
A propensity matched comparison of robotic vs. traditional minimal access approach for mitral valve repair with concomitant cryoablation
Highlight box
Key findings
• Both minimally invasive video-assisted and robotic surgery have been proven to be safe and effective, with outstanding early and mid-term outcomes in mitral or double valve repair with concomitant cryoablation.
What is known and what is new?
• Both minimally invasive video-assisted and robotic surgery have been proven safe and effective mitral valve repair techniques. Concomitant ablation and additional valve surgery ads complexity and increases the potential advantages. Current evidence resides largely from individual observational studies and meta-analyses.
• Literature comparing robotic surgery to other minimally invasive strategies in patients requiring mitral or double valve procedure and additionally concomitant atrial ablation is lacking, reason why our research is unlike any other.
What is the implication, and what should change now?
• Prospective randomized studies comparing the two methods are needed to reinforce our findings, with the addition of high-quality longitudinal health-related quality of life and multi-modality pain assessments, especially given that this is where the robotic approach is likely to have shown advantage.
Introduction
Mitral valve (MV) insufficiency is the second commonest heart valve disease in Europe (1). It represents a very significant public health burden, which is expected to increase as the western population ages. In the 2021 European Society for Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) Guidelines for the management of valvular heart disease, MV repair (MVR) has a Class I recommendation in patients for both symptomatic and asymptomatic (with left ventricular impairment/dilatation) severe primary mitral insufficiency (MI) (1). This has been made possible through the development and international adoption of repair techniques propagated by Carpentier and others in the 1980’s and 1990’s (2). These techniques consistently restore life expectancy to normal in a range of primary mitral pathologies, with outstanding short-term results and long-term freedom from reintervention exceeding 90% (3).
Over the last twenty years, surgeons have continued to push boundaries through development of minimal access approaches including: mini-sternotomy, mini-thoracotomy, port access (non-spreading), and robotic-assisted MV intervention. Concomitant double/triple valve, and atrial fibrillation (AF) ablation procedures are now also commonly done through such approaches. Such interventions have reduced the transfusion requirement, and improved the short- and medium-term health-related quality of life of patients (3).
There are numerous large studies reporting the safety, efficacy and durability of MV surgery in both minimal invasive video-assisted setting via right-sided mini-thoracotomy [minimally invasive mitral valve surgery (MIMVS)] (4-6) or as robotic-assisted procedure (7-14). However, there are fewer reports on rather small patient populations of either MIMVS (15) or robotic-assisted mitral or double valve surgery combined with surgical AF ablation (11,14,16,17).
Since 2007, in Stuttgart we have routinely performed mitral intervention with concomitant ablation of AF during video-assisted MV operations. As of 2019, using the da Vinci XI surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA) we have started performing this procedure with robotic assistance. The purpose of this study is to perform a comparative assessment of the two techniques in terms of the perioperative, early- and mid-term outcomes of mitral intervention with concomitant ablation. The added complexity of the additional ablation makes this an attractive cohort to study. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1306/rc).
Methods
Study design
Between July 2019 and December 2021, we performed 187 robotic-assisted MV procedures. In 64 of them, a concomitant cryoablation was conducted, due to paroxysmal AF (PAF), persistent AF (persAF) or long-standing persistent AF (lspersAF). PAF is classified as an episode of AF that ends on its own and lasts less than 7 days. People with persAF have an arrhythmia event that lasts for more than 7 days and needs electrical or drug-based cardioversion to end. As lspersAF, we described AF that is unresponsive to treatment by cardioversion and has been going on for more than a year.
Using our prospectively collected institutional database, we identified similar cases performed using a MIMVS technique. Propensity score (PS) matching (1:1) was performed with 22 variables [age, gender, body mass index (BMI), EuroSCORE II, history of angina pectoris, New York Heart Association (NYHA) classification, history of acute myocardial infarction, history of percutaneous coronary intervention, redo cardiac surgery, type II diabetes treated with oral medication, arterial hypertension, impaired renal function, serum creatinine value, chronic obstructive pulmonary disease, neurological dysfunction, peripheral vascular disease, type of AF, CHA2DS2-VASc score, left ventricular ejection fraction (LVEF), MV pathology, tricuspid valve pathology, year of the surgery].
The propensity-matched cohort included 104 patients: group 1 (MIMVS) comprised 52 patients operated on between 2017–2022 using a minimally invasive video-assisted right-sided mini-thoracotomy, and group 2 [robotic-assisted mitral valve surgery (RMV)] comprised 52 patients operated on between 2019–2021 using a robotic-assisted approach. We assessed the perioperative, early and mid-term outcomes in terms of morbidity and mortality, as well as the maintenance of sinus rhythm.
The study was conducted in accordance with the ethics principles of the Declaration of Helsinki (as revised in 2013). The study design is observational and has been approved by the ethics board of the Medical University Tübingen (number of ethics registration: 729/2020BO2). Due to the retrospective methodology and the use of anonymised data from regular patient care, patient consent was not thought to be necessary.
Statistical analysis
Continuous variables are presented as median (25th, 75th percentile), categorical variables as absolute and percentages. Because of the nonrandomized group assignment, we performed a PS matching 1:1 nearest neighbour to assess treatment effects. A logistic regression model including the above-mentioned covariates was used to estimate the PS. Balance of the two matched groups was evaluated by standardized mean differences in the matching variables. A maximum standardized mean difference of 0.15 was considered acceptable. In the end, we found and compared 52 pairs. In order to assess the differences between the two groups we used the Wilcoxon signed rank test for continuous variables and the McNemar’s test for categorical variables.
Differences were considered statistically significant if the P value was less than 0.05. The estimation of patient survival was conducted using the Kaplan-Meier curve. The log-rank test was employed to ascertain the disparities between the groups. SPSS 28.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis, and R 3.5.0 (R Foundation for Statistical computational, Vienna, Austria) was used for the computational environment, with the programme MatchIt 3.02 (R Foundation for Statistical Computing, Vienna, Austria) for pairwise matching of instances.
Patient management
Preoperative
Complete medical history, including arrhythmia history (duration of AF, prior ablation, prior electrical cardioversion), 12-lead resting electrocardiography (ECG), determination of the CHA2DS2-VASC score, echocardiography, coronary angiogram, computed tomography (CT) scan of the thorax, carotid sonogram, current medication, written informed consent for the surgical procedure.
Operative
AF ablation
We used the CryoForm® probe (Atricure Europe B.V., Amsterdam, Netherlands) with an application time of 90 seconds for all lesions and an average target temperature of −60 ℃. Two types of lesion sets, left atrial Maze and bi-atrial Maze were reproduced according to the underlying AF pathology.
The first cryolesion is applied on the epicardium across the coronary sinus. After that we perform a longitudinal incision in the interatrial groove toward the posterior wall of the left atrium. The next cryolesion extends from the inferior atriotomy, to the mitral annulus overlapping endocardially with the first lesion performed. A third line starts from the previous one, across the dome of the left atrium and ends at the superior end of the atriotomy (18). A fourth line extending into the left atrial appendage (LAA) completes the left atrial Maze procedure.
For the right atrial lesions, a vertical or transverse right atriotomy is performed depending on whether a concomitant tricuspid valve procedure is planned. The first two lesions extend from the atriotomy to the inferior and then the superior vena cava. A third lesion extends from the atriotomy to the posterior tricuspid leaflet (18). We perform a fourth cryolesion from the superior end of the atriotomy into the right atrial appendage.
The selection of cryolesions set was based on surgeon preference. However, as a general rule, a biatrial Maze would be conducted if there was also a right heart condition, such as tricuspid valve insufficiency or significant right atrial enlargement, as well as in individuals with a history of atrial flutter paired with AF.
Minimally invasive video-assisted cardiac surgery through right anterior mini-thoracotomy
As a routine approach we use a right-sided mini-thoracotomy in the 4th intercostal space (ICS) with two-dimensional (2D) video-assistance. The preparation, setting and approach are similar to that described by Marin Cuartas et al. in their review paper under the ‘Leipzig minimal invasive MV technique’ (19). The only difference is that if a second venous cannula is required, we usually directly cannulate the superior vena cava and connect it to a supplemental line to the venous reservoir. It is inserted through the thoracotomy or through the 3rd ICS next to the Chitwood aortic cross-clamp.
Robotic-assisted cardiac surgery
The inclusion and exclusion criteria for robotic-assisted surgery in our centre, as well as the operative approach have been previously published (20). There are three operating surgeons who have extensive expertise with the MIMVS approach, two of whom have furthered specialized and are now performing the surgeries robotic-assisted. The establishing phase of RMV was reasonably quick after thorough planning, with no elevated risks for the patients (20). The program’s initial extended operative times were greatly lowered by focusing on structured and standardized operation methods (20). As early as 2020, less than a year after the program began, operation times were comparable to standard MIMVS procedures (20).
With growing experience, robotic MVR almost replaced completely mini-MVR in our institute. In the present study we did not exclude patients, who underwent surgery during the establishment phase.
Patients with pectus excavatum, with pulmonary hypertension and of older age were operated on robotic-assisted (20). Patients with severe right-sided diaphragmatic elevation, with extended calcification of the MV and with an aneurysm of the ascending aorta larger than 40 mm were excluded from the robotic approach and operated on using the MIMVS technique. Patients with high-grade obesity and re-operations were excluded from both groups and treated via median sternotomy (20).
The same reconstructive techniques, as well for the mitral as for the tricuspid valve have been implied in both groups. These included triangular resection, quadrangular resection with sliding repair, neochordae implantation, pericardial patch insertion into the anterior mitral leaflet and an annuloplasty with a band or a ring.
For MVR we used two annuloplasty devices, CG Future Band® (Medtronic GmbH, Meerbusch, Germany) and Carpentier-Edwards (CE) Physio II® (Edwards Lifesciences, Irvine, CA, USA). In the few cases where the MV had to be replaced, the patients opted before surgery for a biological prosthesis (CE Perimount®, Edwards Lifesciences, Irvine, CA, USA). For concomitant tricuspid valve repair we used exclusively the Edwards MC3® (Edwards Lifesciences, Irvine, CA, USA) annuloplasty device.
The LAA occlusion was conducted in three different manners: excision with tissue stapler, endocardial closure using two layers suture, and endocardial resection and purse string suture, reinforced by a supplemental two layers suture. The result of the LAA closure was confirmed by transesophageal echocardiography in all patients before leaving the operating theatre.
In-hospital arrhythmia management
Twelve hours after surgery, subcutaneous heparin was initiated. Continuous telemetry was used until discharge. If the patient was in sinus rhythm after surgery, only betablockers would be used (unless amiodarone was one of their pre-operative medications). If AF recurred, amiodarone was initiated for at least 3 months. Haemodynamic instability or failure of medical treatment was managed with electrical cardioversion. If patients developed junctional rhythm or sick sinus syndrome (SSS), all antiarrhythmic drugs were discontinued. A permanent pacemaker (PPM) would only be used after allowing at least 8 days for rhythm recovery. All patients underwent transthoracic echocardiography before discharge.
Follow-up
Patients were followed up at regular intervals by their referring cardiologist, by means of 24-hour Holter monitor at every 3 months up to a year, and then yearly thereafter. Transthoracic echocardiography was performed 6 monthly up to a year, and then yearly thereafter. The results of all investigations were accessible by our institution, and discussed at regular multidisciplinary regional electrophysiology meetings.
In patients with recurrent AF after the surgical ablation procedure the following protocol was used:
- Referral to the cardiac surgery outpatient clinic.
- Participation at our electrophysiology heart team meeting with one of the following therapeutical decisions:
- Escalation of antiarrhythmic drug therapy with repeat electrical cardioversion;
- Interventional transcatheter ablation;
- In case of permanent AF, optimised drug therapy.
Results
Baseline characteristics
The study included 104 patients equally distributed between the two groups.
The overall median age was 68 [interquartile range (IQR): 61–74] years and the median EuroSCORE II was 3.14 (1.93–4.99) for the whole population.
Two thirds of the patients were male. Most patients presented with a NYHA class II/III symptomatic. The majority (81.7%) had impaired renal function, defined as stage 3a or higher chronic kidney disease with a glomerular filtration rate <60 mL/min/1.73 m2. Twenty patients, corresponding to 19.2% had a history of pulmonary disease, which included asthma, bronchitis, chronic obstructive pulmonary disease and pneumonia. Seven patients had a PPM. The groups were well balanced with no statistically significant differences in demographics or comorbidities between (Table 1). Table 2 renders a detailed summary of the heart rhythm history in both groups. Thirty-five-point-five percent presented with PAF and 46.1% with persAF.
Table 1
| Variables | Total (n=104) | MIMVS (n=52) | RMV (n=52) | P value | SMD |
|---|---|---|---|---|---|
| Age (years) | 68 [61–74] | 68 [61–73] | 69 [61.25–75.75] | 0.58 | 0.042 |
| BSA (m2) | 1.97 [1.78–2.13] | 2.01 [1.78–2.16] | 1.96 [1.78–2.10] | 0.56 | |
| BMI (kg/m2) | 26 [23.10–28.10] | 26.30 [23.40–28.70] | 24.85 [22.72–28.10] | 0.24 | −0.091 |
| EuroSCORE II | 3.14 [1.93–4.99] | 2.76 [1.67–4.35] | 3.49 [2.46–5.65] | 0.07 | 0.124 |
| Male gender | 66 (63.5) | 33 (63.5) | 33 (63.5) | >0.99 | <0.001 |
| Angina pectoris | 2 (1.9) | 1 (1.9) | 1 (1.9) | 0.98 | <0.001 |
| NYHA classification | 0.74 | −0.112 | |||
| I | 28 (26.9) | 11 (21.2) | 17 (32.7) | ||
| II | 49 (47.1) | 28 (53.8) | 21 (40.4) | ||
| III | 23 (22.1) | 11 (21.2) | 12 (23.1) | ||
| IV | 4 (3.8) | 2 (3.8) | 2 (3.8) | ||
| History of AMI | 2 (1.9) | 0 | 2 (3.8) | 0.15 | 0.13 |
| History of PCI | 8 (7.7) | 3 (5.8) | 5 (9.6) | 0.46 | 0.142 |
| Redo cardiac surgery | 1 (1.0) | 1 (1.9) | 0 | 0.31 | −0.070 |
| Hyperlipidemia | 39 (37.5) | 17 (32.7) | 22 (42.3) | 0.31 | |
| Type II diabetes treated with oral medication | 4 (3.8) | 2 (3.8) | 2 (3.8) | >0.99 | <0.001 |
| Arterial hypertension | 87 (83.7) | 42 (80.8) | 45 (86.5) | 0.11 | 0.148 |
| Impaired renal function | 85 (81.7) | 43 (82.7) | 42 (80.8) | 0.15 | −0.070 |
| Serum creatinine (mg/dL) | 1.10 [0.90–1.20] | 1.10 [0.90–1.20] | 1.05 [0.90–1.20] | 0.14 | −0.063 |
| History of pulmonary disease | 20 (19.2) | 13 (25.0) | 7 (13.5) | 0.13 | |
| COPD | 4 (3.8) | 2 (3.8) | 2 (3.8) | >0.99 | <0.001 |
| Neurological disease | 0.3 | – | |||
| TIA | 1 (1.0) | 0 | 1 (1.9) | ||
| CVA with deficiency | 1 (1.0) | 1 (1.9) | 0 | ||
| CVA with recovery | 2 (1.9) | 0 | 2 (3.8) | ||
| Neurological dysfunction | 9 (8.7) | 4 (7.7) | 5 (9.6) | 0.72 | 0.094 |
| Peripheral vascular disease | 2 (1.9) | 1 (1.9) | 1 (1.9) | >0.99 | <0.001 |
| History of pulmonary embolism | 1 (1.0) | 1 (1.9) | 0 | 0.31 | – |
| Hemorrhagic events | 2 (1.9) | 1 (1.9) | 1 (1.9) | >0.99 | – |
| Implantable device | 0.24 | – | |||
| Single chamber PPM | 1 (1.0) | 0 | 1 (1.9) | ||
| Dual chamber PPM | 6 (5.8) | 2 (3.8) | 4 (7.7) | ||
| Year of the surgery (considering 5-year intervals) |
– | – | – | – | 0.127 |
Values are presented as n (%) or median [25th, 75th percentile]. MIMVS, minimally invasive mitral valve surgery; RMV, robotic-assisted mitral valve surgery; SMD, standardized mean difference; BSA, body surface area; BMI, body mass index; EuroSCORE, European System for Cardiac Operative Risk Evaluation; NYHA, New York Heart Association; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack; CVA, cerebrovascular accident; PPM, permanent pacemaker.
Table 2
| Variables | Total (n=104) | MIMVS (n=52) | RMV (n=52) | P value | SMD |
|---|---|---|---|---|---|
| Type of AF | 0.83 | 0.052 | |||
| PAF (<7 d) | 37 (35.6) | 18 (34.6) | 19 (36.5) | ||
| PersAF (7 d–1 y) | 48 (46.2) | 26 (50.0) | 22 (42.3) | ||
| LspersAF (>1 y) | 19 (18.3) | 8 (15.4) | 11 (21.2) | ||
| CHA2DS2-VASc score | 2 [1–3] | 2 [2–3] | 2 [1–3] | 0.65 | 0.011 |
| Duration of AF since first diagnosis (months) | 9.5 [2–28] | 7 [3–20] | 12.5 [2–45.5] | 0.22 | – |
| Other SVT arrhythmias | 0.07 | – | |||
| AFL | 7 (6.7) | 2 (3.8) | 5 (9.6) | ||
| AVNRT | 1 (1.0) | 0 | 1 (1.9) | ||
| AF & AFL | 1 (1.0) | 0 | 1 (1.9) | ||
| Prior ablation | 0.17 | – | |||
| AF | 9 (8.7) | 3 (5.8) | 6 (11.5) | ||
| AVNRT | 1 (1.0) | 0 | 1 (1.9) | ||
| No. of ablations | 0.35 | – | |||
| 1 | 7 (6.7) | 1 (1.9) | 6 (11.5) | ||
| 2 | 2 (1.9) | 2 (3.8) | 0 | ||
| No. of ECV | 0.35 | – | |||
| 1 | 36 (34.6) | 19 (36.5) | 17 (32.7) | ||
| 2 | 11 (10.6) | 3 (5.8) | 8 (15.4) | ||
| 3 | 2 (1.9) | 1 (1.9) | 1 (1.9) | ||
| ECG before surgery | |||||
| Heart rhythm | 0.08 | – | |||
| SR | 52 (50.0) | 22 (42.3) | 30 (57.7) | ||
| AF | 47 (45.2) | 26 (50.0) | 21 (40.4) | ||
| AFL | 3 (2.9) | 3 (5.8) | 0 | ||
| PM | 2 (1.9) | 1 (1.9) | 1 (1.9) | ||
| LBBB | 4 (3.8) | 1 (1.9) | 3 (5.8) | 0.31 | – |
| RBBB | 3 (2.9) | 2 (3.8) | 1 (1.9) | 0.56 | – |
| AV block | 0.13 | – | |||
| 1st degree | 12 (11.5) | 4 (7.7) | 8 (15.4) | ||
| 2nd degree Mobitz | 1 (1.0) | 0 | 1 (1.9) |
Values are presented as n (%) or median [25th, 75th percentile]. MIMVS, minimally invasive mitral valve surgery; RMV, robotic-assisted mitral valve surgery; SMD, standardized mean difference; AF, atrial fibrillation; PAF, paroxysmal atrial fibrillation; persAF, persistent atrial fibrillation; lspersAF, long standing persistent atrial fibrillation; d, days; y, years; SVT, supraventricular tachycardia; AFL, atrial flutter; AVNRT, atrioventricular nodal reentry tachycardia; ECV, electrical cardioversion; ECG, electrocardiography; SR, sinus rhythm; PM, pacemaker; LBBB, left bundle branch block; RBBB, right bundle branch block; AV, atrio-ventricular.
Compared to patients in the MIMVS group, those in the RMV group had a longer median duration of AF since first diagnosis (12.5 vs. 7 months, P=0.22).
Only 10% had prior interventional ablation, but 47% had undergone an electrical cardioversion. In the ECG at presentation, 50.0% of the patients presented with SR and 45.2% with AF.
The preoperative echocardiography findings are presented in Table 3. Based on the preoperatively defined LVEF, patients were distributed into three categories: normal LVEF (>55%), moderate LVEF (30–55%), and severe LVEF (<30%). Most patients presented with normal left ventricular function. Eighty-one-point-seven percent presented primary MV pathology with type II insufficiency according to the Carpentier classification as being the most encountered in both study groups. Ninety-five-point-two percent had 3rd degree MI.
Table 3
| Variables | Total (n=104) | MIMVS (n=52) | RMV (n=52) | P value | SMD |
|---|---|---|---|---|---|
| LVEF | 0.89 | 0.093 | |||
| Normal (>55%) | 81 (77.9) | 41 (78.8) | 40 (76.9) | ||
| Moderate (30–55%) | 21 (20.2) | 9 (17.3) | 12 (23.1) | ||
| Severe (<30%) | 2 (1.9) | 2 (3.8) | 0 | ||
| MV pathology | 0.8 | – | |||
| Primary | 85 (81.7) | 42 (80.8) | 43 (82.7) | ||
| Secondary | 19 (18.3) | 10 (19.2) | 9 (17.3) | ||
| Mitral insufficiency | 0.66 | −0.033 | |||
| 2nd degree | 4 (3.8) | 2 (3.8) | 2 (3.8) | ||
| 3rd degree | 99 (95.2) | 50 (96.2) | 49 (94.2) | ||
| 4th degree | 1 (1.0) | 0 | 1 (1.9) | ||
| Type of MV insufficiency (Carpentier classification) | 0.08 | – | |||
| Type I | 27 (26.0) | 10 (19.2) | 17 (32.7) | ||
| Type II | 74 (71.2) | 39 (75.0) | 35 (67.3) | ||
| Type III | 3 (2.9) | 3 (5.8) | 0 | ||
| Aortic valve sclerosis | 3 (2.9) | 1 (1.9) | 2 (3.8) | 0.56 | – |
| Aortic insufficiency | 0.45 | – | |||
| 1st degree | 22 (21.2) | 10 (19.2) | 12 (23.1) | ||
| 2nd degree | 1 (1.0) | 0 | 1 (1.9) | ||
| Tricuspid insufficiency | 0.13 | 0.15 | |||
| 1st degree | 33 (31.7) | 19 (36.5) | 14 (26.9) | ||
| 2nd degree | 22 (21.2) | 8 (15.4) | 14 (26.9) | ||
| 3rd degree | 11 (10.6) | 5 (9.6) | 6 (11.5) | ||
| 4th degree | 4 (3.8) | 1 (1.9) | 3 (5.8) | ||
| LAA thrombus | 2 (1.9) | 1 (1.9) | 1 (1.9) | – | – |
Values are presented as n (%). MIMVS, minimally invasive mitral valve surgery; RMV, robotic-assisted mitral valve surgery; SMD, standardized mean difference; LVEF, left ventricular ejection fraction; MV, mitral valve; LAA, left atrial appendage.
Intraoperative data
All surgeries were elective. Table 4 summarises the intraoperative outcomes: 72.1% underwent MV surgery, and 26.9% a double valve (i.e., additional tricuspid) procedure. There were four replacements of the MV, accounting for 3.8%. One replacement occurred after failed repair. In 76% of the patients, a Future Band device was implanted. The RMV group had a substantially longer median bypass time than the MIMVS group (181 vs. 166 minutes, P=0.02), however this difference is not reflected in the cross-clamp time, which is quite similar between the two groups (99 vs. 101 minutes, P=0.67).
Table 4
| Variables | Total (n=104) | MIMVS (n=52) | RMV (n=52) | P value |
|---|---|---|---|---|
| Conversion to sternotomy | 2 (1.9) | 1 (1.9) | 1 (1.9) | >0.99 |
| Number of operated valves | 0.87 | |||
| 1 (mitral valve) | 75 (72.1) | 38 (73.1) | 37 (71.2) | |
| 2 (mitral and tricuspid valve) | 28 (26.9) | 13 (25.0) | 15 (28.8) | |
| 3 (aortic, mitral and tricuspid valve) | 1 (1.0) | 1 (1.9) | 0 | |
| MV procedure | 0.72 | |||
| Repair | 100 (96.2) | 48 (92.3) | 52 (100.0) | |
| Replacement | 3 (2.9) | 3 (5.8) | 0 | |
| Replacement after failed repair | 1 (1.0) | 1 (1.9) | 0 | |
| Implant type | – | |||
| Future Band | 79 (76.0) | 36 (69.2) | 43 (82.7) | |
| CE Perimount | 4 (3.8) | 4 (7.7) | 0 | |
| Physio II | 21 (20.2) | 12 (23.1) | 9 (17.3) | |
| AF ablation-procedure | 0.76 | |||
| Left atrial Maze | 91 (87.5) | 45 (86.5) | 46 (88.5) | |
| Biatrial Maze | 13 (12.5) | 7 (13.5) | 6 (11.5) | |
| LAA occlusion | <0.001 | |||
| No procedure | 29 (27.9) | 24 (46.0) | 5 (9.6) | |
| Stapler excision | 13 (12.5) | 12 (23.1) | 1 (1.9) | |
| Endocardial suture | 52 (50.0) | 14 (26.9) | 38 (73.1) | |
| Resection and suture | 10 (9.6) | 2 (3.8) | 8 (15.4) | |
| Bypass time (min) | 174 [151.5–216.5] | 166 [130–201] | 181 [158.5–231.5] | 0.02 |
| Cross-clamp time (min) | 99 [79–117] | 101 [77.5–115] | 99 [81–117.5] | 0.67 |
Values are presented as n (%) or median [25th, 75th percentile]. MIMVS, minimally invasive mitral valve surgery; RMV, robotic-assisted mitral valve surgery; MV, mitral valve; AF, atrial fibrillation; LAA, left atrial appendage.
Most (87.5%) received a left atrial Maze and 12.5% a bi-atrial Maze. An LAA occlusion procedure was completed in 72.1% cases. There were two conversions to sternotomy, one in each group. The conversion in the MIMVS group was due to severe aortic valve insufficiency after performing mitral and tricuspid valve repair; consequently, an aortic valve replacement via upper partial sternotomy was performed. The conversion in the RMV group was due to left ventricular posterior wall rupture, which was repaired and the patient made a full recovery thereafter.
Post-operative outcomes
The postoperative course was in the majority of cases uneventful. There were no in-hospital or 30-day deaths. Four patients needed re-exploration for bleeding. Only one patient developed a cerebrovascular accident (CVA). With one exception, there were no significant differences in the postoperative course between the two patient populations (Table 5). The exception constitutes a patient who needed an extracorporeal membrane oxygenation (ECMO) therapy. By the patient in question, the surgical procedure included biatrial Maze ablation in addition to mitral and tricuspid valve repair. Lactate levels as well as vasopressor support kept rising in the early postoperative phase, and due to biventricular dysfunction, we made the decision to set up a veno-arterial ECMO (VA ECMO) to provide hemodynamic and respiratory support. Following 3 days of continuous therapy, the hemodynamic situation stabilized with time, allowing the VA ECMO flow to be decreased. After a week, the VA ECMO was taken out and a veno-venous ECMO (VV ECMO) was installed because pulmonary weaning off the extracorporeal system was not viable. After a further week of weaning, the VV ECMO was removed. The patient made a full recovery and was discharged from our standard ward on the 28th day following the surgical procedure.
Table 5
| Variables | Total (n=104) | MIMVS (n=52) | RMV (n=52) | P value |
|---|---|---|---|---|
| Ventilation (hours) | 6 [4–9.75] | 6 [4–10] | 6 [4–8] | 0.57 |
| ICU stay (hours) | 22 [20–29.5] | 22 [20–27.5] | 20.5 [20–40] | 0.99 |
| In-hospital stay (days) | 8 [7–11.75] | 9 [7.5–12.5] | 8 [6–10.5] | 0.31 |
| Re-exploration for bleeding | 4 (3.8) | 3 (5.8) | 1 (1.9) | 0.31 |
| CVA | 1 (1.0) | 1 (1.9) | 0 | 0.31 |
| Delirium | 10 (9.6) | 5 (9.6) | 5 (9.6) | >0.99 |
| Acute kidney injury | 1 (1.0) | 0 | 1 (1.9) | 0.31 |
| Pneumonia | 3 (2.9) | 0 | 3 (5.8) | 0.08 |
| Wound infection | 2 (1.9) | 1 (1.9) | 1 (1.9) | >0.99 |
| SSI | 2 (1.9) | 0 | 2 (3.8) | 0.15 |
| Pleural effusion | 0.38 | |||
| Puncture | 9 (8.7) | 4 (7.7) | 5 (9.6) | |
| Drainage | 6 (5.8) | 2 (3.8) | 4 (7.7) | |
| Pericardial effusion, conservative treatment | 2 (1.9) | 2 (3.8) | 0 | 0.15 |
| Myocardial infarction treated with PCI | 2 (1.9) | 1 (1.9) | 1 (1.9) | >0.99 |
| LCO | 0.98 | |||
| Vasopressors | 1 (1.0) | 0 | 1 (1.9) | |
| ECMO | 1 (1.0) | 1 (1.9) | 0 | |
| SVT arrhythmias | 0.37 | |||
| AF | 49 (47.1) | 26 (50) | 23 (44.2) | |
| AFL | 2 (1.9) | 2 (3.8) | 0 | |
| AF & AFL | 4 (3.8) | 3 (5.8) | 1 (1.9) | |
| Presence of tachyarrhythmia | 0.25 | |||
| Tachyarrhythmia | 8 (7.7) | 2 (3.8) | 6 (11.5) | |
| Tachyarrhythmia and electrical cardioversion | 3 (2.9) | 2 (3.8) | 1 (1.9) | |
| Bradycardia or conduction block | 0.23 | |||
| Bradycardia | 1 (1.0) | 1 (1.9) | 0 | |
| AV block 3rd degree | 6 (5.8) | 4 (7.7) | 2 (3.8) | |
| SSS | 2 (1.9) | 1 (1.9) | 1 (1.9) | |
| AV block 2nd degree Mobitz | 1 (1.0) | 1 (1.9) | 0 | |
| Tachy-brady syndrome | 2 (1.9) | 1 (1.9) | 1 (1.9) | |
| PPM implantation | 0.12 | |||
| One chamber | 3 (2.9) | 3 (5.8) | 0 | |
| Dual chamber | 8 (7.6) | 5 (9.6) | 3 (5.8) |
Values are presented as n (%) or median [25th, 75th percentile]. MIMVS, minimally invasive mitral valve surgery; RMV, robotic-assisted mitral valve surgery; ICU, intensive care unit; CVA, cerebrovascular accident; SSI, surgical site infection; PCI, percutaneous coronary intervention; LCO, low cardiac output; ECMO, extracorporeal membrane oxygenation; SVT, supra ventricular; AF, atrial fibrillation; AFL, atrial flutter; AV, atrio-ventricular; SSS, sick sinus syndrome; PPM, permanent pacemaker.
Regarding heart rhythm changes in the immediate postoperative course, 52.8% developed supraventricular arrhythmias, but only 2.8% necessitated an electrical cardioversion. A PPM was implanted in 11 patients, 8 in the MIMVS group and 3 in the RMV group.
The results of the discharge echocardiography and ECG are presented in Table 6. The MV intervention was successful in all patients, with no MI greater than 1st degree. More than half of the patients were discharged in sinus rhythm. There was a higher percentage of patients with left bundle branch block (6.7% compared to 3.8% on admission). Also, the percentage of patients with 1st degree AV block was higher (22.1% vs. 11.5% on admission).
Table 6
| Variables | Total (n=104) | MIMVS (n=52) | RMV (n=52) | P value |
|---|---|---|---|---|
| EF | 0.64 | |||
| Normal | 80 (76.9) | 39 (75.0) | 41 (78.8) | |
| Moderate | 24 (23.1) | 13 (25.0) | 11 (21.2) | |
| Mild MV insufficiency | 15 (14.4) | 7 (13.5) | 8 (15.4) | 0.78 |
| Heart rhythm at discharge | 0.64 | |||
| SR | 60 (57.7) | 28 (53.8) | 32 (61.5) | |
| AF | 24 (23.1) | 14 (26.9) | 10 (19.2) | |
| AFL | 6 (5.8) | 4 (7.7) | 2 (3.8) | |
| PM | 8 (7.7) | 5 (9.6) | 3 (5.8) | |
| Junctional rhythm | 6 (5.8) | 1 (1.9) | 5 (9.6) | |
| LBBB | 7 (6.7) | 4 (7.7) | 3 (5.8) | 0.71 |
| RBBB | 5 (4.8) | 4 (7.7) | 1 (1.9) | 0.17 |
| AV block, 1st degree | 23 (22.1) | 11 (21.2) | 12 (23.1) | 0.81 |
Values are presented as n (%). ECG, electrocardiography; MIMVS, minimally invasive mitral valve surgery; RMV, robotic-assisted mitral valve surgery; EF, ejection fraction; MV, mitral valve; SR, sinus rhythm; AF, atrial fibrillation; AFL, atrial flutter; PM, pacemaker; LBBB, left bundle branch block; RBBB, right bundle branch block; AV, atrio-ventricular.
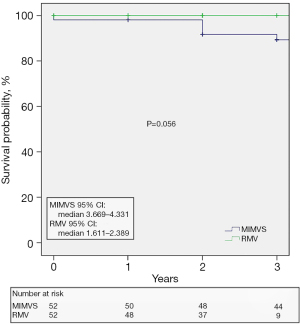
Follow-up was 100% complete; median follow-up time was 2 (IQR, 2–4) years. The patients in the MIMVS group had a longer median follow-up interval [4 (IQR, 2.5–4) years, 95% confidence interval (CI): 3.669–4.331 vs. the patients in the RMV group: 2 (IQR, 1–2) years, 95% CI: 1.611–2.389, respectively]. According to our protocol the patients were periodically checked by means of 24 hours Holter monitoring and transthoracic echocardiography. The gathered follow-up data are summarized in Table 7. Eighteen-point-two percent reported over palpitations. Thirty-one (29.7%) patients had an arrhythmia documented and 6 (5.8%) cases have undergone a ‘touch-up’ interventional ablation. Only one patient necessitated the implantation of a two-chamber PPM during follow-up. Seventy-two (69.2%) patients presented with stable sinus rhythm without amiodarone or other antiarrhythmic drugs class I/III. Another 7 (6.7%) patients were in sinus rhythm but still on antiarrhythmic drugs (class III) treatment. In total, 76% of the patients were in sinus rhythm at follow-up. No myocardial infarction and no stroke occurred during follow-up. One patient needed reoperation due to endocarditis of the MV.
Table 7
| Variables | Total (n=104) | MIMVS (n=52) | RMV (n=52) | P value |
|---|---|---|---|---|
| Palpitations | 19 (18.3) | 9 (17.3) | 10 (19.2) | 0.8 |
| Documented arrhythmia during | 0.42 | |||
| Inside blanking | 5 (4.8) | 3 (5.8) | 2 (3.8) | |
| Outside blanking | 10 (9.6) | 5 (9.6) | 5 (9.6) | |
| Inside & outside blanking | 16 (15.3) | 6 (11.5) | 10 (19.2) | |
| Type of arrhythmia | 0.75 | |||
| PAF | 16 (15.4) | 8 (15.4) | 8 (15.4) | |
| AFL | 2 (1.9) | 1 (1.9) | 1 (1.9) | |
| PersAF | 13 (12.5) | 5 (9.6) | 8 (15.4) | |
| SR kept without amiodarone | 72 (69.2) | 38 (73.1) | 34 (65.4) | 0.39 |
| Myocardial infarction | 0 | 0 | 0 | >0.99 |
| Stroke | 0 | 0 | 0 | >0.99 |
| Redo heart surgery | 1 (1.0) | 1 (1.9) | 0 | 0.31 |
Values are presented as n (%). MIMVS, minimally invasive mitral valve surgery; RMV, robotic-assisted mitral valve surgery; PAF, paroxysmal atrial fibrillation; AFL, atrial flutter; persAF, persistent atrial fibrillation; SR, sinus rhythm.
Five patients died during follow-up. As shown in the Kaplan-Meier survival curves (Figure 1), the survival rate was similar between our two study groups (log-rank test P=0.056).
Discussion
In this study we compared perioperative and mid-term outcomes of MV or double valve surgery with concomitant cryoablation performed through two different minimally invasive surgical approaches (MIMVS versus robotic) using propensity matching analysis. The advantage of our study is that our institutional volume has allowed our group to reach proficiency in both techniques, reducing the bias in comparison. Overall, we demonstrated outstanding outcomes in both techniques. There were no early deaths and the rate of adverse events during follow-up was low.
Literature comparing robotic surgery to other minimally invasive strategies in patients requiring mitral or double valve procedure and additionally concomitant atrial ablation is lacking.
There is growing consensus that in high-risk populations, the minimal access approach to mitral intervention may be of benefit (12). Concomitant ablation and additional valve surgery ads complexity and increases the potential advantages. Given the wide range of evolving minimal access approaches and technologies, further evidence is required to assess the safety and comparative benefits of each as they emerge.
Both, minimally invasive video-assisted and robotic surgery have been proven to be safe and effective MVR techniques (19). They are being performed in an increasing number of centers and with increasing frequency over time (19). However, current evidence resides largely from individual observational studies (19) and meta-analyses (21,22). In a recent 20-year review on MIMVS, Bonatti suggests that robotic assistance resulted in lower stroke rates and mortality compared to the video-assisted right-sided mini-thoracotomy approach (23).
A number of studies compared RMV to video-assisted right mini-thoracotomy. In general, the evidence was of inadequate quality, and long-term outcomes were not sufficiently documented (21). The majority of studies (21) are derived from single centres and involve patients ranging in age from 46 to 63 years who did not necessitate concurrent cardiac procedures.
Wei et al. conducted a comparison of clinical outcomes between 121 robotic and 113 thoracoscopic mitral valve repair patients (24). The bypass and aortic cross-clamp time were significantly shorter in the robotic group (P<0.001), the intensive care unit (ICU) time and intraoperative blood transfusions rate were lower in the thoracoscopic group (P<0.005) and the 30-day mortality rate was 0.9% in both groups (24). In a study including 1,305 patients with isolated mitral valve insufficiency treated using three different surgical approaches (sternotomy: 377, video-assisted right mini-thoracotomy: 481, robotic-assisted: 447), the robotic approach led to a higher rate of MVR, but had longer bypass and aortic cross-clamp times (P<0.001) (25). The adjusted survival rate was similar for all approaches (P=0.357) (25). Fujita et al. found on 335 patients undergoing either robotic or MIMVS, that robotic surgery can be applied to repair more complex mitral lesions (26). The short-term outcomes were excellent and comparable to those with mini-MVR, in this all comers, non-matched population (26).
In a multi-institutional analysis by Hawkins et al., a total of 590 patients were matched between robotic and minimal invasive approaches (27). The authors report over excellent early outcomes with a mortality rate <1% and major morbidity rate <9%. The robotic approach was associated with a higher rate of AF, more transfusions and one day longer postoperative stay compared to the minimally invasive approach (27).
Although these findings are promising, long-term and patient-reported outcomes, including cardiac-specific health-related quality of life, overall survival, and heart failure rates, remain insufficiently supported by evidence. Interpretation of such evidence is hard to conduct, as very few centers are facile in doing both approaches, making comparisons difficult. In a PS matching population of 69 pairs, robotic MVR had longer cross-clamp times and shorter hospital stays that non-robotic minimally invasive MVR, follow-up echocardiogram analysis and midterm survival were similar in both groups (28). In a recently published study by Rao et al. including 124 matched patients receiving robotic or endoscopic mitral valve repair for degenerative disease, there was no difference in early or late mortality at 10 years in either cohort (29).
Overall, the outcomes demonstrated are similar to those seen in other high-volume centres. Marchetto et al. (15) published their findings from 68 patients who underwent cryoablation for AF alongside video-assisted MIMVS. All patients received a left atrial Maze cryoablation. Total cross-clamp time was 97.6±22.8 minutes (15), similar to the 102.13±29.37 minutes in our study (our study cohort underwent more complex interventions and more concomitant procedures). In similar studies published by Aydin et al. (30) and Kadan et al. (17) the reported aortic clamp time and bypass time were in the same range as ours: 105.75±20.03 and 141.8±25.6 minutes, and 188.5±53.8 and 196±25.6 minutes, respectively. Our mean bypass time was 184.17±51.93 minutes. It was slightly higher in the robotic group (as expected) with 194.90±49.02 minutes compared to 173.44±53.00 minutes in the MIMVS group. In the paper published by Nifong et al., the average cross-clamp and cardiopulmonary bypass times in the patients having a concomitant Maze procedure were 130 and 188 minutes, respectively (11).
Our ventilation time, ICU and in-hospital length of stay are similar to those reported by Fujita et al. (26), with the important difference that our patients had a higher EuroSCORE II, median value of 3.14 and were older, median age 68 years. Others reported shorter times for a much younger study population (25,29) or for a population with a very low mortality risk (27). Wei et al. found a mean ventilation time of 13.6±5.3 hours and a mean ICU length of stay of 3.3±2.6 days in their study population of 234 patients with a mean age of 46.5±14 years, data comparable to ours (24).
In terms of rhythm outcomes, our patients developed relapse of AF in 47.1% during the hospital stay and only 57.7% were discharged in sinus rhythm. This fact could be explained by the high percentage (64.3%) of patients presenting with persAF or lspersAF prior to surgery. Marchetto et al. found a 1-year follow-up freedom from AF recurrences rate of 94.8% (15), but their follow-up was mostly ECG and not Holter monitoring based. Nifong et al. report a 96.5% rate of freedom from AF recurrence and off antiarrhythmic drugs class I/III at 351 days (11). Ad et al. published long-term results in 473 patients following concomitant MV surgery and Cox maze procedure for AF (31): they found return to sinus rhythm rates regardless of antiarrhythmic drugs at 1, 5, and 7 years of 90%, 80% and 66%, respectively (31). The sinus rhythm rates in patients off antiarrhythmic drugs at the above-defined intervals were 83%, 69%, and 55%, respectively (31). The patients included in our study had a 75% rate of freedom from AF recurrence outside blanking period and at a median follow-up time of 2 (IQR, 2–4) years.
The 2021 ESC/EACTS Guidelines on valve pathology (1) recommend after mitral valve repair, respectively tricuspid valve repair with other indications for oral anticoagulation, a long-term treatment with oral anticoagulation (Class I). Our study patients suffered from different stages of AF and were concomitantly treated by means of ablation, therefore, according to the above-mentioned guidelines, were discharged with continued oral anticoagulation. However, the postoperative oral anticoagulation regime varied depending on the operating surgeon. Two of the 3 operating surgeons in the MIMVS group prefer for 3 months after surgery treatment with a coumadin. The third surgeon in the MIMVS group and also the leading surgeon in the RMV group prefers reinstating after surgery the same oral anticoagulation as preoperatively. In cases without oral anticoagulation prior to surgery we started either coumadin or rivaroxaban.
At last follow-up, 72 (69.2%) patients presented with stable sinus rhythm and without amiodarone treatment. However, oral anticoagulation was discontinued only in 14 cases. We suspect that this decision by the referring cardiologists is either due to clinical reasons (e.g., lack of atrial contraction), or due to lack of consensus in the community on whether anticoagulation should be stopped after successful ablation.
We registered just one in-hospital CVA and no strokes during follow-up. Marchetto et al. reported CVAs at an annual rate of 1.7% (15). We hypothesize that our unusually low stroke rate is due to our multidisciplinary guided close follow-up and investigation of all patients post ablation.
Bogachev-Prokophiev et al. reported their outcomes of 242 patients undergoing MV surgery and concomitant cryoablation. Seventy-point-seven percent were operated on using a median sternotomy and 29.3% minimally invasive (32). The 30-day mortality was 0.4%, the rate for re-exploration for bleeding was 6.2%, the rate of myocardial infarction was 2.1% and the CVA rate was 1.2% (32). Chitwood reported in 321 RMV surgeries with concomitant Maze procedure an early mortality of 4.7% (33). Major complications rates were 1.7% for myocardial infarction, 2.6% for stroke and 4.3% for re-explorations for bleeding (33). In our study group, as well as in the MIMVS as in the RMV population the rates of the above-mentioned complications were lower and we registered no casualties in the first 30 days. This most likely reflects the fact that these authors were pioneers in the field, introducing new techniques at the dawn of the minimal access era.
The incidence of PPM implantation after concomitant surgical AF ablation varies between 5% and 25% (32). Bogachev-Prokophiev et al. identified by means of multivariate logistic regression model that concomitant tricuspid valve surgery was a significant risk factor for PPM implantation (32). From the 11 patients requiring PPM implantation in our study group, eight received double valve surgery.
The indications for PPM in our study population were: high atrioventricular block in seven patients, SSS in two patients and tachy-brady syndrome in two other patients.
In 2020, the patients receiving a robotic-assisted MV surgery in our clinic had a 25% reduction in hospital stay compared to MIMVS patients (20). However, in our current study groups of MV surgery combined with concomitant AF cryoablation, the ICU and in-hospital length of stay did not differ between the two groups. This is reflective of the maturity of our enhanced recovery programs.
This was an observational, retrospective, single-centre study. The statistical power of the analysis may have been impacted by the patient screening process and the restricted sample size. The limited occurrence of clinical events precluded the possibility of conducting a multivariate analysis to identify any independent risk factors. The relatively short follow-up period may have influenced the statistical results. The lack of health-related quality of life and multi-modality pain assessments also limit the interpretation of the results, especially given that this is where the robotic approach is likely to have shown advantage.
Conclusions
Robotic-assisted mitral or double valve repair with concomitant cryoablation is safe, feasible and has excellent early- and mid-term follow-up results. Sinus rhythm rate during follow-up was acceptable and did not differ between the two groups. Prospective randomized studies comparing the two methods are needed to reinforce our findings, with the addition of high-quality longitudinal health-related quality of life and health economic assessments.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1306/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1306/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1306/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1306/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of the Medical University Tübingen (number of ethics registration: 729/2020BO2) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2021;60:727-800. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery: the “French correction.” J Thorac Cardiovasc Surg 1983;86:323-37.
- Tan MK, Jarral OA, Thong EH, et al. Quality of life after mitral valve intervention. Interact Cardiovasc Thorac Surg 2017;24:265-72. [Crossref] [PubMed]
- Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91. [Crossref] [PubMed]
- Davierwala PM, Seeburger J, Pfannmueller B, et al. Minimally invasive mitral valve surgery: "The Leipzig experience". Ann Cardiothorac Surg 2013;2:744-50. [Crossref] [PubMed]
- McClure RS, Athanasopoulos LV, McGurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199-206. [Crossref] [PubMed]
- Seco M, Cao C, Modi P, et al. Systematic review of robotic minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:704-16. [Crossref] [PubMed]
- Mandal K, Alwair H, Nifong WL, et al. Robotically assisted minimally invasive mitral valve surgery. J Thorac Dis 2013;5:S694-703. [Crossref] [PubMed]
- Yoo JS, Kim JB, Jung SH, et al. Mitral durability after robotic mitral valve repair: analysis of 200 consecutive mitral regurgitation repairs. J Thorac Cardiovasc Surg 2014;148:2773-9. [Crossref] [PubMed]
- Ricci D, Pellegrini C, Aiello M, et al. Port-access surgery as elective approach for mitral valve operation in re-do procedures. Eur J Cardiothorac Surg 2010;37:920-5. [Crossref] [PubMed]
- Nifong LW, Rodriguez E, Chitwood WR Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-43. [Crossref] [PubMed]
- Bonatti J, Kiaii B, Alhan C, et al. The role of robotic technology in minimally invasive surgery for mitral valve disease. Expert Rev Med Devices 2021;18:955-70. [Crossref] [PubMed]
- Mihaljevic T, Koprivanac M, Kelava M, et al. Value of robotically assisted surgery for mitral valve disease. JAMA Surg 2014;149:679-86. [Crossref] [PubMed]
- Kim HJ, Kim JB, Jung SH, et al. Clinical outcomes of robotic mitral valve repair: a single-center experience in Korea. Ann Cardiothorac Surg 2017;6:9-16. [Crossref] [PubMed]
- Marchetto G, Anselmino M, Rovera C, et al. Results of Cryoablation for Atrial Fibrillation Concomitant With Video-Assisted Minimally Invasive Mitral Valve Surgery. Semin Thorac Cardiovasc Surg 2016;28:271-80. [Crossref] [PubMed]
- Ju MH, Huh JH, Lee CH, et al. Robotic-Assisted Surgical Ablation of Atrial Fibrillation Combined With Mitral Valve Surgery. Ann Thorac Surg 2019;107:762-8. [Crossref] [PubMed]
- Kadan M, Kubat E, Erol G, et al. Early- and mid-term results of cryoablation of atrial fibrillation concomitant with robotic mitral valve surgery. Anatol J Cardiol 2021;25:266-72. [Crossref] [PubMed]
- McCarthy PM. The maze IV operation is not always the best choice: Matching the procedure to the patient. JTCVS Tech 2023;17:79-83.
- Marin Cuartas M, Javadikasgari H, Pfannmueller B, et al. Mitral valve repair: Robotic and other minimally invasive approaches. Prog Cardiovasc Dis 2017;60:394-404. [Crossref] [PubMed]
- Franke UFW, Huether F, Ghinescu M, et al. Robotically assisted mitral valve surgery-experience during the restart of a robotic program in Germany. Ann Cardiothorac Surg 2022;11:596-604. [Crossref] [PubMed]
- Fatehi Hassanabad A, Nagase FNI, Basha AM, et al. A Systematic Review and Meta-Analysis of Robot-Assisted Mitral Valve Repair. Innovations (Phila) 2022;17:471-81. [Crossref] [PubMed]
- Husen TF, Kohar K, Angelica R, et al. Robotic vs other surgery techniques for mitral valve repair and/or replacement: A systematic review and meta-analysis. Hellenic J Cardiol 2023;71:16-25. [Crossref] [PubMed]
- Bonatti J, Crailsheim I, Grabenwöger M, et al. Minimally Invasive and Robotic Mitral Valve Surgery: Methods and Outcomes in a 20-Year Review. Innovations (Phila) 2021;16:317-26. [Crossref] [PubMed]
- Wei S, Zhang X, Cui H, et al. Comparison of clinical outcomes between robotic and thoracoscopic mitral valve repair. Cardiovasc Diagn Ther 2020;10:1167-74. [Crossref] [PubMed]
- Stevens LM, Rodriguez E, Lehr EJ, et al. Impact of timing and surgical approach on outcomes after mitral valve regurgitation operations. Ann Thorac Surg 2012;93:1462-8. [Crossref] [PubMed]
- Fujita T, Kakuta T, Kawamoto N, et al. Benefits of robotically-assisted surgery for complex mitral valve repair. Interact Cardiovasc Thorac Surg 2021;32:417-25. [Crossref] [PubMed]
- Hawkins RB, Mehaffey JH, Mullen MG, et al. A propensity matched analysis of robotic, minimally invasive, and conventional mitral valve surgery. Heart 2018;104:1970-5. [Crossref] [PubMed]
- Zheng CR, Mazur P, Arghami A, et al. Robotic vs. minimally invasive mitral valve repair: A 5-year comparison of surgical outcomes. J Card Surg 2022;37:3267-75. [Crossref] [PubMed]
- Rao A, Tauber K, Szeto WY, et al. Robotic and endoscopic mitral valve repair for degenerative disease. Ann Cardiothorac Surg 2022;11:614-21. [Crossref] [PubMed]
- Aydin U, Sen O, Kadirogullari E, et al. Robotic Mitral Valve Surgey Combined with Left Atrial Reduction and Ablation Procedures. Braz J Cardiovasc Surg 2019;34:285-9. [Crossref] [PubMed]
- Ad N, Holmes SD, Massimiano PS, et al. Long-term outcome following concomitant mitral valve surgery and Cox maze procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2018;155:983-94. [Crossref] [PubMed]
- Bogachev-Prokophiev A, Sharifulin R, Karadzha A, et al. Results of concomitant cryoablation for atrial fibrillation during mitral valve surgery. Interact Cardiovasc Thorac Surg 2022;34:540-7. [Crossref] [PubMed]
- Chitwood WR Jr. Robotic mitral valve surgery: overview, methodology, results, and perspective. Ann Cardiothorac Surg 2016;5:544-55. [Crossref] [PubMed]


