
A real-world study of pneumonitis in non-small cell lung cancer patients receiving durvalumab following concurrent chemoradiation
Highlight box
Key findings
• Pneumonitis secondary to durvalumab administration in patients with non-small cell lung cancer does not impact overall survival or progression-free survival.
• Obesity is a significant predictor of pneumonitis in this patient population.
What is known and what is new?
• Incidence of pneumonitis is higher in real world patients receiving durvalumab than the PACIFIC trial and causes discontinuation of therapy.
• Pneumonitis secondary to durvalumab does not lead to higher rates of death.
• Patients with a higher body mass index (BMI) are more likely to develop pneumonitis secondary to durvalumab therapy.
What is the implication, and what should change now?
• Incident pneumonitis was not associated with increased risk of death in this population, even among patients with severe chronic obstructive pulmonary disease. Higher risks of pneumonitis should be discussed with patients with a high BMI.
Introduction
Locally advanced non-small cell lung cancer (LA-NSCLC) comprises approximately one-third of new NSCLC diagnoses. After the results of the PACIFIC trial, unresectable disease is treated with definitive intent concurrent chemoradiation (CRT) followed by the programmed death-ligand 1 (PD-L1) inhibitor durvalumab given higher overall survival (OS) and progression-free survival (PFS) with the addition of durvalumab (1-3). As such, durvalumab following completion of CRT has become standard of care.
Immune checkpoint inhibitors are associated with a variety of complications. In patients receiving durvalumab for NSCLC, pneumonitis has been shown to be one of the most common (4). This inflammatory condition of the lungs is a potentially fatal adverse effect of immunotherapy or radiation therapy, therefore the risk of pneumonitis is a key concern. The incidence of clinically important, grade 3/4 pneumonitis in the landmark PACIFIC trial was 3.4% (compared to 2.6% with placebo), and the rate of any grade pneumonitis was 19% (1). In clinical practice, the observed rate of clinically significant pneumonitis is higher (5). Smaller real-world cohorts have reported the rate of any grade pneumonitis as anywhere from 19–35% (6-8). The reported incidence of clinically significant grade 3 or higher pneumonitis has been reported from 6% to 15% in smaller real-world cohorts and larger meta-analyses (7,9,10). Death from pneumonitis (grade 5 toxicity) while rare (reported incidence around 1%) represents a serious consequence of therapy (11). Altogether these studies suggest that pneumonitis is more common in real-world settings than reported in clinical trials. Our study sought to explore the frequency of pneumonitis, the impact of pneumonitis on survival, and explore clinical predictors of pneumonitis in a real-world cohort of US veterans.
Previous studies have evaluated some clinical and laboratory predictors of pneumonitis. The following characteristics including chemotherapy choice (6), lung volume receiving >20 Gy (V20), sex, age, smoking status, presence of baseline pneumonitis, type of radiation, location of lesion (12), PD-L1 expression (13), dose of durvalumab, Eastern Cooperative Oncology Group (ECOG) performance status, histology, time between radiation and durvalumab, relevant co-morbidities (14), and Brinkman index (15) have not been found to be predictive of pneumonitis. There has been some incongruity in the literature however as some studies have found that V20, V40, V5, mean lung dose, and history of pneumonitis prior to durvalumab administration (16) are risk factors for grade 2 or greater pneumonitis, while others have found the opposite (11,17-19). Taken together, reliable clinical and laboratory markers predictive of pneumonitis development have not been consistently reported. No large multi-institutional studies have evaluated chronic obstructive pulmonary disease (COPD) severity, though in a single institution presence of baseline COPD was found to be a risk factor for developing pneumonitis (20). Body mass index (BMI) has never been evaluated as a potential clinical predictor of pneumonitis development.
The clinical impact of pneumonitis on patients receiving durvalumab is that they have higher rates of discontinuation of therapy (5,21). Both discontinuation of therapy and development of pneumonitis may impact survival. One real world study showed that patients that experienced any grade pneumonitis had a lower 12-month OS, while others have shown that grade 2 or higher pneumonitis does not appear to be associated with worse OS or PFS (10,21). Given the high incidence of pneumonitis in this patient population and unclear impact on survival, this study evaluates the incidence of pneumonitis and survival in a large nationwide real-world cohort of United States Veterans. This will become increasingly important in order to help make decisions about continuing vs. stopping durvalumab therapy in patients with pneumonitis. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1604/rc).
Methods
Veterans receiving durvalumab between 12/5/2017 and 4/15/2020 were identified using VA Informatics and Computing Infrastructure (VINCI) data services. Only patients with NSCLC who received CRT and at least once dose of durvalumab were included. Individual patient records were reviewed using the Compensation and Pension Records Interchange software system/Joint Legacy Viewer. Patients were followed through 9/14/2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Veterans Affairs St. Louis Healthcare System Institutional Review Boards (IRB No. 1625948-6) and individual consent for this retrospective analysis was waived.
This is a multi-center, population-based, retrospective cohort study evaluating patients with NSCLC treated with durvalumab following completion of concurrent CRT. Oncologic treatment history including chemotherapy received, dates and doses of durvalumab administration, radiation treatment history, date of progression, pulmonary function tests, PD-L1 percentage, BMI, and date of death were recorded through manual chart review (N.A. and T.S.T.).
The primary outcome of interest was development of clinical pneumonitis. This was assessed through review of documentation from oncology and pulmonology providers. Imaging reports were reviewed to assess for the presence of infiltrates. Pneumonitis grade was obtained directly from the medical record when available. If missing, clinical documents in combination of prescription of corticosteroids and supplemental oxygen administration were interpreted and graded using Common Terminology Criteria for Adverse Events (CTCAE) 4.0 criteria. Patients with new radiographic infiltrates without documented clinical pneumonitis are considered asymptomatic, potential pneumonitis patients. Receipt of corticosteroids was confirmed through pharmacy records. COPD severity was graded based on the American Thoracic Society categories from 2005. The Romano adaptation of the Charlson comorbidity index was calculated using International Classification of Diseases, Ninth Revision (ICD-9) codes to develop a composite comorbidity score (22).
Statistical analyses
Univariate logistic regression analysis evaluated for associations between age, race, Romano co-morbidity score, chemotherapy regimen, COPD severity [determined by recorded forced expiratory volume in 1 second (FEV1)], BMI, and development of clinical pneumonitis. Cox proportional hazards analysis was used to estimate hazard ratios for risk of death up to 1 and 2 years from durvalumab start date and controlling for potentially confounding variables (age, clinical stage, co-morbidities, and pneumonitis). Cox proportional hazards analysis was performed to estimate hazard ratios to evaluate risk of death at 2 years including age, co-morbidities, race, chemotherapy, time from radiation to durvalumab initiation, COPD severity, and obesity (<30 vs. ≥30 kg/m2 BMI). PFS and OS (stratified by clinical pneumonitis) were evaluated using Kaplan-Meier methods. Survival curves were made using date of first durvalumab through 9/14/2021 as the specified time period. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
There were 284 patients with NSCLC at the Veterans Health Administration (VHA) who received CRT followed by durvalumab between 12/5/2017 and 4/15/2020. Of these patients, 1 was stage I, 21 were stage II, 228 were stage III, the rest were unknown (Table 1). The majority of patients had either adenocarcinoma (125 patients) or squamous cell carcinoma (132 patients). The median age at diagnosis was 68 for all patients included. Of the patients that PD-L1 expression was checked and recorded (112 patients), 39 had <1% PD-L1 expression, 41 had between 11% and 49% expression, and 32 had greater than 50% expression. Regarding co-morbidities, of this patient population 33% had type 2 diabetes, 10% had renal failure, and 18% had a prior autoimmune disease. Baseline FEV1 data was also collected on these patients. Fifty-one patients had a normal FEV1, 33 had a mild impairment, 31 had a moderate impairment, 37 had a moderately severe impairment, 31 had a severe impairment and 17 had a very severe impairment, 84 were unknown. Chemotherapy regimen and radiation dose are summarized in Table 1. Carboplatin and paclitaxel was administered most commonly to 80% of patients. In total, 59% of patients received >54 Gy of radiation. Durvalumab was completed in 101 (35%) of patients and was ongoing at time of review in 2 patients (1%). Durvalumab was discontinued due to disease progression in 84 patients (30%) and toxicity in 59 patients (21%).
Table 1
| Characteristics | Value (n=284) |
|---|---|
| Age (years), median [range] | 68 [39–88] |
| Male, n [%] | 271 [96] |
| Charlson score, mean | 4.6 |
| BMI (kg/m2), median | 26.0 |
| BMI (kg/m2), n [%] | |
| ≥30 | 67 [24] |
| <30 | 217 [76] |
| Stage, n [%] | |
| I | 1 [<1] |
| II | 21 [7] |
| III | 228 [80] |
| Unknown | 35 [12] |
| Histology, n [%] | |
| Adenocarcinoma | 125 [44] |
| Squamous cell | 132 [46] |
| Poorly differentiated | 21 [7] |
| Mixed | 1 [<1] |
| Large cell | 2 [1] |
| NOS | 2 [1] |
| PD-L1 expression, n [%] | |
| <1% | 39 [14] |
| 11–49% | 41 [14] |
| >50% | 32 [11] |
| Unknown | 172 [61] |
| PFTs, n [%] | |
| No impairment | 51 [18] |
| Mild (FEV1 70–79%) | 33 [11] |
| Moderate (FEV1 60–69%) | 31 [11] |
| Moderately severe (FEV1 50–59%) | 37 [13] |
| Severe (FEV1 35–49%) | 31 [11] |
| Very severe (FEV1 <35%) | 17 [6] |
| Unknown | 84 [30] |
| Chemotherapy regimen, n [%] | |
| Carboplatin/paclitaxel | 226 [80] |
| Platinum/pemetrexed | 25 [9] |
| Platinum/etoposide | 20 [7] |
| Platinum/vinorelbine | 1 [<1] |
| Unknown or none | 12 [4] |
| Radiation dose, n [%] | |
| <54 Gy | 9 [3] |
| 54–66 Gy | 140 [49] |
| >66 Gy | 28 [10] |
| Unknown | 107 [38] |
| Reason for durvalumab discontinuation, n [%] | |
| Therapy completed | 101 [35] |
| Progression | 84 [30] |
| Toxicity | 59 [21] |
| Patient decision | 23 [8] |
| Death | 128 [45] |
| Therapy ongoing | 2 [1] |
| Unknown | 4 [1] |
BMI, body mass index; NOS, not otherwise specified; PD-L1, programmed death-ligand 1; PFTs, pulmonary function tests; FEV1, forced expiratory volume in 1 second.
All reported toxicities are summarized in Table 2. Sixty-one patients developed clinically significant pneumonitis, defined as grade 2 or higher. One hundred and six patients developed imaging changes possibly consistent with pneumonitis, but of these only 9 were clinically defined as grade 1 pneumonitis in electronic medical record (EMR) notes. Of the total cohort 9% developed grade 2 pneumonitis, 9% developed grade 3 pneumonitis, 1% developed grade 4 pneumonitis, and 2% developed grade 5 pneumonitis. Most patients who developed pneumonitis did not resume durvalumab. Nineteen patients (27%) who developed clinically significant pneumonitis were re-challenged. Of these re-challenged patients, 15 patients (79%) tolerated durvalumab without re-developing pneumonitis. Two patients got pneumonitis a second time and therapy was stopped again. Other toxicities noted in this study were endocrinopathies (13 patients), hepatitis (2 patients), colitis (2 patients), hypersensitivity reactions (5 patients), and arthritis (1 patient). Characteristics of patients that developed grade 5 pneumonitis are shown in Table 3. Of note, median time to pneumonitis in these patients was 26 days and the majority were treated with steroids.
Table 2
| Toxicity | Value (n=284) |
|---|---|
| Pneumonitis, n [%] | |
| Grade 2 | 25 [9] |
| Grade 3 | 26 [9] |
| Grade 4 | 3 [1] |
| Grade 5 | 7 [2] |
| Hepatitis, n [%] | 2 [1] |
| Endocrinopathy, n [%] | 13 [5] |
| Colitis, n [%] | 2 [1] |
| Hypersensitivity reaction, n [%] | 5 [2] |
| Arthralgia, n [%] | 1 [<1] |
Table 3
| No. | Stage | Histology | Time to pneumonitis (days) | Treatment | Other irAE |
|---|---|---|---|---|---|
| 1 | T3N2M0 | SCC | 28 | Steroids + infliximab | Arthritis |
| 2 | T4NxMx | SCC | 209 | Steroids | No |
| 3 | T2N2M0 | Adeno | 26 | Unknown | No |
| 4 | T0N3M0 | Adeno | 6 | Steroids | No |
| 5 | Unknown | SCC | 20 | Steroids | No |
| 6 | T4N2M0 | Mixed (SCC + adeno) | 20 | Steroids | No |
| 7 | T3N2M0 | SCC | 28 | Infliximab | No |
irAE, immune related adverse event; SCC, squamous cell carcinoma; adeno, adenocarcinoma.
The median OS in patients that developed pneumonitis (including 9 defined as grade 1), compared to those who did not was 27.8 and 36.9 months (P=0.22), respectively (Figure 1). Similarly PFS was not significantly different in patients who developed pneumonitis vs. those that did not (14.4 vs. 17.4 months, P=0.38) (Figure 2). Our study additionally looked at clinical and laboratory predictors of pneumonitis and found that patients with a BMI ≥30 kg/m2 were more likely to develop pneumonitis than patients with a BMI <30 kg/m2 [odds ratio (OR): 1.87; 95% confidence interval (CI): 1.01 to 3.47; P=0.04]. Other clinical predictors including COPD severity, race, age at durvalumab start date, chemotherapy regimen, and Romano comorbidity score were not significant predictors of pneumonitis (Table 4). Cox proportional hazards analysis failed to demonstrate an association between the development of pneumonitis and risk of death. Our study also evaluated age, co-morbidities, race, chemotherapy, time from radiation to durvalumab initiation, COPD Severity, and obesity (<30 vs. ≥30 kg/m2 BMI) as risk factors for death at 2 years and found that they were not clinically significant predictors.

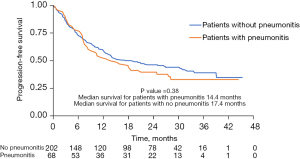
Table 4
| Characteristics | Risk factors | OR | 95% CI |
|---|---|---|---|
| BMI (kg/m2) | ≥30 (vs. <30) | 1.87 | 1.01–3.47 |
| Age (years) | 60–69 (vs. <60) | 1.15 | 0.43–3.10 |
| >70 (vs. <60) | 1.67 | 0.63–4.46 | |
| Co-morbidities | Romano co-morbidity index | 1.03 | 0.93–1.15 |
| Race | Black (vs. White) | 0.81 | 0.38–1.74 |
| Other (vs. White) | 0.97 | 0.10–9.52 | |
| Chemotherapy | Platinum + pemetrexed (vs. carbo/taxol) | 2.43 | 1.02–5.82 |
| Platinum + etoposide (vs. carbo/taxol) | 1.46 | 0.53–4.00 | |
| Time from radiation to durvalumab initiation (days) | 30 (vs. 31–45) | 1.26 | 0.56–2.87 |
| 46–60 (vs. 31–45) | 1.94 | 0.85–4.39 | |
| 61 (vs. 31–45) | 1.46 | 0.67–3.18 | |
| COPD severity | Mild impairment (vs. none) | 2.29 | 0.87–6.00 |
| Moderate impairment (vs. none) | 0.66 | 0.21–2.11 | |
| Moderate to severe impairment (vs. none) | 1.79 | 0.70–4.58 | |
| Severe impairment (vs. none) | 0.86 | 0.28–2.63 | |
| Very severe impairment (vs. none) | 0.97 | 0.27–3.56 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Discussion
In this multi-center, population-based retrospective cohort study of US veterans with NSCLC treated with durvalumab, the incidence of clinically significant pneumonitis (defined as grade 2 or higher) was 21% which included 13% with grade 3 or higher pneumonitis. In the landmark PACIFIC trial, grade 3/4 pneumonitis occurred in 3.4% of patients (2). Prior studies have similarly demonstrated a higher incidence of pneumonitis then reported in clinical trials (7,9,10). Interestingly, in our cohort, 7 patients had grade 5 pneumonitis and died from these complications. The PACIFIC trial did not report grade 5 pneumonitis (1). Our study also shows a higher rate of grade 5 pneumonitis than what has been reported in other real-world studies, with others reporting the rate of grade 5 pneumonitis around 1% (11). This highlights the fact that in real-world populations, pneumonitis is more prevalent and more severe. This may be due to the fact that real-world populations include a more frail patient population with more co-morbidities than clinical trial populations. However, our study shows that pneumonitis does not affect OS or PFS across all patients. The current literature has not been consistent if pneumonitis has an effect on survival, our study is one the largest populations in which survival has been evaluated, with no clear association found between OS and pneumonitis incidence among this population.
Our study also found that obesity is a predictor of pneumonitis development in patients receiving durvalumab. Patients with a BMI ≥30 kg/m2 had a significantly increased risk of developing pneumonitis. Obesity has not been previously evaluated as a clinical predictor of pneumonitis in this patient population. This suggests that providers should monitor for development of pneumonitis when administering durvalumab in patients with elevated BMIs. The mechanism of this is not yet known, however, a study by Katsui et al. suggests dysfunctional adipocytes in obese patients have increased adipocytokine production including interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), leading to a state of chronic inflammation (23). This baseline elevated cytokine production may lower the threshold needed to cause the inflammatory response for durvalumab mediated pneumonitis. Other clinical predictors evaluated in this study including COPD severity, race, age at durvalumab start date, chemotherapy regimen, and Romano comorbidity score were not found to be significant predictors of pneumonitis development. COPD severity has not been previously evaluated as a predictor of pneumonitis in this population, however it does not appear to be a risk factor, meaning a severe impairment should not prohibit clinicians from administering durvalumab to these patients. This is in contrast to Thomas et al. (20) who showed that baseline COPD was a risk factor in the development of radiation of immune related pneumonitis in their cohort of 39 patients. Our study shows in a larger patient population and taking into account the severity of COPD, this risk factor does not have an impact of the development of pneumonitis.
Together this study confirms higher rates of clinically significant pneumonitis in a multi-center real world population that other studies have shown. This study also suggests that the risk of potentially fatal pneumonitis is not minimal, and clinicians should carefully discuss this risk with individual patients while closely monitoring for this potentially deadly adverse event. Our study found there is no association between pneumonitis and risk of death up to 1 or 2 years when age, cancer stage and co-morbidities are taken into account. However, durvalumab was discontinued in most patients who developed pneumonitis. Whether there will be long term impacts on survival due to this discontinuation requires longer follow up. There was no difference in PFS suggesting that disease control was not impacted by the discontinuation of durvalumab.
Additionally, our study reports 5 patients who had a hypersensitivity reaction (2%) while receiving durvalumab. The incidence of hypersensitivity reactions has not been previously reported in the literature in NSCLC patients receiving durvalumab to our knowledge. This is clinically significant as two reactions resulted in hospitalizations. Hypersensitivity reactions to durvalumab are potentially under recognized. This is an important aspect of treatment that clinicians should be aware of as it is treatable with prompt interventions during infusion.
This study included patients that were majority male given the patient population was patients receiving care at the VA, meaning this data may not be generalizable to a female patient population. Additionally, grade 1 immune related pneumonitis and radiation pneumonitis do not have clear guidelines to differentiate the two, meaning our study may have underestimated the prevalence of grade 1 pneumonitis due to durvalumab therapy in this patient population. Additionally, our study may have included pneumonitis secondary to radiation vs. durvalumab for the same reason.
Conclusions
In conclusion, this study confirms that rates of clinically significant pneumonitis are higher than noted in the PACIFIC trial in NSCLC patients receiving durvalumab. However as has been inconsistent in the literature, this high rate of pneumonitis does not have an impact on OS or PFS. This study also found obesity (defined as a BMI ≥30 kg/m2) to be a clinical predictor of pneumonitis.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1604/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1604/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1604/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1604/coif). K.M.S. is an Expert Case Review for Quinn Johnston, not related to the published work, American Society of Hematology Junior Faculty Scholar Award, Research Funds Paid to the institution, not related to the published work and a Consultant for Health Services Advisory Group, not related to the published work. J.W.K.’s work is supported by the Congressionally Directed Medical Research Program (Department of Defense) Career Development Award #W81XWH-20-1-0785, CA191184 [2020–2023]. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Veterans Affairs St. Louis Healthcare System Institutional Review Boards (IRB No. 1625948-6) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021;16:860-7. [Crossref] [PubMed]
- Zhang W, Gu J, Bian C, et al. Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Advanced Non-small Cell Lung Cancer: A Network Meta-Analysis of Randomized Clinical Trials. Front Pharmacol 2021;12:686876. [Crossref] [PubMed]
- Sankar K, Bryant AK, Strohbehn GW, et al. Real World Outcomes versus Clinical Trial Results of Durvalumab Maintenance in Veterans with Stage III Non-Small Cell Lung Cancer. Cancers (Basel) 2022;14:614. [Crossref] [PubMed]
- Hassanzadeh C, Sita T, Savoor R, et al. Implications of pneumonitis after chemoradiation and durvalumab for locally advanced non-small cell lung cancer. J Thorac Dis 2020;12:6690-700. [Crossref] [PubMed]
- Wang Y, Zhang T, Huang Y, et al. Real-World Safety and Efficacy of Consolidation Durvalumab After Chemoradiation Therapy for Stage III Non-small Cell Lung Cancer: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys 2022;112:1154-64. [Crossref] [PubMed]
- Voong KR, Hazell SZ, Fu W, et al. Relationship Between Prior Radiotherapy and Checkpoint-Inhibitor Pneumonitis in Patients With Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer 2019;20:e470-9. [Crossref] [PubMed]
- Sugimoto T, Fujimoto D, Sato Y, et al. Durvalumab for patients with unresectable stage III non-small cell lung cancer and grade 1 radiation pneumonitis following concurrent chemoradiotherapy: a multicenter prospective cohort study. Invest New Drugs 2021;39:853-9. [Crossref] [PubMed]
- Desilets A, Blanc-Durand F, Lau S, et al. Durvalumab therapy following chemoradiation compared with a historical cohort treated with chemoradiation alone in patients with stage III non-small cell lung cancer: A real-world multicentre study. Eur J Cancer 2021;142:83-91. [Crossref] [PubMed]
- Saito G, Oya Y, Taniguchi Y, et al. Real-world survey of pneumonitis and its impact on durvalumab consolidation therapy in patients with non-small cell lung cancer who received chemoradiotherapy after durvalumab approval (HOPE-005/CRIMSON). Lung Cancer 2021;161:86-93. [Crossref] [PubMed]
- Inoue H, Ono A, Kawabata T, et al. Clinical and radiation dose-volume factors related to pneumonitis after treatment with radiation and durvalumab in locally advanced non-small cell lung cancer. Invest New Drugs 2020;38:1612-7. [Crossref] [PubMed]
- Shaverdian N, Thor M, Shepherd AF, et al. Radiation pneumonitis in lung cancer patients treated with chemoradiation plus durvalumab. Cancer Med 2020;9:4622-31. [Crossref] [PubMed]
- LeClair JN, Merl MY, Cohenuram M, et al. Real-World Incidence of Pneumonitis in Patients Receiving Durvalumab. Clin Lung Cancer 2022;23:34-42. [Crossref] [PubMed]
- Saito S, Abe T, Kobayashi N, et al. Incidence and dose-volume relationship of radiation pneumonitis after concurrent chemoradiotherapy followed by durvalumab for locally advanced non-small cell lung cancer. Clin Transl Radiat Oncol 2020;23:85-8. [Crossref] [PubMed]
- Nishimura A, Ono A, Wakuda K, et al. Prognostic impact of pneumonitis after durvalumab therapy in patients with locally advanced non-small cell lung cancer. Invest New Drugs 2022;40:403-10. [Crossref] [PubMed]
- Shintani T, Kishi N, Matsuo Y, et al. Incidence and Risk Factors of Symptomatic Radiation Pneumonitis in Non-Small-Cell Lung Cancer Patients Treated with Concurrent Chemoradiotherapy and Consolidation Durvalumab. Clin Lung Cancer 2021;22:401-10. [Crossref] [PubMed]
- Mayahara H, Uehara K, Harada A, et al. Predicting factors of symptomatic radiation pneumonitis induced by durvalumab following concurrent chemoradiotherapy in locally advanced non-small cell lung cancer. Radiat Oncol 2022;17:7. [Crossref] [PubMed]
- Landman Y, Jacobi O, Kurman N, et al. Durvalumab after concurrent chemotherapy and high-dose radiotherapy for locally advanced non-small cell lung cancer. Oncoimmunology 2021;10:1959979. [Crossref] [PubMed]
- Thomas HMT, Hippe DS, Forouzannezhad P, et al. Radiation and immune checkpoint inhibitor-mediated pneumonitis risk stratification in patients with locally advanced non-small cell lung cancer: role of functional lung radiomics? Discov Oncol 2022;13:85. [Crossref] [PubMed]
- Bruni A, Scotti V, Borghetti P, et al. A Real-World, Multicenter, Observational Retrospective Study of Durvalumab After Concomitant or Sequential Chemoradiation for Unresectable Stage III Non-Small Cell Lung Cancer. Front Oncol 2021;11:744956. Erratum in: Front Oncol 2021;11:802949. [Crossref] [PubMed]
- Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075-9; discussion 1081-90. [Crossref] [PubMed]
- Katsui K, Ogata T, Sugiyama S, et al. Visceral Adipose Mass and Radiation Pneumonitis After Concurrent Chemoradiotherapy in Patients With Non-small-cell Lung Cancer. Cancer Diagn Progn 2021;1:61-7. [Crossref] [PubMed]


