shorter smoking histories and higher smoking intensity, which are not accounted for in the current screening guidelines (12-14). The lack of eligibility for screening within this population, contributes to the lower rates of screening (44% less likely compared to NHWs) (15), and screening rate comparisons are worsened for AA/B residing in historically redlined areas (61% less likely compared to NHW)—suggesting structural racial discrimination influence on lung cancer screening uptake (15). Additional barriers to health care access remain a challenge, demonstrated by AA/B being 30% less likely to be asked about smoking cessation programs compared to NHW patients (16). These multimodal factors lead to AA/B being diagnosed younger and with more advanced/metastatic lung cancer at diagnosis (10,12). Once diagnosed, AA/B are less likely to receive radiation treatment, systemic therapy, surgical resection, clinical trial access, and comprehensive next-generation sequencing (NGS) testing compared to NHW (4,10,17,18). One specific example is surgery, which is performed at earlier stages and can often be curative. In one study, AA/B residing in urban areas had a 45% lower odds of undergoing surgery; and in rural counties, AA/B were disproportionally impacted, where there was a 67% lower odds of receiving surgery (19). Another example of a treatment barrier is the utilization of immunotherapy. AA/B treated with immunotherapy have a 15% lower death rate than NHW (6), however, they are less likely to be treated with immunotherapy, regardless of insurance status (20). Limited access to lifesaving and innovative treatment options in conjunction with additional barriers, contribute to AA/B being 12% less likely to survive five years compared to NHW (10), with worsening mortality outcomes in racially segregated areas of the country (21). Interestingly, under conditions of equal access to treatment, data have demonstrated similar outcomes or a reduction in disparities, reinforcing the need to improve access to quality care (18,22,23). The high mortality rates demonstrate the critical need for end-of-life care, but unfortunately, AA/B are less likely to be enrolled in hospice and receive medication for symptom control at end-of-life. Additionally, AA/B have higher end-of-life costs than NHW; partly driven by higher hospital and ICU admission rates in the final month of life (24).
Table 2
| Race | Count | Rate/100,000 | |||
|---|---|---|---|---|---|
| Incidence | Mortality | Incidence | Mortality | ||
| Non-Hispanic Whites | 177,518 | 114,012 | 58.5 | 38 | |
| African American/Blacks | 23,462 | 14,945 | 55.8 | 37.2 | |
| American Indian/Alaska Native | 1,380 | 787 | 52 | 30.2 | |
| Asian American and Pacific Islander | 6,882 | 3,917 | 33 | 19.8 | |
| Hispanic/Latinx | 10,682 | 5,660 | 27.7 | 15.4 | |
Many barriers are contributing to the disparities in AA/B populations due to the history of state and local governments denying adequate public services to AA/B neighborhoods; re-zoning residential areas for mixed use with industrial or toxic sites (25), and creating residential segregation from Jim Crow laws (rural) and ‘redlining’ (urban) (26). These structural and systemic barriers have led to unequal opportunities to build wealth through high-wage jobs, access to home equity, and access to quality education which created a drastic wealth gap between NHW and AA/B (27,28). Many of these segregated areas have higher environmental exposures exhibited by AA/B being 75% more likely than other Americans to live close to facilities that produce hazardous waste and being exposed to 1.5× more sooty pollution (29). The lower level of educational attainment within these communities has also led to lower health literacy scores compared to NHW (30), and has positioned AA/B communities to have access to lower-paying jobs with poor health insurance coverage, further contributing to health care access challenges. For example, 25% of AA/B do not have a personal doctor and were more likely to report not seeing a doctor in the past year due to cost compared to NHW (31). In addition, due to structural and systemic racism, there is a shortage of primary care providers in AA/B communities (32), which impacts quality care and negatively impacts patient-provider engagement, patient experience and trust, and the delivery of culturally competent care (33). The lack of representation of health care providers can further create barriers within these communities, especially because of the historic unethical treatment of the AA/B population within the U.S. health care system (e.g., medical experimentation, forced sterilization, and denial of treatment). While recognizing the barriers within the healthcare system, it is imperative to also acknowledge that AA/B communities have been specifically targeted by tobacco manufacturers' marketing. This targeting is evident through the sponsorship of athletic, cultural, and entertainment events, as well as a high concentration of billboards (34). These, among other factors, contribute to the elevated rates of lung cancer within this community.
AI/AN
The AI/AN population has the second highest lung cancer incidence rate when compared to other racial/ethnic minority groups at a rate of 50/100,000 (Table 2) (1). AI/AN also has the highest smoking rate in the United States (35), which is historically embedded in religious practices and social relationships (36). Limited data on lung cancer screening are available within AI/AN populations due to lung cancer screening not being a mandatory reporting measure by the Indian Health Service (IHS) (37). However, only 23% of AI/AN patients are diagnosed early (10), which may infer that lung cancer screening is also low within AI/AN populations. Once diagnosed, 22% of the AI/AN population do not receive lung cancer treatment (10), and they are 21% less likely to receive surgical treatment compared to NHW (10). Additionally, only 0.2% of enrollees in precision oncology trials are AI/AN, suggesting low biomarker testing rates within AI/AN populations (5). AI/AN are also 13% less likely to survive five years compared to NHW, likely due to lack of access to or receipt of treatment (10). With higher mortality rates and lack of treatment, end-of-life care becomes essential for the AI/AN population. However, there are limited options on reservations (38), which reemphasizes the need to expand care and policies to increase health care access.
Barriers exist within the AI/AN population because federally established reservations have led to residential segregation and concentrated/persistent poverty. On the reservations, where ~13% of AI/AN resides (39), there can be inadequate medical facilities, shortages in health care providers, and lack of access to preventative screenings. IHS chronically receives lower per capita funding than the Veteran’s Health Administration (VHA), Medicare, and Medicaid which creates limited access to specialty services and chronic physician shortages within the IHS (40). Furthermore, if AI/AN populations seek care outside of IHS, they often face barriers such as transportation, financial burdens including lack of insurance coverage, and interactions with non-IHS physicians who are often unaware of unique AI/AN needs (41,42). Furthermore, some states with high concentrations of rural AI/AN residents have not expanded Medicaid, which results in a higher percentage of low-income AI/AN individuals not being qualified for health insurance. In addition to access barriers, mistrust of the health system prevents many AI/AN from seeking care (43). These barriers all continue to impact these communities, exhibited by 34% of the AI/AN community not having a personal doctor and being 17% more likely to report not seeing a doctor in the past year due to cost (44).
AAPI
As an aggregated population, AAPI are less commonly diagnosed with lung cancer, with a rate of 33/100,000 compared to NHW of 57/100,000 (1). However, there is a higher percentage of Asian American women diagnosed with lung cancer who have never smoked (57%), compared to 16% of all women (45). This highlights a critical need to further investigate non-smoking risk factors contributing to a lung cancer diagnosis within the AAPI group. Drivers for high non-smoking lung cancer rates in AAPI are not well understood, but previous literature has shown that for Chinese women exposure to secondhand smoke and cooking oils at high heat may contribute to high lung cancer incidence rates (46). Unfortunately, many AAPI women who fall within this category do not qualify for lung cancer screening due to risk prediction models not including a direct measure of genetic risk as a variable, therefore, unable to be included in lung cancer screening eligibility criteria (47). Barriers to screening AAPI populations may explain why data indicate that AAPI individuals are 16% less likely to be diagnosed early when compared to NHW (10). Once diagnosed, AAPI are 3% more likely to not receive any treatment compared to NHW (10). However, AAPI are 16% more likely to receive surgical treatment compared to NHW (10). Similar to other minority groups, there is low enrollment (4.8%) in precision medicine lung cancer clinical trials (5). However, when survival is assessed, AAPI were equally likely to survive 5 years compared to NHW (10). This may be due to the high prevalence of targetable alterations [such as epidermal growth factor receptor (EGFR)] in Asian descendants. Contrarily, when the data are disaggregated for non-small cell lung cancer (NSCLC), Japanese people, Vietnamese men, Korean men, and smaller AAPI communities (e.g., Cambodian, Laotian, Samoan) have worse two-year survival rates than other AAPI subgroups (48). This highlights the importance of data disaggregation to further understand populations.
The literature has highlighted fewer barriers within AAPI populations compared to other racial/ethnic groups, which could be due to the higher educational attainment than any other group, with 56% of AAPI achieving a bachelor’s degree education or higher (44). This is reflected by a higher representation of AAPI in the clinical workforce compared to other racial/ethnic minority groups, where 17.1% of AAPI are active physicians (49). AAPI populations also have higher insurance coverage and are 35% more likely to report getting a routine checkup in the past year compared to NHW (44). However, there are some known challenges within AAPI communities such as language barriers. Within the AAPI population, there are 50 ethnic groups and 100 languages (50). However, there are limited translators available for 100+ languages, and more than often, educational health materials are not translated. There is also provider bias and cultural differences within AAPI populations that can create additional barriers. For example, the rise in anti-Asian hate crimes and increased discrimination resulting from the coronavirus disease 2019 (COVID-19) pandemic may negatively affect experiences with health and health care (51). In regards to cultural differences, there are cultural attitudes and perspectives around discussing bad news with elders and cultural taboos against talking about death or dying (52). This can potentially create challenges with discussing end-of-life care and/or participating in support groups to cope with a lung cancer diagnosis.
HL
The HL population has the lowest lung cancer incidence in the United States at a rate of 28/100,000 (1). HL also have a low smoking rate compared to the general population (53), and have demonstrated a higher interest in lung cancer screening once made aware of this intervention (54). However, there is a lower prevalence of lung cancer screening referrals in states with a greater HL population (55). This may allude to why HL are 15% less likely to be diagnosed with early-stage lung cancer compared to NHW (10). Once diagnosed, HL populations are 28% more likely to not receive any treatment (10) and are less likely to receive guideline-concordant care compared to NHW (24). However, when compared to NHWs, they are equally likely to receive surgical treatment. Another critical part of treatment is having access to clinical trials, yet only 1.1% of HL chose to enroll (5), and physicians are less likely to recommend clinical trials to HL patients relative to NHW patients. Furthermore, when HL are enrolled in clinical trials, they are more likely to not fully understand the aims of the trial (56), demonstrating communication gaps and opportunities to address language and health literacy barriers. Treatment access and disparities contribute to lower lung cancer survival rates of HL, in which they are 12% less likely to survive five years compared to NHW (10). With advanced disease and lower survival, palliative care at the end of life becomes an important factor within the HL population. However, literature demonstrates that HL report greater symptom burden but are less likely to receive palliative care and medications for symptom control and have higher end-of-life costs (57,58).
One major barrier within the HL population is health care access. Currently, 42% of HL do not have a personal doctor and are 18% more likely to have not seen a doctor in the past 12 months because of cost (31). Similar to other minority populations, HL populations are impacted by structural racism/racist policies that create a negative environment for accessing health care among those with public insurance. Currently, for the HL population, 19% are uninsured and 33% are enrolled in Medicaid or other public insurance (59). Several states with large HL populations (e.g., TX, FL) have not expanded Medicaid (60). In addition, restrictive immigration policies contribute to the high numbers of Hispanics who are non-U.S. citizens and consequently undocumented to utilize health care (61). It remains a significant barrier for HL with limited English proficiency to work in occupations that offer employer-based health insurance and paid work leave. It is estimated that 28% of HL do not speak English well or at all and 13% live in a limited English-speaking household (62). With limited representation of HL in the oncology workforce (<5% in hematology/oncology and <1% of radiation oncologists), there are challenges with addressing language barriers for patients and caregivers (63).
Low income
There is an overall increased risk of lung cancer incidence among people with low economical, low educational, and low occupational socioeconomic position (64). Additionally, low SES populations face tremendous barriers to obtaining lung cancer screening because of a lack of/poor insurance coverage and under-resourced clinics in the community (65). These barriers contribute to the higher prevalence of lung cancer diagnosis during an unscheduled, emergency, or unplanned hospital admission (9). Once diagnosed, low SES populations have 16–30% lower odds of receiving traditional treatments, such as surgery, chemotherapy (66), immunotherapy (67), stereotactic body radiotherapy (68), or receiving next-generation sequencing-directed treatment (69). These challenges lead to poorer survival in low SES populations diagnosed with lung cancer (7). For example, patients from communities with household incomes below $30,000, are 25% more likely to die within 30 days of lung surgery than wealthier patients (70). This can also generationally impact children born and raised in lower SES, exhibited by higher lung cancer mortality in adulthood, in part due to smoking exposure (71). Gaps in research on end-of-life care, as well as engagement with survivorship resources, limit our understanding of the needs of low SES populations.
Low SES communities face worse outcomes in part due to having under-resourced health care settings (e.g., shortages of providers, including specialty providers) (65), coupled with significant concerns about life necessities, including food, shelter, and personal safety. Additionally, there are delays in using health-care services because of costs and significant barriers around insurance (72). Although Medicaid was developed to provide health coverage for people below the federal poverty level (26), it still created coverage gaps for many workers employed in low-wage jobs with inadequate health coverage because they were ineligible to switch to Medicaid and also did not qualify for federal subsidies offered through the Affordable Care Act (ACA) Marketplaces, resulting in being locked into employer plans that provide less protection (26). This by default causes many low-income employees to fall into the Medicaid coverage gap—people too poor to afford private insurance and not eligible for Medicaid. Medicaid expansion allows for more low-income individuals to qualify for Medicaid, without additional stipulations of pregnancy, children, elderly, or having a disability. However, many states have not expanded Medicaid, which leaves significant coverage gaps for many low SES patients to go without insurance coverage, who are at risk for lung cancer.
Rural
In areas of high rurality, there is almost twice the smoking prevalence and lung cancer incidence when compared to the largest metropolitan areas (8). Additionally, late-stage lung cancer is diagnosed at a higher rate in rural areas (73). Rural residents are more likely to not receive treatment (74), including chemotherapy or radiotherapy (75). When treatment is provided, rural populations have lower odds of receiving cancer-directed surgery (76), and receive less guideline-concordant treatment when compared to metropolitan residents (75). In general, there are delays in treatment and increased time from diagnosis to surgery, especially for early-stage patients in small/isolated rural areas (77). These barriers and access challenges contribute to worsening survival rates for rural/small-town patients compared to urban/metro counties at 1 year (85% vs. 87%), 5 years (48% vs. 54%), and 10 years (26% vs. 31%) (78). In general, lung cancer mortality rates are higher among all rural racial/ethnic groups vs. urban (e.g., 54% higher for AA/B) (79). This makes it critical for rural populations to have access to palliative care end-of-life options, however, patients who reside in rural areas are at an increased risk for underutilizing palliative care (80), presumably because of palliative care provider scarcity.
Thirteen percent of US counties are persistent poverty counties, which are predominantly rural and located in the south (81). Therefore, affordability to access care and insurance becomes a priority. However, health care access is a critical gap in rural communities due to industries and employers being less likely to offer health insurance coverage to their employees (82), and the lack of Medicaid expansion in large rural populations (83). Additionally, lack of public transportation and distance remain significant access barriers within rural communities (82). For example, distance plays a role in lung cancer screening, noted by rural communities being less likely to have access to a low-dose CT scan (LDCT) screening center within 30 miles (48% rural vs. 94% urban) or a 30-minute drive (22% rural vs. 83% urban) (84). Accessing screening sites is crucial because tobacco is deeply ingrained in the culture, serving as a significant source of income for many rural areas (85). Additionally, longer travel distances are adversely associated with receipt of guideline-concordant care (86) and can limit representation in clinical research (87-89) due to the majority of clinical trial sites being located in metropolitan areas. Furthermore, many rural areas are classified as health professional shortage areas (90) with fewer primary and specialty care providers per capita and increasing rates of hospital closures (91). Although telehealth may be a partial solution, there is often limited access to broadband internet in rural communities (82). This ultimately limits access to health care and leads many patients to travel farther for care.
LGBTQIA+
Sexual identity and gender minority data are largely untracked in national health and cancer databases, which has resulted in many gaps in our understanding of the experiences of LGBTQIA+ populations, including barriers across the lung cancer care continuum. Although lung cancer prevalence within the LGBTQIA+ population is unknown, currently, about 1 in 5 LGB adults smoke cigarettes, compared with about 1 in 7 heterosexual adults. For transgender adults, the percentage of cigarette smokers is 35% higher than those identifying as cisgender adults (92). Big Tobacco was one of the first major consumer industries to target LGBTQIA+ communities (93), groups that had been largely ignored by mainstream advertising. Although there are other risk factors for developing lung cancer, the high incidence of smoking within the LGBTQIA+ populations warrants education on smoking cessation and lung cancer screening. In one study, males who self-identified as gay/lesbian and who had no medical cost burden had higher odds of receiving lung cancer screening, whereas being bisexual was associated with a lower likelihood of screening, suggesting there are sexual-identity disparities in utilizing lung cancer screening (94). After diagnosis, treatment options for gender minority individuals appeared to have similar proportions of surgical, chemotherapy, and radiation therapies as gender majority (95). Additionally, one study highlighted that LGB survivors are more likely to participate in cancer clinical trials than heterosexual survivors, but the data were not stratified by cancer type (96). Lung cancer clinical trial enrollment in these populations remains unknown. Although data are limited in LGBTQIA+ populations, there is a positive association between lung cancer incidence and mortality rates in areas with higher densities of sexual minority men (97). This indicates the need to tailor and develop interventions that would raise awareness and support services for groups within the LGBTIA+ population.
The lack of data on LGBTQIA+ populations are a result of social marginalization and exclusion, which has resulted in legal, economic, political, and social structure barriers. In general, data have demonstrated that sexual minorities were more likely than heterosexual individuals to delay seeking healthcare (98). In addition, external and internalized homophobia and transphobia negatively affect health, mental health, and experiences with the health care system (99). Four in five transgender adults report being treated with less courtesy or respect than their cisgender heterosexual counterparts (100). Furthermore, medical students receive under 5 hours of training on LGBT issues on average creating a lack of cultural competence in the health care system (101). This ultimately leads to provider bias and lack of knowledge about population-specific needs, which is a snowball effect for why many LGBTQIA+ “chosen” families are often not recognized by the health care system, limiting their support, especially in times of end-of-life care.
Women
When stratified by sex, lung cancer incidence, and mortality rates are higher within males (1). However, lung cancer diagnoses have risen a startling 84% among females over the past 42 years, while dropping 36% among males over the same period (102). Females are more likely to be diagnosed at a younger age, at an earlier stage, with adenocarcinoma, and with no history of smoking (103). This makes many females ineligible for screening according to the guidelines. Interestingly, females are slightly more likely to be screened compared to males (6.3% vs. 5.6%) (104), indicating a potential for higher uptake in screening if eligibility criteria would become more inclusive. This is critical because females who do not smoke are more than two times as likely to get lung cancer compared to men who do not smoke (102). Once diagnosed with lung cancer, females are also more likely to have identifiable genetic changes in their cancer (105). This is important as many of the newer therapies target genetic changes (e.g., EGFR, ALK, ROS, etc.), and result in improved survival compared to traditional chemotherapy. Furthermore, females have better responses to certain chemotherapy such as platinum agents (106). Taken together, treatment differences may contribute to why females typically have higher survival rates at 5 years (25.5 vs. 18.5) (107). However, females are less likely to be enrolled in clinical trials for lung cancer (108). Minimal research has focused on sex/gender barriers across the lung cancer care continuum, however, institutionalized sexism affects how both women and men experience gender roles and discrimination and the intersectionality between sex, gender, race/ethnicity, rurality, SES, and other factors may compound disparities for certain groups.
Veterans and active duty
Military service members are 25% more likely to receive a lung cancer diagnosis (109), and more likely to be diagnosed early (110). Although many Veteran Administration (VA) clinics and hospitals provide access to lung cancer screening (109) less than two-thirds of veterans received timely recommended follow-up after initial lung cancer screening, with a higher risk of delayed or absent follow-up for individuals who were Black; those with psychiatric diagnoses; and those with low incomes (111). However, for veterans with access to the VA system, favorable outcomes were noted in patients with lung cancer receiving care through the VHA, presumably because of a wide range of treatment options, and access to advanced therapies in a closed-loop and integrated system. For example, veterans diagnosed with stage I NSCLC were more likely to receive minimally invasive surgical procedures compared to non-VHA patients (112). In addition, within the system, veterans have higher lung cancer survival rates than the general population, including significantly better 30-day mortality outcomes and median overall survival rates after NSCLC surgery (111).
The high rates of lung cancer in the veteran and active-duty populations are largely linked to several occupational, environmental, and smoking exposures which increase lung cancer risk within the veteran population. Occupational exposures to carcinogens such as Agent Orange, asbestos, carbon monoxide in diesel exhaust, heavy smog, pesticides, oil well fires, burn pits, and detonated or destroyed chemical weapons can lead to lung cancer (109). In addition, military service members had easy access to cheap tobacco products until 1976 when soldiers were given free cigarettes in C and K rations (109). These factors all contribute to the recent passage of the PACT Act, which allows veterans exposed to toxic substances to be eligible for toxic screening every five years. However, only 55% of veterans live within 40 miles of VA oncology services (113), causing many to not utilize the services due to proximity and widening the gaps in accessing care.
SCLC
SCLC is typically more advanced and aggressive compared to NSCLC (114). Only two percent of cases arise in people with no smoking history (115). SCLC is among the cancers most correlated with smoking, yet current lung cancer screening protocols are ineffective at catching SCLC in early stages relative to NSCLC due to rapid tumor growth, making early detection and diagnosis challenging. Out of the SCLC cases diagnosed, 71% are diagnosed at metastatic stage vs. 38–55% for all lung and bronchus cases [2011–2020] (116). Available treatment options have lagged behind NSCLC advancements. At every stage, the prognosis of SCLC is worse than that of NSCLC (117). For Medicare patients, it takes on average, six weeks between diagnosis to first-line treatment, but only 60% of Medicare patients get treatment (118), compared to nearly 80% for lung cancer overall (119). In contrast to NSCLC, biomarker testing is not available for SCLC due to the lack of availability of targeted treatment options (120). Due to the aggressiveness and low prevalence of the disease, there are limited tissue banks to drive basic & translational research and limited funding available. Therefore, these factors and disparities contributes to the median overall survival of SCLC to be half of NSCLC patients (8.5 vs. 17.5 months) (121). People with SCLC also face high levels of comorbidities and side effect burden of treatment, which heightens the need for palliative care, caregiver support, and patient support. Data gaps limit full understanding of equity in access to existing innovations within SCLC population, but literature informs us that more research, education, and advancement in treatment is needed.
Conclusions
To our knowledge, this is the first review that includes ten critical marginalized populations affected throughout the lung cancer continuum of care and provides an overview of disparate outcomes and root causes that impact accessing quality health care. There were key differences, similarities, and data gaps for several populations across the lung cancer continuum (Tables 3,4; Figure 2), but consistent barriers across all groups with accessing, being referred, or being eligible for lung cancer screening. Although data are limited to nationally compare lung cancer screening rates across marginalized populations, the average national uptake is less than 6% in the United States (122). The low uptake in lung cancer screening has led to higher rates of advanced/metastatic lung cancer at diagnosis, with even higher rates for racial/ethnic minorities (10). Furthermore, the majority of marginalized populations assessed in this narrative review, were more likely to not receive treatment (i.e., surgery, radiation, chemotherapy, immunotherapy, targeted therapy, etc.) or comprehensive biomarker testing which could potentially provide patients with innovative treatments (i.e., targeted therapy, clinical trials) that may improve survival outcomes.
Table 3
| Population | Key differences |
|---|---|
| African American/Black | • Diagnosed younger and with more advanced/metastatic lung cancer at diagnosis |
| • Deep inequities across the patient journey, especially in racially segregated and rural counties | |
| Hispanic/Latinx | • Large immigrant population; language barriers and lack of insurance limit access to timely, quality care |
| • Data disaggregation needed for distinct subgroups | |
| Asian American and Pacific Islander | • Lower overall incidence, but higher for Southeast Asians and very high rates of female never-smoker lung cancer diagnoses |
| • Language barriers, healthcare access, and cultural taboos around death and dying affect lung cancer outcomes | |
| American Indian/Alaska Native | • Highest smoking/tobacco use of all racial/ethnic groups, but extremely under researched and under resourced |
| • Significant geographic variation in lung cancer burden and distinct rural/urban barriers in access to care | |
| LGBTQIA+ | • Higher smoking rates, particularly for transgender people and gay and bisexual women |
| • Face healthcare access barriers re: insurance & discrimination | |
| Sex and gender | • Females diagnosed younger, with lower smoking history |
| • Higher male mortality, potentially due to health-seeking behaviors | |
| Low socioeconomic status | • Variable Medicaid coverage for core lung cancer detection and treatment; access worse for uninsured |
| • More likely to present as an emergency, experience treatment delays, or not receive treatment | |
| • Intersectionality with race/rurality worsens inequities | |
| Rural/remote | • Higher overall and late-stage incidence rates but similar screening rates to non-rural, despite barriers |
| • Lower likelihood of any, quality, or timely treatment contributes to low survival and poor survivor outcomes | |
| Military | • Higher rates of smoking and occupational exposure |
| • Despite higher incidence, veterans have higher survival rates than the general population, likely due to greater access to integrated care | |
| Small cell lung cancer | • Highly correlated with significant smoking history; significant comorbidities often present |
| • Lower survival rate than non small cell lung cancer |
Table 4
| Summary of research data gaps |
| • Screening rates and utilization are a data gap across populations—no national aggregation exists |
| • Sexual identity and veteran status largely untracked in national health and cancer databases |
| • AI/AN race often misclassified at birth and time of death, lack of AI/AN specific cancer registry, and AI/AN needs largely under-researched |
| • Mixed outcomes within AAPI and HL populations require more nuanced data disaggregation to understand key drivers and opportunities |
| • Clinical research in process to understand stark and specific inequities for female Asian never smokers (FANS study) |
| • Access to and need for palliative/end of life care is not understood for most underserved populations (low SES, HL, AI/AN, etc.), and requires specific cultural considerations for LGBTQIA+ and AAPI populations |
| • Understanding of SCLC patient demographics and inequities is restricted by limitations in available data, including small number sizes, underrepresentation of groups with inequitable access to care, and a paucity of studies on key patient groups |
AI/AN, American Indian/Alaska Native; AAPI, Asian American and Pacific Islander; HL, Hispanic/Latinx; SES, socioeconomic status; SCLC, small cell lung cancer.
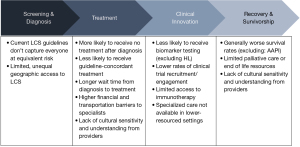
Significant themes contributing to health disparities in lung cancer were SES, transportation, language, historic trauma, provider bias or lack of cultural training, and lack of health care access, in part due to insurance coverage. Many of these barriers are caused by structural/systemic racial policies. For example, redlining has caused many communities to suffer from physician shortages, environmental exposures, food deserts, and underfunded public schools which contribute to poor health literacy and lack of education advancement for employment, etc. Additionally, with Medicaid expansion being optional for states, this reinforces racial hierarchy and results in inequities in coverage, which is evident in southern states with large numbers of Black and Latino residents that have not expanded Medicaid.
As long as structural racism continues to shape health care policy, marginalized populations will suffer from inequitable access across the lung cancer care continuum resulting in poorer health outcomes. The aforementioned disparities are complex and rooted in historic and contemporary inequities and involve factors both within and external to the health care delivery system. We must combat social determinants of health such as poverty, food insecurity, discrimination, lack of access to quality education, and lack of stable housing and health care, among other social inequities—the factors that ultimately have the biggest say in health and disproportionately affect certain groups at risk, diagnosed with, or surviving lung cancer. More research is needed to further develop meaningful solutions to disparities in health outcomes and access for those who are at risk, diagnosed with, or surviving lung cancer from marginalized populations.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-727/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-727/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-727/coif). S.M. and J.C.K. report that research funding for this study was directly paid to GO2 for Lung Cancer by AstraZeneca, Daiichi Sankyo, and Merck. GO2 for Lung Cancer is recipient of the funds. S.M.’s Diversity, Equity, and Inclusion (DEI) lung cancer research is supported by Bristol-Myers Squib, Merck, Sanofi, Janssen, Takeda, and Mirati in which GO2 is the direct recipient of research funds. Maureen Rigney and N.P. were co-leads on the design of the project and funds requested to support this work was sponsored by AstraZeneca, Daiichi, Sankyo, and Merck. GO2 is the direct recipient of research funds to support this project. Maureen Rigney also received travel support for a presentation in 2019 for AstraZeneca; received honorarium in 2020 for serving on the advisory board for Jazz and Novartis in 2021. Maureen Rigney was also an invited speaker for International Association for the Study of Lung Cancer, and received partial airfare and hotel coverage. Maureen Rigney also volunteers at Joe’s House and for the Association of Oncology Social Workers. C.C., T.A. and Mikayla Redding are employees of FSG, a consultant agency that provides consulting and research services related to health equity and access to several biopharmaceutical and healthcare services companies, including Bristol Myers Squibb and Quest Diagnostics, that address lung cancer. FSG was hired by GO2 for Lung Cancer to conduct the data collection and analysis to support GO2 for Lung Cancer’s DEI work. J.C.K. serves as the PI on research funding to GO2 from Bristol Myers Squibb and Genetech, unrelated to this project, in which funds are paid to GO2 for Lung Cancer. J.C.K. provided consultation to Amgen, Boehringer Ingelheim, and EQRX, in which all funds were paid to GO2 for Lung Cancer. J.C.K. also participated on an advisory board for Bristol Myers Squibb in which all funds were paid directly to GO2 for Lung Cancer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2021 submission data (1999-2019). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; released in November 2022. Available online: https://www.cdc.gov/cancer/dataviz
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer. 2022. Available online: https://seer.cancer.gov/statfacts/html/lungb.html
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Breast Cancer. 2022. Available online: https://seer.cancer.gov/statfacts/html/breast.html
- Bruno DS, Hess LM, Li X, et al. Disparities in Biomarker Testing and Clinical Trial Enrollment Among Patients With Lung, Breast, or Colorectal Cancers in the United States. JCO Precis Oncol 2022;6:e2100427. [Crossref] [PubMed]
- Aldrighetti CM, Niemierko A, Van Allen E, et al. Racial and Ethnic Disparities Among Participants in Precision Oncology Clinical Studies. JAMA Netw Open 2021;4:e2133205. [Crossref] [PubMed]
- Gupta A, Akinyemiju T. Abstract PO-107: Racial differences in survival among advanced-stage non-small cell lung cancer patients who received immunotherapy: An analysis of the U.S. National Cancer Database (NCDB). Cancer Epidemiol Biomarkers Prev 2022;31:PO-107. [Crossref] [PubMed]
- Finke I, Behrens G, Weisser L, et al. Socioeconomic Differences and Lung Cancer Survival-Systematic Review and Meta-Analysis. Front Oncol 2018;8:536. [Crossref] [PubMed]
- Atkins GT, Kim T, Munson J. Residence in Rural Areas of the United States and Lung Cancer Mortality. Disease Incidence, Treatment Disparities, and Stage-Specific Survival. Ann Am Thorac Soc 2017;14:403-11. [Crossref] [PubMed]
- Redondo-Sánchez D, Petrova D, Rodríguez-Barranco M, et al. Socio-Economic Inequalities in Lung Cancer Outcomes: An Overview of Systematic Reviews. Cancers (Basel) 2022;14:398. [Crossref] [PubMed]
- American Lung Association. State of Lung Cancer: Racial and Ethnic Disparities. 2022. Available online: https://www.lung.org/research/state-of-lung-cancer/racial-and-ethnic-disparities
- Rivera MP, Katki HA, Tanner NT, et al. Addressing Disparities in Lung Cancer Screening Eligibility and Healthcare Access. An Official American Thoracic Society Statement. Am J Respir Crit Care Med 2020;202:e95-e112. [Crossref] [PubMed]
- Aldrich MC, Mercaldo SF, Sandler KL, et al. Evaluation of USPSTF Lung Cancer Screening Guidelines Among African American Adult Smokers. JAMA Oncol 2019;5:1318-24. [Crossref] [PubMed]
- Li CC, Matthews AK, Rywant MM, et al. Racial disparities in eligibility for low-dose computed tomography lung cancer screening among older adults with a history of smoking. Cancer Causes Control 2019;30:235-40. [Crossref] [PubMed]
- Ryan BM. Differential eligibility of African American and European American lung cancer cases using LDCT screening guidelines. BMJ Open Respir Res 2016;3:e000166. [Crossref] [PubMed]
- Poulson MR, Kenzik KM, Singh S, et al. Redlining, structural racism, and lung cancer screening disparities. J Thorac Cardiovasc Surg 2022;163:1920-1930.e2. [Crossref] [PubMed]
- Cokkinides VE, Halpern MT, Barbeau EM, et al. Racial and ethnic disparities in smoking-cessation interventions: analysis of the 2005 National Health Interview Survey. Am J Prev Med 2008;34:404-12. [Crossref] [PubMed]
- Shugarman LR, Mack K, Sorbero ME, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care 2009;47:774-81. [Crossref] [PubMed]
- Chen VE, Lombardo JF, Castaneda SA, et al. Disparities in Lung Cancer for Black Patients in the US: An Overview of Contributing Factors and Potential Strategies. Appl Rad Oncol 2020;9:16-20.
- Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non-small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc 2011;103:711-8. [Crossref] [PubMed]
- Verma V, Haque W, Cushman TR, et al. Racial and Insurance-related Disparities in Delivery of Immunotherapy-type Compounds in the United States. J Immunother 2019;42:55-64. [Crossref] [PubMed]
- Landrine H, Corral I, Lee JGL, et al. Residential Segregation and Racial Cancer Disparities: A Systematic Review. J Racial Ethn Health Disparities 2017;4:1195-205. [Crossref] [PubMed]
- Zheng L, Enewold L, Zahm SH, et al. Lung cancer survival among black and white patients in an equal access health system. Cancer Epidemiol Biomarkers Prev 2012;21:1841-7. [Crossref] [PubMed]
- Annesi CA, Poulson MR, Mak KS, et al. The Impact of Residential Racial Segregation on Non-Small Cell Lung Cancer Treatment and Outcomes. Ann Thorac Surg 2022;113:1291-8. [Crossref] [PubMed]
- Stein JN, Rivera MP, Weiner A, et al. Sociodemographic disparities in the management of advanced lung cancer: a narrative review. J Thorac Dis 2021;13:3772-800. [Crossref] [PubMed]
- Maantay J. Zoning law, health, and environmental justice: what's the connection? J Law Med Ethics 2002;30:572-93. [Crossref] [PubMed]
- Yearby R, Clark B, Figueroa JF. Structural Racism In Historical And Modern US Health Care Policy. Health Aff (Millwood) 2022;41:187-94. [Crossref] [PubMed]
- South E, Venkataramani A, Dalembert G. Building Black Wealth - The Role of Health Systems in Closing the Gap. N Engl J Med 2022;387:844-9. [Crossref] [PubMed]
- Muvuka B, Combs RM, Ayangeakaa SD, et al. Health Literacy in African-American Communities: Barriers and Strategies. Health Lit Res Pract 2020;4:e138-43. [Crossref] [PubMed]
- Mikati I, Benson AF, Luben TJ, et al. Disparities in Distribution of Particulate Matter Emission Sources by Race and Poverty Status. Am J Public Health 2018;108:480-5. [Crossref] [PubMed]
- Kutner M, Greenberg E, Jin Y, et al. The Health Literacy of America's Adults: Results from the 2003 National Assessment of Adult Literacy. National Center for Education Statistics: U.S. Department of Education; 2006.
- KFF. Figure 7. Percent of Nonelderly Adults Who do not have a Personal Doctor or Health Care Provider by Race/Ethnicity, 2021. Available online: https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/
- Gaskin DJ, Dinwiddie GY, Chan KS, et al. Residential segregation and the availability of primary care physicians. Health Serv Res 2012;47:2353-76. [Crossref] [PubMed]
- Hamel LM, Chapman R, Malloy M, et al. Critical Shortage of African American Medical Oncologists in the United States. J Clin Oncol 2015;33:3697-700. [Crossref] [PubMed]
- Flenaugh EL, Henriques-Forsythe MN. Lung cancer disparities in African Americans: health versus health care. Clin Chest Med 2006;27:431-9. vi. [Crossref] [PubMed]
- Cornelius ME, Loretan CG, Wang TW, et al. Tobacco Product Use Among Adults — United States, 2020. MMWR Morb Mortal Wkly Rep 2022;71:397-405. [Crossref] [PubMed]
- Kunitz SJ. Historical Influences on Contemporary Tobacco Use by Northern Plains and Southwestern American Indians. Am J Public Health 2016;106:246-55. [Crossref] [PubMed]
- Rhoades DA. Abstract IA-48: A brief overview of lung and colorectal cancer disparities in American Indian and Alaska Native populations. Cancer Epidemiol Biomarkers Prev 2022;31:IA-48.
- Isaacson MJ, Lynch AR. Culturally Relevant Palliative and End-of-Life Care for U.S. Indigenous Populations: An Integrative Review. J Transcult Nurs 2018;29:180-91. [Crossref] [PubMed]
- Profile: American Indian/Alaska Native. U.S. Department if Health and Human Services Office of Minority Health 2023.
- U.S Government Accountability Office. Indian Health Service: Spending Levels and Characteristics of IHS and Three Other Federal Health Care Programs. 2018. Available online: https://www.gao.gov/products/gao-19-74r
- Artiga S, Arguello R, Duckett P. Health Coverage and Care for American Indians and Alaska Natives. 2013. Available online: https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-and-care-for-american-indians-and-alaska-natives/
- Guadagnolo BA, Cina K, Helbig P, et al. Assessing cancer stage and screening disparities among Native American cancer patients. Public Health Rep 2009;124:79-89. [Crossref] [PubMed]
- Guadagnolo BA, Cina K, Helbig P, et al. Medical mistrust and less satisfaction with health care among Native Americans presenting for cancer treatment. J Health Care Poor Underserved 2009;20:210-26. [Crossref] [PubMed]
- KFF. Key Facts on Health and Health Care by Race and Ethnicity. Available online: https://www.kff.org/racial-equity-and-health-policy/press-release/key-facts-on-health-and-health-care-by-race-and-ethnicity/
- Siegel DA, Fedewa SA, Henley SJ, et al. Proportion of Never Smokers Among Men and Women With Lung Cancer in 7 US States. JAMA Oncol 2021;7:302-4. [Crossref] [PubMed]
- Xue Y, Jiang Y, Jin S, et al. Association between cooking oil fume exposure and lung cancer among Chinese nonsmoking women: a meta-analysis. Onco Targets Ther 2016;9:2987-92. [Crossref] [PubMed]
- Lebrett MB, Crosbie EJ, Smith MJ, et al. Targeting lung cancer screening to individuals at greatest risk: the role of genetic factors. J Med Genet 2021;58:217-26. [Crossref] [PubMed]
- Chang ET, Shema SJ, Wakelee HA, et al. Uncovering disparities in survival after non-small-cell lung cancer among Asian/Pacific Islander ethnic populations in California. Cancer Epidemiol Biomarkers Prev 2009;18:2248-55. [Crossref] [PubMed]
- AAMC. Figure 18. Percentage of all active physicians by race/ethnicity, 2018. In: Diversity in Medicine: Facts and Figures 2019. Available online: https://www.aamc.org/data-reports/workforce/data/figure-18-percentage-all-active-physicians-race/ethnicity-2018
- Chau V, Chan P. One size does not fit all: Appreciating the diversity of Asian Americans, Native Hawaiians, and Pacific Islanders (AANHPIs) and the Implications for Mental Health. 2021. Available online: https://www.samhsa.gov/blog/one-size-does-not-fit-all-appreciating-diversity-asian-americans-native-hawaiians-pacific
- Ta Park VM, Dougan MM, Meyer OL, et al. Discrimination Experiences during COVID-19 among a National, Multi-Lingual, Community-Based Sample of Asian Americans and Pacific Islanders: COMPASS Findings. Int J Environ Res Public Health 2022;19:924. [Crossref] [PubMed]
- Lee MC, Hinderer KA, Alexander CS. What Matters Most at the End-of-Life for Chinese Americans? Gerontol Geriatr Med 2018;4:2333721418778195. [Crossref] [PubMed]
- CDC. Fast Facts and Fact Sheets. 2020. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm#:~:text=CDC%20recommends%20that%20states%20spend%2012%25%20of%20those,day%2C%20about%201%2C600%20youth%20try%20their%20first%20cigarette
- Percac-Lima S, Ashburner JM, Atlas SJ, et al. Barriers to and Interest in Lung Cancer Screening Among Latino and Non-Latino Current and Former Smokers. J Immigr Minor Health 2019;21:1313-24. [Crossref] [PubMed]
- Fedewa SA, Kazerooni EA, Studts JL, et al. State Variation in Low-Dose Computed Tomography Scanning for Lung Cancer Screening in the United States. J Natl Cancer Inst 2021;113:1044-52. [Crossref] [PubMed]
- Ramirez AG, Wildes K, Talavera G, et al. Clinical trials attitudes and practices of Latino physicians. Contemp Clin Trials 2008;29:482-92. [Crossref] [PubMed]
- Chen Y, Criss SD, Watson TR, et al. Cost and Utilization of Lung Cancer End-of-Life Care Among Racial-Ethnic Minority Groups in the United States. Oncologist 2020;25:e120-9. [Crossref] [PubMed]
- Saphire ML, Prsic EH, Canavan ME, et al. Patterns of Symptom Management Medication Receipt at End-of-Life Among Medicare Beneficiaries With Lung Cancer. J Pain Symptom Manage 2020;59:767-777.e1. [Crossref] [PubMed]
- KFF. Figure 2. Health Coverage of Nonelderly Population by Race and Ethnicity, 2021 and 2022. Available online: https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity/
- KFF. Figure 3. Health Coverage of the Nonelderly Population by Race/Ethnicity and Medicaid Expansion Status, 2022. Available online: https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity/
- Hacker K, Anies M, Folb BL, et al. Barriers to health care for undocumented immigrants: a literature review. Risk Manag Healthc Policy 2015;8:175-83. [Crossref] [PubMed]
- KFF. Figure 43. Percent of Individuals Living in a Household Where No One Ages 14 and Older Speaks English Well by Race/Ethnicity. Available online: https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/
- AAMC. Table 13. Practice specialty, males by race/ethnicity, 2018. Diversity in Medicine: Facts and Figures 2019. Available online: https://www.aamc.org/data-reports/workforce/data/table-13-practice-specialty-males-race/ethnicity-2018
- Sidorchuk A, Agardh EE, Aremu O, et al. Socioeconomic differences in lung cancer incidence: a systematic review and meta-analysis. Cancer Causes Control 2009;20:459-71. [Crossref] [PubMed]
- Castro S, Sosa E, Lozano V, et al. The impact of income and education on lung cancer screening utilization, eligibility, and outcomes: a narrative review of socioeconomic disparities in lung cancer screening. J Thorac Dis 2021;13:3745-57. [Crossref] [PubMed]
- Forrest LF, Adams J, Wareham H, et al. Socioeconomic inequalities in lung cancer treatment: systematic review and meta-analysis. PLoS Med 2013;10:e1001376. [Crossref] [PubMed]
- Osarogiagbon RU, Sineshaw HM, Unger JM, et al. Immune-Based Cancer Treatment: Addressing Disparities in Access and Outcomes. Am Soc Clin Oncol Educ Book 2021;41:1-13. [Crossref] [PubMed]
- Lin Y, Mak KS. Racial and socioeconomic disparities in the use of stereotactic body radiotherapy for treating non-small cell lung cancer: a narrative review. J Thorac Dis 2021;13:3764-71. [Crossref] [PubMed]
- Norris RP, Dew R, Sharp L, et al. Are there socio-economic inequalities in utilization of predictive biomarker tests and biological and precision therapies for cancer? A systematic review and meta-analysis. BMC Med 2020;18:282. [Crossref] [PubMed]
- Figure 3. Disparities in Lung Cancer Throughout the Patient Pathway. In: Bhavaraju N, Nanni J, Carlson C, et al. Breaking the Barriers to Specialty Care. FSG; 2016.
- Zou X, Wang R, Yang Z, et al. Family Socioeconomic Position and Lung Cancer Risk: A Meta-Analysis and a Mendelian Randomization Study. Front Public Health 2022;10:780538. [Crossref] [PubMed]
- KFF. Figure 7. Percent of Nonelderly Adults who did not See a Doctor Due to Cost in the Past 12 Months by Race/Ethnicity, 2021. Available online: https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/
- Zahnd WE, Fogleman AJ, Jenkins WD. Rural-Urban Disparities in Stage of Diagnosis Among Cancers With Preventive Opportunities. Am J Prev Med 2018;54:688-98. [Crossref] [PubMed]
- Ray M, Faris NR, Derrick A, et al. The relative impact of patient and institutional rurality on lung cancer disparities. J Clin Oncol 2019;37:e20052.
- Johnson AM, Hines RB, Johnson JA 3rd, et al. Treatment and survival disparities in lung cancer: the effect of social environment and place of residence. Lung Cancer 2014;83:401-7. [Crossref] [PubMed]
- Loehrer AP, Chen L, Wang Q, et al. Rural Disparities in Lung Cancer-directed Surgery: A Medicare Cohort Study. Ann Surg 2023;277:e657-63. [Crossref] [PubMed]
- Fairfield KM, Black AW, Lucas FL, et al. Association Between Rurality and Lung Cancer Treatment Characteristics and Timeliness. J Rural Health 2019;35:560-5. [Crossref] [PubMed]
- Logan CD, Feinglass J, Halverson AL, et al. Rural-urban survival disparities for patients with surgically treated lung cancer. J Surg Oncol 2022;126:1341-9. [Crossref] [PubMed]
- Johnson AM, Johnson A, Hines RB, et al. Neighborhood context and non-small cell lung cancer outcomes in Florida non-elderly patients by race/ethnicity. Lung Cancer 2020;142:20-7. [Crossref] [PubMed]
- Huo J, Hong YR, Turner K, et al. Geographic variation in palliative care delivery among patients diagnosed with metastatic lung cancer in the USA: Medicare population-based study. Support Care Cancer 2021;29:813-21. [Crossref] [PubMed]
- U.S. Government Accountability Office. Targeting Federal Funds: Information on Funding to Areas with Persistent or High Poverty. 2020. Available online: https://www.gao.gov/products/gao-20-518
- Yabroff KR, Han X, Zhao J, et al. Rural Cancer Disparities in the United States: A Multilevel Framework to Improve Access to Care and Patient Outcomes. JCO Oncol Pract 2020;16:409-13. [Crossref] [PubMed]
- Turrini G, Branham DK, Chen L, et al. Access to Affordable Care in Rural America: Current Trends and Key Challenges. 2021. Available online: https://aspe.hhs.gov/sites/default/files/2021-07/rural-health-rr.pdf
- Eberth JM, Bozorgi P, Lebrón LM, et al. Geographic Availability of Low-Dose Computed Tomography for Lung Cancer Screening in the United States, 2017. Prev Chronic Dis 2018;15:E119. [Crossref] [PubMed]
- American Lung Association. Special concerns in tobacco growing regions. 2012.
- Lin CC, Bruinooge SS, Kirkwood MK, et al. Association Between Geographic Access to Cancer Care, Insurance, and Receipt of Chemotherapy: Geographic Distribution of Oncologists and Travel Distance. J Clin Oncol 2015;33:3177-85. [Crossref] [PubMed]
- Unger JM, Moseley A, Symington B, et al. Geographic Distribution and Survival Outcomes for Rural Patients With Cancer Treated in Clinical Trials. JAMA Netw Open 2018;1:e181235. [Crossref] [PubMed]
- Virani S, Burke L, Remick SC, et al. Barriers to recruitment of rural patients in cancer clinical trials. J Oncol Pract 2011;7:172-7. [Crossref] [PubMed]
- Mudaranthakam DP, Gajewski B, Krebill H, et al. Barriers to Clinical Trial Participation: Comparative Study Between Rural and Urban Participants. JMIR Cancer 2022;8:e33240. [Crossref] [PubMed]
- Gong G, Phillips SG, Hudson C, et al. Higher US Rural Mortality Rates Linked To Socioeconomic Status, Physician Shortages, And Lack Of Health Insurance. Health Aff (Millwood) 2019;38:2003-10. [Crossref] [PubMed]
- Kaufman BG, Thomas SR, Randolph RK, et al. The Rising Rate of Rural Hospital Closures. J Rural Health 2016;32:35-43. [Crossref] [PubMed]
- American Lung Association. Lung Disease and LGBTQ+ Communities. 2021. Available online: https://www.lung.org/blog/lung-disease-lqbtq
- Acosta-Deprez V, Jou J, London M, et al. Tobacco Control as an LGBTQ+ Issue: Knowledge, Attitudes, and Recommendations from LGBTQ+ Community Leaders. Int J Environ Res Public Health 2021;18:5546. [Crossref] [PubMed]
- Xie H, Li Y, Wang Q, et al. MA04.02 Lung Cancer Screening Utilization and Its Correlates in Sexual Minorities: An Analysis of the BRFSS 2018. J Thorac Oncol 2021;16:S144.
- Liu L, Rwigema JN, Song Z, et al. Assessing Disparities in Lung Cancer Incidence for Gender Minority Individuals Using California Cancer Registry Data. Chest 2021;159:2491-3. [Crossref] [PubMed]
- Jabson JM, Blosnich JR. Representation of lesbian, gay, and bisexual people in clinical cancer trials. Ann Epidemiol 2012;22:821-3. [Crossref] [PubMed]
- Boehmer U, Ozonoff A, Miao X. An ecological approach to examine lung cancer disparities due to sexual orientation. Public Health 2012;126:605-12. [Crossref] [PubMed]
- Bosworth A, Turrini G, Pyda S, et al. Table 5: Healthcare Access by Sexual Orientation. Available online: https://aspe.hhs.gov/sites/default/files/2021-07/lgbt-health-ib.pdf
- Alencar Albuquerque G, de Lima Garcia C, da Silva Quirino G, et al. Access to health services by lesbian, gay, bisexual, and transgender persons: systematic literature review. BMC Int Health Hum Rights 2016;16:2. [Crossref] [PubMed]
- Center for American Progress. Figure 3. Shares of transgender and cisgender heterosexual adults who reported poor treatment in various spaces, 2016–2018. In: Medina C, Santos T, Mahowald L, et al. Protecting and Advancing Health Care for Transgender Adult Communities. Available online: https://www.americanprogress.org/wp-content/uploads/sites/2/2021/08/Advancing-Health-Care-For-Transgender-Adults.pdf
- Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA 2011;306:971-7. [Crossref] [PubMed]
- Women's Health Access Matter. Did you know? Available online: https://thewhamreport.org/report/lung/
- Ragavan M, Patel MI. The evolving landscape of sex-based differences in lung cancer: a distinct disease in women. Eur Respir Rev 2022;31:210100. [Crossref] [PubMed]
- Warner ET, Lathan CS. Race and sex differences in patient provider communication and awareness of lung cancer screening in the health information National Trends Survey, 2013-2017. Prev Med 2019;124:84-90. [Crossref] [PubMed]
- Xiao D, Pan H, Li F, et al. Analysis of ultra-deep targeted sequencing reveals mutation burden is associated with gender and clinical outcome in lung adenocarcinoma. Oncotarget 2016;7:22857-64. [Crossref] [PubMed]
- Barrera-Rodriguez R, Morales-Fuentes J. Lung cancer in women. Lung Cancer (Auckl) 2012;3:79-89. [Crossref] [PubMed]
- SEER. Lung and Bronchus Recent Trends in SEER Relative Survival Rates, 2000-2018.
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004;291:2720-6. [Crossref] [PubMed]
- Lung Cancer Center. Risk of Lung Cancer in the Military: 2023. Available online: https://www.lungcancercenter.com/who-lung-cancer-affects/military/
- Nations JA, Brown DW, Shao S, et al. Comparative Trends in the Distribution of Lung Cancer Stage at Diagnosis in the Department of Defense Cancer Registry and the Surveillance, Epidemiology, and End Results data, 1989-2012. Mil Med 2020;185:e2044-8. [Crossref] [PubMed]
- Núñez ER, Caverly TJ, Zhang S, et al. Adherence to Follow-up Testing Recommendations in US Veterans Screened for Lung Cancer, 2015-2019. JAMA Netw Open 2021;4:e2116233. [Crossref] [PubMed]
- Heiden BT, Eaton DB Jr, Chang SH, et al. Comparison Between Veteran and Non-Veteran Populations With Clinical Stage I Non-small Cell Lung Cancer Undergoing Surgery. Ann Surg 2023;277:e664-9. [Crossref] [PubMed]
- Farmer CM, Hosek SD, Adamson DM. Balancing Demand and Supply for Veterans' Health Care: A Summary of Three RAND Assessments Conducted Under the Veterans Choice Act. Rand Health Q 2016;6:12.
- Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021;7:3. [Crossref] [PubMed]
- Varghese AM, Zakowski MF, Yu HA, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014;9:892-6. [Crossref] [PubMed]
- Small cell carcinoma of the Lung and Bronchus Stage Distribution of SEER Incidence Cases, 2011-2020. SEER. 2020. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=611&data_type=1&graph_type=4&compareBy=sex&chk_sex_1=1&race=1&age_range=1&advopt_precision=1&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0
- Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:300-11.
- Ramirez RA, Boyd M, Cockrum P. Trends in treatment patterns associated with small cell lung cancer in a U.S. Medicare population. J Clinic Oncol 2022;40:8584.
- American Lung Association. Lack of Treatment. 2022. Available online: https://www.lung.org/research/state-of-lung-cancer/key-findings#:~:text=Nationally%2C%2021%25%20of%20cases%20did%20not%20receive%20any%20treatment.%20Treatment%20rates%20improved%2015%25%20over%20the%20last%20five%20years%20and%20were%20best%20in%20North%20Dakota%20(14%25)%20and%20worst%20in%20Arizona%20(32%25)
- Liguori NR, Lee Y, Borges W, et al. Absence of Biomarker-Driven Treatment Options in Small Cell Lung Cancer, and Selected Preclinical Candidates for Next Generation Combination Therapies. Front Pharmacol 2021;12:747180. [Crossref] [PubMed]
- Roof L, Wei W, Stevenson JP. HSR22-166: Patient Demographic and Socioeconomic Differences in Non-Small Cell and Small Cell Lung Cancers: Impact on Outcomes. A National Cancer Database Analysis. Journal of the National Comprehensive Cancer Network ; [Crossref]
- American Lung Association. Lung Cancer Key Findings. 2022. Available online: https://www.lung.org/research/state-of-lung-cancer/key-findings



