
Transbronchial cryobiopsy using an ultrathin cryoprobe with a guide sheath for the diagnosis of pulmonary mucosa-associated lymphoid tissue lymphoma
Introduction
Primary pulmonary lymphoma, which is characterized by clonal proliferation of lymphoid tissue, is a rare disease that accounts for less than 1% of all pulmonary neoplasms and 3–4% of all extra-nodal non-Hodgkin lymphomas (1). Pulmonary mucosa-associated lymphoid tissue (MALT) lymphoma is the most common type of primary pulmonary lymphoma (2). The diagnosis of MALT lymphoma is based on morphological, immunological, and molecular biological assessments; thus, maintenance of the cellular architecture and acquisition of a sufficient amount of tissue are essential for the diagnosis of these tumors (3,4).
The methods used to diagnose pulmonary MALT lymphoma include transbronchial biopsy (TBB), computed tomography (CT)-guided needle aspiration biopsy, and surgery. A diagnosis using noninvasive examinations is preferable; however, the diagnostic yield of TBB for pulmonary MALT lymphoma is low, with Borie et al. and Farrell et al. reporting a diagnostic yield of 31% (19/61 cases) and 25% (2/8 cases), respectively (3,5). Moreover, diagnosis based on conventional transbronchial lung biopsies for pulmonary lymphoma is often hampered by the presence of crush artifacts in small samples (5).
Bianchi et al. retrospectively reported the usefulness of transbronchial lung cryobiopsy (TBLC) in 13 cases of lymphoproliferative disorders of the lungs, including four cases of pulmonary MALT lymphoma (6). In all cases, TBLC was performed under deep sedation with rigid bronchoscopic intubation using a 2.4 mm cryoprobe in combination with a Fogarty catheter; thus, the diagnostic effectiveness of TBLC can be explained by the fact that this approach yielded significantly larger samples with fewer crushed artifacts and more preserved architecture than that achieved with TBB. The samples obtained using this approach were suitable for subsequent molecular testing. However, TBLC is associated with the risk of bleeding owing to the removal of the entire bronchoscope when obtaining specimens (6,7).
With the advent of the 1.1 mm single-use cryoprobe, specimens can be pulled through a guide sheath (GS) placed in the working channel of the bronchoscope, allowing cryobiopsy with the GS and bronchoscope left in place. Although the specimens obtained with this approach are smaller than those obtained with conventional TBLC, they were much larger than those obtained with TBB and can be safely obtained without crush artifacts (8). Since cryobiopsy using a GS is expected to yield sufficient tissue samples without artifacts, we report the efficacy and safety of using a 1.1 mm cryoprobe with a GS for the diagnosis of pulmonary MALT lymphoma. Furthermore, there have been previous reports on the usefulness of conventional TBLC in the diagnosis of pulmonary MALT lymphoma; however, this is the first report describing the use of a 1.1 mm cryoprobe with a GS. We present this article in accordance with the STROBE and AME Case Series reporting checklists (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1074/rc).
Methods
Patients
We retrospectively analyzed the CT findings for four patients who underwent cryobiopsy using a GS for suspected pulmonary MALT lymphoma at our institution between February 2022 and January 2023. Notably, all patients had CT findings characteristic of pulmonary MALT lymphoma, such as lung nodules, ground-glass opacity, lung mass, and/or airspace consolidation. The lesions had peribronchovascular distributions with air bronchograms (9). This study was conducted in accordance with the Declaration of Helsinki (revised in 2013), and was approved by the Yamagata Prefectural Central Hospital Review Board (approval No.: 2023-028). The requirement for informed consent was waived due to the retrospective design of the study.
Procedures
TBLC was performed using a BF-1T290Q (working channel diameter, 3.0 mm; Olympus, Tokyo, Japan), a 1.1 mm single-use probe (Erbe, Tuebingen, Germany), a GS (Machida® SG-27092; outer diameter, 2.7 mm; inner diameter, 2.6 mm; length, 920 mm; Abiko, Japan), a radial endobronchial ultrasound (EBUS) probe (UM-S20-17S, Olympus), virtual bronchoscopic navigation (VBN), and fluoroscopy. The patient was placed under moderate conscious sedation with propofol and fentanyl. Similar to the approach used for general EBUS with a GS (EBUS-GS), we selected the bronchial branches on the basis of VBN and defined the target lesion using an ultrasound probe with a GS. After obtaining favorable findings on EBUS, the ultrasound probe was replaced with a 1.1 mm cryoprobe and biopsies were obtained with a freezing time of 2–5 seconds. Specimens were collected through the GS. An extra-large GS (outer diameter, 2.7 mm; inner diameter, 2.6 mm; length, 920 mm), which has a larger diameter than a conventional GS (outer diameter, 2.55 mm; length, 1,050 mm), can be used to obtain larger specimens (10), the extra-large size GS was used in all cases in this study. If the freezing time is too long with a 1.1 mm cryoprobe, the sample is too large to pass through the 2.7 mm GS and gets stuck. However, a 1.1 mm cryoprobe was used because the specimens would not pass through the GS unless the freezing time was shortened, although it is theoretically possible to combine a 1.7 or 2.4 mm cryoprobe with a GS. TBB using single-use 1.8 mm diameter flexible biopsy forceps (Radial Jaw™ 4 Pulmonary Biopsy Forceps; Boston Scientific, Tokyo, Japan) was added only in case 1 to compare the size and quality of the specimens obtained using TBLC and TBB. Complications during the procedure were monitored, and the follow-up period for complications was mainly based on interviews until the next visit.
Results
The patient in case 1 was a 70-year-old male former smoker with hypertension and hyperlipidemia who was referred to our hospital due to multiple consolidations on chest radiography without any accompanying symptoms. High-resolution CT revealed multiple consolidations with an air bronchogram distributed along the bronchus (Figure 1A). The EBUS findings confirmed that the probe reached the inside of the lesion, and we performed TBB three times and TBLC 16 times in combination with a GS for a 28 mm lesion in the right B4a (Figure 1B,1C). No adverse events such as bleeding or pneumothorax were observed.
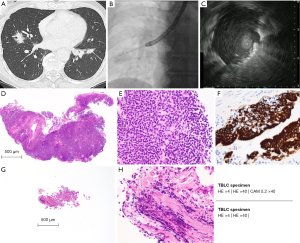
Four TBLC and three TBB specimens were formalin-fixed for pathological diagnosis. Histological examination of the TBLC specimens revealed small lymphoid proliferations accompanied by lymphoepithelial lesions (LELs) (Figure 1D-1F). Immunohistochemical staining of the TBLC specimens revealed positivity for CD10, CD20, CD79a, and BCL-2 and negative results for CD3 and CD5. Additionally, immunohistochemical assessments using anti-kappa and anti-lambda antibodies revealed light-chain restriction. On the basis of these findings, the patient was diagnosed as showing pulmonary MALT lymphoma.
Flow cytometry and immunoglobulin gene rearrangement (IGR) were performed using polymerase chain reaction on the remaining 12 fresh TBLC samples. Flow cytometry analysis showed a low proportion of CD3 expression and elevated expression levels of CD19 and CD20. Furthermore, clonal IGR was also demonstrated.
Case 2 was a 69-year-old male former smoker with hypertension. He was asymptomatic and noticed abnormal shadows during the routine physical examination. Case 3 was a 78-year-old female never-smoker with a history of osteoporosis. She had symptoms of shortness of breath. Case 4 was an 86-year-old female who had never smoked. She had Sjogren’s syndrome with symptoms of dry eyes and dry mouth, hypertension and previous chronic subdural hematoma. All cases had the CT findings described in the Methods section and were performed using a 1.1 mm cryoprobe in combination with a GS described in the Methods section.
Table 1 presents the findings for all four cases. In each case, biopsies were performed on one or two peripheral pulmonary lesions (PPLs) ranging from 28 to 67 mm in size. The number of specimens obtained ranged from 8 to 16. No complications such as pneumothorax or uncontrollable bleeding were observed. Formalin-fixed specimens were subjected to pathological examination, and fresh specimens were subjected to molecular testing.
Table 1
| Case No. | Age, years | Sex | CT findings | Examination | Diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample lesion, mm |
Diameter longest, mm |
Distance from pleura, mm | Number of TBLC | Complication | Proliferation of B-cells† | LELs‡ | LCR | IGR | FCM | ||||||
| 1 | 70 | Male | Rt. B4a | 28 | 35 | 16 | − | ++ | ++ | Positive | Positive | Positive | |||
| 2 | 69 | Male | Rt. B2b | 45 | 7 | 8 | − | + | + | Non-specific | Positive | N/A | |||
| 3 | 78 | Female | Rt. B6b, Rt. B9a | 61, 67 | 8, 0 | 8 | − | ++ | ++ | Positive | N/A | N/A | |||
| 4 | 86 | Female | Rt. B4b, Rt. B9b | 44, 40 | 0, 13 | 10 | − | + | − | Non-specific | Positive | N/A | |||
†, ++: ≥50%, +: 1–49% at each specimen. ‡, ++: ≥5 lesions, +: 1–4 lesions at each specimen. CT, computed tomography; TBLC, transbronchial lung cryobiopsy; LELs, lymphoepithelial lesions; LCR, light-chain restriction; IGR, immunoglobulin gene rearrangement; FCM, flow cytometry; N/A, not available.
Cases 1 and 3 showed proliferation of B cells and LELs. Immunohistochemical assessments revealed clear light-chain restriction, indicating a definitive pathological diagnosis. In cases 2 and 4, the specimens primarily showed intracavitary organization and fibrosis, resulting in a small tumor area. Therefore, a histopathological diagnosis of pulmonary MALT lymphoma was challenging. IGR was performed in three cases, excluding case 3, which aimed to diagnose recurrence. B-cell clonality was confirmed in all the cases. In case 1, flow cytometry could be performed due to the large number of specimens and surplus of specimens.
TBB was also performed in case 1 to compare the specimen size and quality. The TBLC specimen obtained with a 1.1 mm probe (Figure 1D) was much larger than the TBB specimen (Figure 1G) (maximum diameter: 3.9 vs. 0.9 mm). The TBLC specimen (Figure 1E) was also of higher quality with no crush artifacts, and its cell morphology and tissue structure were clearly observable. The TBB specimen (Figure 1H) showed a crushed cell morphology owing to crush artifacts, and its structure could not be evaluated.
Discussion
Diagnosis
Pulmonary MALT lymphoma is diagnosed on the basis of morphological, immunological, and molecular biological characteristics. For this reason, obtaining a sufficient amount of tissue that is free of crush artifacts and has an intact cellular architecture is important (3,5). TBLC has been reported to allow the collection of large specimens without crush artifacts and thereby improve the diagnosis rate of lymphoproliferative diseases such as pulmonary MALT lymphoma (6). Furthermore, CT images of pulmonary MALT lymphoma show a direct route from the bronchus to the lesion, making it easily accessible for biopsy. However, TBB specimens from the lateral aspect of the bronchus may be occasionally difficult to obtain (11) because the lesion is located along a relatively central and large-diameter bronchus. TBLC after EBUS is useful for confirming the target lesions and the presence of surrounding vessels prior to biopsy (12).
With the recent introduction of a 1.1 mm single-use cryoprobe, cryobiopsy can be performed in combination with an oversheath, and with the oversheath and bronchoscope left in place, specimens larger than those obtained in TBB can be safely collected (8). However, for PPL, the oversheath attached to the cryoprobe does not reach the target lesion because of the short distance from the working channel. For this reason, three studies have reported the effectiveness of the 1.1 mm cryoprobe using the Olympus GS. This effectiveness has been attributed to the possibility of repeated sampling from the same site, as confirmed by ultrasound (13-15).
In cases 1 and 3, a definitive diagnosis could be obtained with pathological findings alone, and the large, high-quality specimens obtained by TBLC with a 1.1 mm cryoprobe contributed to the diagnosis. In contrast, in cases 2 and 4, histopathological diagnosis of pulmonary MALT lymphoma was difficult because of the small size of the tumors, which were mostly occupied by organization and fibrosis. Organizing pneumonia has been reported to be associated with low-grade lymphoma (16), which may have contributed to the difficulty in diagnosis using microscopic specimens in TBB. However, repeated TBLC under a GS yielded a large number of specimens and allowed molecular testing, potentially facilitating the diagnosis. Bianchi et al. reported that immunohistochemical staining and molecular testing are available, even in TBLC specimens (6). We performed immunohistochemical staining in all cases and evaluated the proliferation of B-cells and light-chain restriction by immunohistochemical staining. Clonal IGR was also demonstrated using molecular testing in cases 1, 2, and 4. There have been no previous reports of flow cytometry using TBLC specimens. Although flow cytometry also contributed to the diagnosis in case 1, more cases need to be accumulated to determine whether TBLC specimens can be used for flow cytometry. It may be better to perform flow cytometry with non-frozen TBB specimens.
In this study, more than eight TBLC specimens were collected from all patients and a definitive diagnosis of pulmonary MALT lymphoma was obtained in all four cases. Since 10 TBLC samples were collected in case 4, in which fibrosis accounted for the majority of the lesions, the results of this study suggest that acquisition of 10 or more TBLC samples may be sufficient for a definitive diagnosis of pulmonary MALT lymphoma.
Safety
In comparison with conventional TBB, TBLC is associated with a higher risk of bleeding and pneumothorax (7). Therefore, another device such as a balloon blocker could be used (17). However, a GS allows easier hemostasis (18). No bleeding or pneumothorax-related complications were observed in the cases evaluated in this study. The GS was inserted into the procedural bronchoscope and remained in the tracheal lumen after biopsy, thereby reducing the risk of bleeding. The use of the GS also allows many TBLC procedures to be performed in a short period of time, ensuring a short interval between biopsies and precluding the need for a hemostasis period. The best point is that the procedure is almost the same as TBB in EBUS-GS and can be performed under moderate sedation without the use of general anesthesia.
Limitations
The present study was retrospective in nature and included the findings from only four cases evaluated at a single institution. Data from a larger number of cases is needed to further advance the simplification and safety of this diagnostic method.
Conclusions
TBLC with GS for pulmonary MALT lymphoma is a useful and safe method, and can reproducibly yield sufficient quantities of good-quality biopsy specimens.
Acknowledgments
We presented this study in Congress of the TSANZ 2023.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE and AME Case Series reporting checklists. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1074/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1074/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1074/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Yamagata Prefectural Central Hospital Review Board (approval No.: 2023-028) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sanguedolce F, Zanelli M, Zizzo M, et al. Primary Pulmonary B-Cell Lymphoma: A Review and Update. Cancers (Basel) 2021;13:415. [Crossref] [PubMed]
- Borie R, Wislez M, Antoine M, et al. Lymphoproliferative Disorders of the Lung. Respiration 2017;94:157-75. [Crossref] [PubMed]
- Borie R, Wislez M, Antoine M, et al. Pulmonary mucosa-associated lymphoid tissue lymphoma revisited. Eur Respir J 2016;47:1244-60. [Crossref] [PubMed]
- Liu X, He H, Li Y, et al. The application of antigen receptor gene rearrangement of BIOMED-2 in the pathologic diagnosis of 348 cases with non-Hodgkin lymphoma in a single institution in Southwest of China. Pathol Res Pract 2019;215:152615. [Crossref] [PubMed]
- Farrell AM, Farrell SA, Kennedy MP, et al. Pulmonary Mucosa-Associated Lymphoid Tissue Lymphoma- A Single Center Review of the Diagnostic Approach. Clin Oncol Res 2020; [Crossref]
- Bianchi R, Dubini A, Asioli S, et al. Transbronchial cryobiopsy: an effective tool in the diagnosis of lymphoproliferative disorders of the lung. ERJ Open Res 2020;6: [Crossref] [PubMed]
- Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respiration 2018;95:188-200. [Crossref] [PubMed]
- Hetzel J, Linzenbold W, Boesmueller H, et al. Evaluation of Efficacy of a New Cryoprobe for Transbronchial Cryobiopsy: A Randomized, Controlled in vivo Animal Study. Respiration 2020;99:248-56. [Crossref] [PubMed]
- Deng W, Wan Y, Yu JQ. Pulmonary MALT Lymphoma has variable features on CT. Sci Rep 2019;9:8657. [Crossref] [PubMed]
- Niwa T, Baba T, Tabata E, et al. A pilot study of endobronchial ultrasound guided cryobiopsy using extra-large size guide sheath. Eur Respir J 2019;54:OA1620.
- Kho SS, Chan SK, Yong MC, et al. Performance of transbronchial cryobiopsy in eccentrically and adjacently orientated radial endobronchial ultrasound lesions. ERJ Open Res 2019;5:00135-2019. [Crossref] [PubMed]
- Gupta A, Youness H, Dhillon SS, et al. The value of using radial endobronchial ultrasound to guide transbronchial lung cryobiopsy. J Thorac Dis 2019;11:329-34. [Crossref] [PubMed]
- Jiang S, Liu X, Chen J, et al. A pilot study of the ultrathin cryoprobe in the diagnosis of peripheral pulmonary ground-glass opacity lesions. Transl Lung Cancer Res 2020;9:1963-73. [Crossref] [PubMed]
- Kho SS, Chai CS, Nyanti LE, et al. Combination of 1.1 mm flexible cryoprobe with conventional guide sheath and therapeutic bronchoscope in biopsy of apical upper lobe solitary pulmonary nodule. BMC Pulm Med 2020;20:158. [Crossref] [PubMed]
- Herath S. Use of the 1.1 mm cryoprobe through the radial EBUS GS (without the need for a bronchial blocker) to obtain samples safely in diagnosing PPL. Respirol Case Rep 2023;11:e01128. [Crossref] [PubMed]
- Mirili C, Paydas S, Ogul A, et al. Pulmonary MALT lymphoma with underlying interstitial lung disease: A case report and review of the literature. J Oncol Sci 2018;4:56-9.
- Echevarria-Uraga JJ, Pérez-Izquierdo J, García-Garai N, et al. Usefulness of an angioplasty balloon as selective bronchial blockade device after transbronchial cryobiopsy. Respirology 2016;21:1094-9. [Crossref] [PubMed]
- Zhang L, Wu H, Wang G. Endobronchial ultrasonography using a guide sheath technique for diagnosis of peripheral pulmonary lesions. Endosc Ultrasound 2017;6:292-9. [Crossref] [PubMed]


