
Pleural fluid soluble Fas ligand and tuberculous pleural effusion: a prospective diagnostic test accuracy study
Highlight box
Key findings
• Pleural fluid soluble FasL ligand (sFasL) has moderate diagnostic accuracy for tuberculous pleural effusion (TPE).
What is known and what is new?
• Preliminary studies revealed pleural sFasL had a high diagnostic value for TPE. It remains unknown whether sFasL provides additional diagnostic value beyond adenosine deaminase (ADA).
• This study indicates that sFasL does not provide added diagnostic value beyond ADA. Age may affect the diagnostic accuracy of sFasL for TPE.
What is the implication, and what should change now?
• Age should be considered when interpreting the diagnostic value of sFasL for TPE. sFasL is an alternative diagnostic tool for TPE.
Introduction
Background
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis (M.tb), has a high prevalence and annual incidence worldwide (1). According to the report released by the World Health Organization (WHO), the number of newly diagnosed TB was 6.4 million worldwide in 2021, and approximately 1.6 million people died from TB (2). TB can be classified as pulmonary TB (PTB) and extrapulmonary TB (EPTB), and the latter accounts for 16% of TB cases (3). The common types of EPTB are tuberculous lymphadenitis, pleuritis, and spondylitis (4). A multicenter cross-sectional study showed that tuberculous pleuritis accounts for about 50% of EPTB in China (5). Pleural effusion is a common sign of tuberculous pleuritis, and the pleural effusion caused by tuberculous pleuritis is thus termed tuberculous pleural effusion (TPE). Diagnosing TPE is challenging for pulmonologists because pleural effusion is not a specific sign of TPE. It can also be caused by malignancy, pneumonia, and heart failure (HF) (6). The gold standards for TPE are pleural fluid M.tb culture, Ziehl-Neelsen staining, or pleural biopsy (7). However, these tools have some limitations. M.tb culture has a long turn-around time and thus does not facilitate early diagnosis. In addition, the sensitivity of M.tb culture is <20% (8). Ziehl-Neelsen staining is an easy-to-perform tool with a short turn-around time; however, its sensitivity is <3% (8). Ultrasound- or CT-guided percutaneous pleural biopsy and medical thoracoscopy have high diagnostic accuracy for TPE, but they are invasive tools that can cause operating-related complications (9,10). In addition, medical thoracoscopy needs special training, which limits its clinical application in remote areas. The nucleic acid amplification test (NAAT) has high specificity for TPE, but its sensitivity is only around 50% (11-13). By contrast, pleural fluid or serum biomarkers are of clinical value because of low cost, feasibility, minimal invasiveness, and short turn-around time (14,15).
Some pleural biomarkers have been reported to be useful diagnostic tools for TPE, such as adenosine deaminase (ADA), interferon-gamma (IFN-γ), and interleukin-27 (IL-27) (14). The sensitivities and specificities of these biomarkers are >90%, as indicated by meta-analyses (16-18). However, these biomarkers have limitations. For example, the diagnostic performance of ADA for TPE is influenced by many factors, such as age (19). Pleural IFN-γ and IL-27 are not standardized, and the comparability between different commercial kits is poor (14). Therefore, developing novel pleural biomarkers to improve the diagnostic accuracy of available markers or replace them remains valuable.
Rationale and knowledge gap
Fas ligand (FasL, CD95) is a type II transmembrane protein of approximately 40 kDa. It belongs to the tumor necrosis factor (TNF) family (20). The binding of Fas receptor and FasL can induce cellular apoptosis via caspase-8 (21). There are two types of FasL, named membrane-bound Fas ligand (mFasL) and the soluble Fas ligand (sFasL), respectively. The mFasL is cleaved by the activated metalloproteinases (MMPs) to form the sFasL (22). sFasL can competitively bind to Fas and thus has a protective effect on apoptosis (20).
Preliminary studies revealed that pleural sFasL had a high diagnostic value for TPE (14,23-25). However, it remains unknown: (I) whether sFasL has an additional diagnostic value to the traditional markers (e.g., ADA); (II) whether sFasL provides a net benefit in patients with undiagnosed pleural effusion; (III) factors affecting the diagnostic accuracy of sFasL for TPE. This study aimed to investigate the additional diagnostic accuracy and net benefit of pleural fluid sFasL for TPE. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1076/rc) (26).
Methods
Participants
Participants in this study were from a prospective, pre-registered, and double-blind study termed SIMPLE (Chinese Clinical Trial Registry: ChiCTR1800017449) (27). The design details of the SIMPLE study have been introduced previously (27,28). Briefly, we prospectively enrolled the patients with undiagnosed pleural effusion who visited the Affiliated Hospital of Inner Mongolia Medical University (AHIMMU, 2018–2021) and the Affiliated Changshu Hospital of Nantong University (ACHNU, 2020–2021).
The inclusion criteria for participant enrollment were: (I) patients with undiagnosed pleural effusion; (II) diagnostic thoracentesis was performed, and the pleural fluid specimen was obtained. The presence of pleural effusion was confirmed by chest X-ray, CT, or ultrasound. The exclusion criteria were: (I) patients who had a history of pleural effusion with known etiology in the last three months at the point of diagnosis; (II) age <18 years old; (III) pregnant woman; (IV) patients with insufficient pleural fluid specimen; (V) patients who developed pleural effusion during hospitalization; and (VI) patients with pleural effusion caused by trauma or surgery.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committees of the AHIMMU (No. 2018011) and the ACHNU (No. KY2021014). Informed consent was obtained from all participants.
Diagnostic criteria
TPE was diagnosed with pleural fluid M.tb culture, Ziehl-Neelsen staining, and pleural biopsy. Positive biopsy was defined as the presence of pleural granuloma with the exclusion of other granulomatous diseases. In some patients who has a high probability of TPE and other types of pleural effusion can be excluded, TPE was diagnosed by treatment response to antituberculosis therapies and follow-up. Parapneumonic pleural effusion (PPE) was diagnosed by pleural fluid bacterial culture, imaging (loculated effusions), and response to antibiotic therapy. Malignant pleural effusion (MPE) was diagnosed by effusion cytology, pleural biopsy, or evidence of primary cancer with the exclusion of benign pleural effusion. HF was diagnosed by history, laboratory findings, and response to diuretics. The concentration of sFasL was masked to the clinicians who made the diagnosis.
Routine biomarkers
The pleural fluid specimen of the participants was obtained at the time of admission. After centrifugation at 2,000 rpm for 10 minutes, the pleural fluid supernatant was aliquoted and stored at −80 to −70 °C for further analysis.
The sFasL concentrations were determined by an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) following the manufacturer’s instructions. Concentrations of white blood cells (WBC), protein, lactate dehydrogenase (LDH), and ADA in the pleural fluid were collected from the participant’s medical records. ADA and LDH were measured by Beckman AU5831 (AHIMMU) and ADVIA 2400 (ACHNU). All tests were performed by technicians who were unaware of the clinical diagnosis.
Statistical analysis
Continuous variables were expressed as the median and interquartile range (IQR). We used the Mann-Whitney U test to compare continuous variables between the TPE and the non-TPE groups. The Kruskal-Wallis H tests were used to compare continuous data in more than two groups. Categorical variables were compared with the Chi-squared test. A receiver operating characteristic (ROC) curve analysis was used to evaluate the diagnostic accuracy of sFasL. A decision curve analysis (DCA) was used to assess the net benefit of sFasL. The added diagnostic value of sFasL was estimated using net reclassification improvement (NRI) and integrated discriminant improvement (IDI) (29). The mathematical basis of NRI and IDI has been introduced in an easy-to-understand way in a previous publication (30). All statistical analyses and graphs were performed using SPSS 25.0, GraphPad Prism 8.3.0, Stata 15.1, and R 4.2.2. P<0.05 was considered statistically significant.
Results
Basic characteristics of the participants
Figure 1 is a flowchart of participant selection. A total of 232 participants were recruited, including 62 in ACHNU and 170 in AHIMMU. Twenty-one participants were excluded, and 211 were used for data analysis. Table 1 lists the baseline characteristics of the participants. There were 33 TPEs (15.7%) and 178 non-TPEs (84.3%). The non-TPEs comprised 92 patients with MPE, 42 patients with PPE, 28 patients with HF, and 16 other types of pleural effusion. The median [IQR] ages of patients with TPE and non-TPE were 72 [64–80] and 73 [65–79] years, respectively.
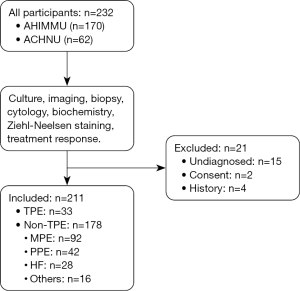
Table 1
| Variables | TPE (n=33) | Non-TPE (n=178) | P |
|---|---|---|---|
| Age (years) | 72 [64–80] | 73 [65–79] | 0.579 |
| Sex (F/M) | 14/19 | 62/116 | 0.404 |
| Pleural fluid WBC (×106/mL) | 1,574.0 [876.5–2,849.0] | 851.5 [411.8–1,833.5] | 0.001 |
| Pleural fluid protein concentration (g/L) | 44.0 [36.4–47.6] | 35.8 [23.4–43.1] | 0.001 |
| Serum protein (g/L) | 65.1±8.3 | 61.6±8.1 | 0.027 |
| Pleural fluid/serum protein ratio | 0.66 [0.61–0.74] | 0.55 [0.38–0.69] | 0.005 |
| Pleural fluid glucose (mmol/L) | 5.3 [4.7–6.4] | 6.0 [4.8–6.9] | 0.182 |
| Pleural fluid LDH (U/L) | 283.0 [176.0–394.0] | 232.00 [141.0–486.0] | 0.468 |
| Pleural fluid ADA (U/L) | 42.2 [14.6–53.5] | 9.2 [5.3–16.1] | <0.001 |
Continuous data are presented as the median and interquartile range, or mean and standard deviation, and compared with Mann-Whitney or Student’s t-test. TPE, tuberculous pleural effusion; WBC, white blood cell; LDH, lactate dehydrogenase; ADA, adenosine deaminase.
sFasL concentration and etiologies of pleural effusion
As shown in Figure 2A, the median concentration of sFasL in TPE patients (147.7 pg/mL) was significantly higher than that in non-TPE (98.2 pg/mL) (P<0.001 by Mann-Whitney U test). A subgroup analysis of non-TPE patients showed that the sFasL concentration significantly differed among PPE, MPE, HF, and other pleural effusion (Figure 2B, P<0.001 by Kruskal-Wallis H test).

Diagnostic performance of sFasL and ADA
Figure 3 shows the ROC curves of ADA, sFasL, and their combination. The area under the ROC curve (AUC) was 0.74 (95% CI: 0.65–0.83) and 0.80 (95% CI: 0.71–0.90) for sFasL and ADA, respectively (P=0.17 by Delong’s test). A logistic model combining ADA and sFasL had an AUC of 0.80 (95% CI: 0.72–0.89), but its AUC did not significantly differ from that of ADA (P=0.97 by Delong’s test). The prespecified thresholds and their corresponding sensitivities and specificities were summarized in Table 2. At the 110 pg/mL threshold, sFasL had a sensitivity of 0.73 (95% CI: 0.58–0.88) and specificity of 0.60 (95% CI: 0.53–0.67). In addition, we analyzed the AUCs of sFasL in the AHIMMU and ACHNU separately and found that their AUCs were 0.72 (95% CI: 0.61–0.85) and 0.74 (95% CI: 0.57–0.90), respectively.
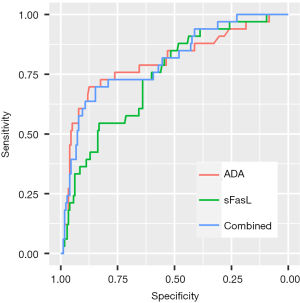
Table 2
| Biomarkers | AUC (95% CI) | Cut-off | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| sFasL | 0.74 (0.65–0.83) | 110 pg/mL | 0.73 (0.58–0.88) | 0.60 (0.53–0.67) |
| ADA | 0.80 (0.71–0.90) | 35 U/L | 0.61 (0.42–0.76) | 0.92 (0.88–0.95) |
| sFasL + ADA | 0.80 (0.72–0.89) | 0.20 | 0.64 (0.45–0.79) | 0.85 (0.80–0.90) |
sFasL, soluble Fas ligand; ADA, adenosine deaminase; TPE, tuberculous pleural effusion; AUC, area under the ROC curve; CI, confidence interval.
We performed NRI and IDI analysis to examine whether sFasL provides added diagnostic value beyond ADA. The continuous NRI (95% CI) and IDI (95% CI) were 0.36 (0.00–0.72, P=0.05) and 0.02 (−0.01–0.06, P=0.18), respectively.
The net benefit of sFasL in patients with undiagnosed pleural effusion
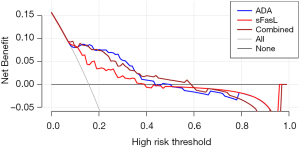
Figure 4 shows the decision curves of pleural fluid sFasL, ADA, and their combination. Compared with ADA, the decision curve of sFasL was closer to two reference lines, indicating that the net benefit of sFasL is inferior to that of ADA.
Discussion
Key findings
The primary findings of this study can be summarized as follows: (I) pleural fluid sFasL concentration was significantly higher in TPE than in non-TPE, which is consistent with previous studies (31-33); (II) pleural fluid sFasL had moderate diagnostic accuracy for TPE; (III) measurement of sFasL in pleural effusion patients provides no additional diagnostic value beyond ADA, despite its net benefit. These results indicate that pleural sFasL is an alternative test for TPE.
Strengths and limitations
Compared with previous studies, our study has some strengths. First, we analyzed the distribution of sFasL in non-TPE patients and found PPE and MPE patients had higher sFasL than HF patients (P<0.001). By contrast, previous studies did not investigate the distribution of sFasL in non-TPE patients. This result indicates that in clinical settings with a high prevalence of MPE and PPE, the diagnostic accuracy of sFasL may decrease. Second, this is the first study investigating the added diagnostic value of sFasL on ADA. Investigating the added value of sFasL is of high clinical relevance. ADA is a guideline-endorsed biomarker for TPE (34). If sFasL could not provide added diagnostic value beyond ADA, measuring sFasL and ADA together is meaningless. Third, we performed a DCA and revealed the net benefit of sFasL.
Our study has some limitations. First, the sample size of our study is small, and only 33 TPEs were included. The small sample size may decrease the precision of our study. Second, we used the stored pleural fluid specimens to determine sFasL, and the long-term stability of sFasL remains unknown. Third, we did not investigate the cellular origin of sFasL. However, this study provides a novel insight into the diagnostic accuracy of sFasL for TPE.
Comparison with similar research
We searched the PubMed database and found that three studies have investigated the diagnostic accuracy of sFasL for TPE (23-25). The characteristics and primary findings of these studies are summarized in Table 3. They concluded that the diagnostic accuracy of sFasL had sensitivities and specificities of >90% (23-25). We found that sFasL had an AUC of approximately 0.74, which is lower than that in previous studies. We speculate that the inconsistency between previous studies and our work is partially due to differences in the study population. Notably, the average age of TPE patients was higher in our study, while the age of TPE was lower in previous studies (23-25). Indeed, age can affect the diagnostic accuracy of pleural biomarkers for TPE (19,23,35). Notably, a previous study and our work found that age was negatively correlated with sFasL (23). Therefore, we conclude that in clinical settings where TPE patients are younger than non-TPE patients, sFasL has high diagnostic accuracy. In contrast, in settings where TPE patients’ ages are comparable to those of non-TPE patients, the diagnostic accuracy of sFasL decreases. Notably, the participants’ mean age in a study is around 76 years, but the AUC in that study was 0.88 (24), indicating that age can only partially explain the heterogeneity across previous studies.
Table 3
| Study | Year | Country | TPE/non-TPE | Diagnostic criteria | Mean or medium age (years) | AUC | |
|---|---|---|---|---|---|---|---|
| TPE | Non-TPE | ||||||
| Wu (24) | 2010 | China | 23/56 | CRS | 76 | 76 | 0.88 |
| Klimiuk (25) | 2015 | Poland | 44/159 | CRS | 52 | 68 | 0.95 |
| Korczynski (23) | 2019 | Poland | 60/162 | CRS | 54 | NR | ~0.93 |
| This study | 2023 | China | 33/178 | CRS | 72 | 73 | 0.74 |
sFasL, soluble Fas ligand; TPE, tuberculous pleural effusion; AUC, area under the ROC curve; CRS, complex reference standard; NR, not reported.
We found that sFasL in non-TPE patients varied according to their diagnosis. MPE and PPE patients had higher sFasL than HF patients. Therefore, the composition of non-TPE is a potential source of inconsistency between previous studies and our study. When interpreting the diagnostic value of sFasL for TPE, it is crucial to consider the underlying causes of pleural effusion in a clinical setting.
We found that the diagnostic accuracy of sFasL decreased in elderly patients. This finding is biologically plausible. The Fas/FasL system plays a regulatory role in immune cell apoptosis (36) and has thus been involved in the pathogenesis of TB. sFasL, the soluble form of FasL, is released after being cleaved from its membrane partner by metalloproteinases (37). FasL expression has been detected in activated T cells and natural killer (NK) cells (37,38). T cells and NK cells are enriched in the pleural fluid of TPE patients (39). Therefore, we hypothesized that the sFasL in the pleural fluid of TPE patients were cleaved from the lymphocyte. The expression of FasL in lymphocytes is regulated by several transcription factors, such as c-JUN, c-FOS, Y-box binding protein 1 (YB-1), nuclear factor of activated T cells (NFAT), NF-κB, specificity protein-1 (SP1) (37). Previous studies indicated aging decreased the activity of these transcription factors (37,40-42). Animal studies also revealed that old mice had fewer Fas+FasL+CD8+ T cells than young mice (43).
Conclusions
In conclusion, we find the diagnostic accuracy of sFasL is moderate and comparable to that of ADA. sFasL has net benefits in patients with pleural fluid. Therefore, sFasL is an alternative test for diagnosing TPE. Due to the limitations of this study, further studies are needed to evaluate the diagnostic value of sFasL for TPE in different clinical settings.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1076/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1076/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1076/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1076/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed following the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committees of the Affiliated Hospital of Inner Mongolia Medical University (No: 2018011) and the Affiliated Changshu Hospital of Nantong University (No. KY2021014). Informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Furin J, Cox H, Pai M. Tuberculosis. Lancet 2019;393:1642-56. [Crossref] [PubMed]
- Golbal Tuberculosis Report 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022
- Global tuberculosis report 2020. Available online: https://www.who.int/publications/i/item/9789240013131
- Baykan AH, Sayiner HS, Aydin E, et al. Extrapulmonary tuberculosıs: an old but resurgent problem. Insights Imaging 2022;13:39. [Crossref] [PubMed]
- Kang W, Liu S, Du J, et al. Epidemiology of concurrent extrapulmonary tuberculosis in inpatients with extrapulmonary tuberculosis lesions in China: a large-scale observational multi-centre investigation. Int J Infect Dis 2022;115:79-85. [Crossref] [PubMed]
- Porcel JM, Esquerda A, Vives M, et al. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol 2014;50:161-5. [Crossref] [PubMed]
- Gopi A, Madhavan SM, Sharma SK, et al. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest 2007;131:880-9. [Crossref] [PubMed]
- Bielsa S, Acosta C, Pardina M, et al. Tuberculous Pleural Effusion: Clinical Characteristics of 320 Patients. Arch Bronconeumol (Engl Ed) 2019;55:17-22. [Crossref] [PubMed]
- Wang Z, Xu LL, Wu YB, et al. Diagnostic value and safety of medical thoracoscopy in tuberculous pleural effusion. Respir Med 2015;109:1188-92. [Crossref] [PubMed]
- Wang XJ, Yang Y, Wang Z, et al. Efficacy and safety of diagnostic thoracoscopy in undiagnosed pleural effusions. Respiration 2015;90:251-5. [Crossref] [PubMed]
- Soni A, Guliani A, Nehra K, et al. Insight into diagnosis of pleural tuberculosis with special focus on nucleic acid amplification tests. Expert Rev Respir Med 2022;16:887-906. [Crossref] [PubMed]
- Kohli M, Schiller I, Dendukuri N, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2021;1:CD012768. [Crossref] [PubMed]
- Wen XH, Han YL, Cao XS, et al. Diagnostic value of nucleic acid amplification tests for tuberculous pleural effusion. Future Microbiol 2023;18:971-83. [Crossref] [PubMed]
- Zhang M, Li D, Hu ZD, et al. The diagnostic utility of pleural markers for tuberculosis pleural effusion. Ann Transl Med 2020;8:607. [Crossref] [PubMed]
- Zheng WQ, Hu ZD. Pleural fluid biochemical analysis: the past, present and future. Clin Chem Lab Med 2023;61:921-34. [Crossref] [PubMed]
- Liang QL, Shi HZ, Wang K, et al. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med 2008;102:744-54. [Crossref] [PubMed]
- Greco S, Girardi E, Masciangelo R, et al. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis 2003;7:777-86.
- Wang W, Zhou Q, Zhai K, et al. Diagnostic accuracy of interleukin 27 for tuberculous pleural effusion: two prospective studies and one meta-analysis. Thorax 2018;73:240-7. [Crossref] [PubMed]
- Jiang CG, Wang W, Zhou Q, et al. Influence of age on the diagnostic accuracy of soluble biomarkers for tuberculous pleural effusion: a post hoc analysis. BMC Pulm Med 2020;20:178. [Crossref] [PubMed]
- Volpe E, Sambucci M, Battistini L, et al. Fas-Fas Ligand: Checkpoint of T Cell Functions in Multiple Sclerosis. Front Immunol 2016;7:382. [Crossref] [PubMed]
- Lettau M, Paulsen M, Kabelitz D, et al. Storage, expression and function of Fas ligand, the key death factor of immune cells. Curr Med Chem 2008;15:1684-96. [Crossref] [PubMed]
- Mariani SM, Matiba B, Bäumler C, et al. Regulation of cell surface APO-1/Fas (CD95) ligand expression by metalloproteases. Eur J Immunol 1995;25:2303-7. [Crossref] [PubMed]
- Korczynski P, Klimiuk J, Safianowska A, et al. Impact of age on the diagnostic yield of four different biomarkers of tuberculous pleural effusion. Tuberculosis (Edinb) 2019;114:24-9. [Crossref] [PubMed]
- Wu SH, Li CT, Lin CH, et al. Soluble Fas ligand is another good diagnostic marker for tuberculous pleurisy. Diagn Microbiol Infect Dis 2010;68:395-400. [Crossref] [PubMed]
- Klimiuk J, Krenke R, Safianowska A, et al. Diagnostic performance of different pleural fluid biomarkers in tuberculous pleurisy. Adv Exp Med Biol 2015;852:21-30. [Crossref] [PubMed]
- Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Clin Chem 2015;61:1446-52. [Crossref] [PubMed]
- Han YQ, Yan L, Li P, et al. A Study Investigating Markers in PLeural Effusion (SIMPLE): a prospective and double-blind diagnostic study. BMJ Open 2019;9:e027287. [Crossref] [PubMed]
- Yan Z, Wen JX, Wang H, et al. Diagnostic accuracy of pleural fluid lactate dehydrogenase to adenosine deaminase ratio for tuberculous pleural effusion: an analysis of two cohorts. BMC Pulm Med 2022;22:428. [Crossref] [PubMed]
- Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157-72; discussion 207-12. [Crossref] [PubMed]
- Pickering JW, Endre ZH. New metrics for assessing diagnostic potential of candidate biomarkers. Clin J Am Soc Nephrol 2012;7:1355-64. [Crossref] [PubMed]
- Wu SH, Chu JJ, Chiang CD. Increased soluble Fas ligand concentration in tuberculous pleural effusion. J Formos Med Assoc 2001;100:32-4.
- Budak F, Uzaslan EK, Cangür S, et al. Increased pleural soluble fas ligand (sFasL) levels in tuberculosis pleurisy and its relation with T-helper type 1 cytokines. Lung 2008;186:337-43. [Crossref] [PubMed]
- Cui HY, Zhang Q, Su B, et al. Differential levels of cytokines and soluble Fas ligand between tuberculous and malignant effusions. J Int Med Res 2010;38:2063-9. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii4-17. [Crossref] [PubMed]
- Abrao FC, de Abreu IR, Miyake DH, et al. Role of adenosine deaminase and the influence of age on the diagnosis of pleural tuberculosis. Int J Tuberc Lung Dis 2014;18:1363-9. [Crossref] [PubMed]
- Yamada A, Arakaki R, Saito M, et al. Dual Role of Fas/FasL-Mediated Signal in Peripheral Immune Tolerance. Front Immunol 2017;8:403. [Crossref] [PubMed]
- Lagunas-Rangel FA. Fas (CD95)/FasL (CD178) system during ageing. Cell Biol Int 2023;47:1295-313. [Crossref] [PubMed]
- Sharma K, Wang RX, Zhang LY, et al. Death the Fas way: regulation and pathophysiology of CD95 and its ligand. Pharmacol Ther 2000;88:333-47. [Crossref] [PubMed]
- Tong ZH, Shi HZ. Subpopulations of helper T lymphocytes in tuberculous pleurisy. Tuberculosis (Edinb) 2013;93:279-84. [Crossref] [PubMed]
- Song L, Stephens JM, Kittur S, et al. Expression of c-fos, c-jun and jun B in peripheral blood lymphocytes from young and elderly adults. Mech Ageing Dev 1992;65:149-56. [Crossref] [PubMed]
- Cartwright T, Perkins ND. L Wilson C. NFKB1: a suppressor of inflammation, ageing and cancer. FEBS J 2016;283:1812-22. [Crossref] [PubMed]
- Kim SY, Kang HT, Han JA, et al. The transcription factor Sp1 is responsible for aging-dependent altered nucleocytoplasmic trafficking. Aging Cell 2012;11:1102-9. [Crossref] [PubMed]
- Hsu HC, Shi J, Yang P, et al. Activated CD8(+) T cells from aged mice exhibit decreased activation-induced cell death. Mech Ageing Dev 2001;122:1663-84. [Crossref] [PubMed]


