Ginsenoside Rg1 attenuates hypoxia and hypercapnia-induced vasoconstriction in isolated rat pulmonary arterial rings by reducing the expression of p38
Introduction
Chronic obstructive pulmonary disease (COPD) is a major ongoing public health problem, and is considered as a major contributor to morbidity and mortality worldwide (1). Recent study has shown a high prevalence of pulmonary arterial hypertension (PAH) in COPD, especially in cases of advanced COPD, with the probability between 30% and 70% (2). Meanwhile, COPD patients with PAH demonstrate more severe symptoms, increased exacerbation risk and poorer outcomes (3). Hypoxic vasoconstriction, pulmonary hyperinflation and endothelial dysfunction are considered the most important mechanisms of PAH (4). Pulmonary vasoconstriction is the intrinsic response to hypoxia of pulmonary arterial smooth muscle cells (PASMCs) and endothelial cells. However, the effect of CO2 on pulmonary vascular tone is controversial, with available evidences on both vasoconstriction (5) and vasodilatation (6). Despite the existing contradictory reports, it is a widely accepted that chronic hypercapnia is a common finding in patients with progressive hypoxic lung disease (7). Thus, in the present study, a model of hypoxia and hypercapnia, rather than only hypoxia, was chosen, in order to match the clinical setting of quite a number of COPD patients.
Panax notoginseng saponin (PNS), the active ingredient of traditional Chinese medicine Panax notoginseng (Burk.) F. H. Chen, is a compound form of several saponins, its major bioactive saponins are ginsenoside Rg1 (Figure 1), ginsenoside Rb1, and notoginsenoside R1. Previously, it has been have been identified that PNS exerts beneficial effects on vessel dilation, myocardial consumption of oxygen reduction, platelet aggregation inhibition, free radicals removal, and antioxidation (8-11). The preventive effect of PNS on chronic hypoxic pulmonary hypertension in rats has been reported previously (12). Recent research shows that ginsenoside Rb1 and Rg1 may decrease hypertension via the stimulation of endothelial-dependent vessel dilation (10). However, whether ginsenoside Rg1 exerts any effect on hypoxia and hypercapnia-induced vasoconstriction is still a matter of research.
p38 mitogen-activated protein kinase (p38) is one of the important members in mitogen-activated protein kinases (MAPK) families, it has been documented to mediate a variety of biomedical functions regulating such as cell proliferation, migration (13), and apoptosis (14). Based on our previous study, PNS might also attenuate hypoxia and hypercapnia-induced pulmonary vasoconstriction (HHPV) by inhibiting the p38 pathway (15), we intended to explore the role of ginsenoside Rg1 in attenuating hypoxia and hypercapnia-induced vasoconstriction and the related underlying mechanism of p38.
Methods
Experimental materials
Male adult Sprague-Dawley rats were provided by Experimental Animal Center of Wenzhou Medical University, weighing (380±20) g. Cells were isolated from rat lungs. Twenty-five rats were used for isolation of arterial rings and ten rats were used for cell isolated. All experimental procedures were carried out in accordance with international accepted guidelines on laboratory animal use, the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health.
Chemicals and antibodies
Ginsenoside Rg1 (>98% pure determined by HPLC) was provided by the Department of Organic Chemistry, College of Preclinical Medicine, Jilin University (Jilin, China). The p38 specific inhibitor SB203580 was purchased from Biosource (Foster City, CA, USA). Dulbecco’s minimal essential medium (DMEM), fetal bovine serum (FBS) and bovine serum albumin (BSA) were purchased from Gibco (California, U.S Carlsbad, CA, USA). SABC-FITC (POD) kit, DAB staining kit, phosphate buffered saline (PBS), sodium dodecyl sulfonate (SDS) and Tris-buffered saline were purchased from Takara Biotechnology (Dalian, Shandong, China). Anti-phospho-p38 (rabbit anti-mouse) and anti-p38 (rabbit anti-mouse) monoclonal antibodies and goat anti-rabbit IgG/HRP were purchased from Cell Signal Technology (Genetimes Technology Inc., Shanghai, China). BCA protein assay kit and enhanced chemiluminescent kit were purchased from Pierce (Genetimes Technology Inc.). Collagenase from Clostridium histolyticum (Type I) (CAS number 9001-12-1) and acetycholine were purchased from Sigma (St. Louis, MO, USA). All other reagents were of analytical reagent grade or HPLC grade where applicable.
Tension measurements
All animals were housed under temperature-controlled conditions (21–23 °C), and were allowed free access to food and water and exposed to a 12:12 h light-dark cycles.
Rats were anesthetized by pentobarbital sodium (50 mg/kg i.p.). The second order branches (<200 µm) were separated from the pulmonary trunk and cut into 3 mm long ring segments at 4 °C in oxygenated Krebs buffer that contained (in mM): NaCl, 130; KCl, 4.7; CaCl2, 2.5; KH2PO4, 1.2; MgSO4, 1.2; NaHCO3, 25; glucose, 11; and EDTA, 0.1. Subsequently, each segment was suspended between two triangular stainless steel wires in organ bath filled with Krebs buffer maintained at 37 °C. One of the triangular wires was mounted to a stable hook, while the other was attached to a force displacement transducer (Panlab, Cornellà, Spain) connected to PowerLab 4 passages electrophysiolograph (Ad Instruments, NSW, Australia). The arteries were stretched to a resting tension of 450 mg (which tested as the optimal preload by a preliminary experiment) for 1 h under a normoxic condition (95% O2 + 5% CO2; pH =7.35). Endothelium-intact rings were tested by the endothelium-dependent relaxation of acetylcholine (10−5 M) after pre-constriction with phenylephrine (10−5 M). After the rings were washed with Krebs buffer and equilibrated for 30 min, the equilibrium contractile force (P0) was recorded. Isometric contraction was determined and contractile force (P0) was recorded incessantly when the rings were gassed either with normoxia (95% O2 + 5% CO2) or with hypoxia and hypercapnia (92% N2 + 8% CO2). Consequently, the rate of the contractile force change, P% = [(P-P0)/P0 ×100%] was calculated.
To explore the role played by ginsenoside Rg1, the monomer was dissolved in DMSO at 8, 40, and 100 mg/L, respectively. Pulmonary arterial rings, after pretreatment with different dosage of ginsenoside Rg1 for 20 min, were exposed to conditions of hypoxia and hypercapnia with ginsenoside Rg1 solution in the organ bath. A significant effect of ginsenoside Rg1 on hypoxia and hypercapnia-induced vasoconstriction in pulmonary arterial rings was observed and the optimal concentration of ginsenoside Rg1 was determined based on the obtained data.
Our previous study showed that p38 inhibitor SB203580 contributed toward the release of HHPV by PNS. To determine whether ginsenoside Rg1 has an imperative role in this pathway, SB203580 (10−5 mol/L) was added into the organ bath for a further 20 min of incubation after the treatment of rings with the selected optimal Rg1 (8 mg/L) solution. Subsequently, hypoxic and hypercapnic conditions were employed and the changes in contractile force were recorded.
Preparation and culture pulmonary arterial smooth muscle cells (PASMCs)
Primary cultures of PASMCs were prepared as previously described (16). The purity of the cells was >95%, confirmed by observing the cells under an inverted phase contrast microscope or by fluorescence microscopy after fluorescence staining with specific monoclonal antibody raised against smooth muscle α-actin. Passage 3–5 PASMCs were used for experiments.
PASMCs were planted at a density of 500,000 cells/well in a six-well plate with 10% FBS DMEM. After reaching 80–90% confluence, the cells were starved for with serum-free DMEM 24 h (17) before pharmacological intervention was enforced. All the scheduled medication was given within 30 min of the initiation of experiment. PASMCs were randomly divided into four groups: (I) normoxia group (N group); (II) hypoxia + hypercapnia group (H group); (III) hypoxia + hypercapnia + DMSO (0.05%) group (HD group); (IV) ginsenoside Rg1 (8 mg/L) treatment group (RgL group). N group was incubated under normoxia (5% CO2, 21% O2, 37 °C) for 24 h, H group, HD group, and RgL group were incubated under hypoxia and hypercapnia (6% CO2, 1% O2, 37 °C) atmosphere for 24 h.
Western blot analysis
PASMCs in six-well plates were gently washed thrice with cold PBS, then, incubated in RAPI lysate containing 1 mM protease inhibitor, phenylmethyl sulfonyl fluoride (PMSF) and 10 mM NaF for 30 min on ice. The lysate was next centrifuged at 12,000 rpm for 5 min at 4 °C. The supernatant were collected. The total protein concentration in the supernatant was determined by the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA) with BSA as the standard. Protein samples (40 µg) from different groups were boiled in SDS sample buffer for 5 min before the initiation of separation process on a 10% SDS-polyacrylamide gel. Separated proteins were transferred to PVDF membranes by electroblotting. After being blocked with 5% skim milk in Tris-buffered saline plus Tween 0.05% (TBST) for 1 h at room temperature, the membranes were then washed and incubated with an anti-phosphospecific p38 antibody (1:1,000) at 4 °C overnight. The next day, membrane was incubated with anti-rabbit IgG:horseradish peroxidase conjugate (1:4,000) for 2 h after washed with TBST thrice. The Enhanced Chemiluminescence (ECL) System (Amersham Pharmacia Biotech, Piscataway, NJ, USA) was used to detect the bound antibody (18). Experiment was repeated thrice by employing total p38 as an internal control.
Statistical analysis
Data are expressed as means ± SE, and n corresponds to the number of tissue rings or cells examined. Analysis of variance for tension change rate was conducted by repeated measurements and multivariate analysis of variance was used for multiple comparisons between different groups at every time point. As for the comparison of multiple groups, statistical analyses were performed using one-way ANOVA followed by Dunnett’s test. Differences were considered significant when P<0.05 and highly significant when P<0.01.
Results
Direct impact of hypoxia and hypercapnia on the contractile force of pulmonary arterial rings
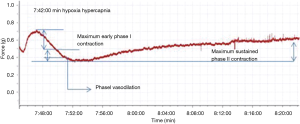
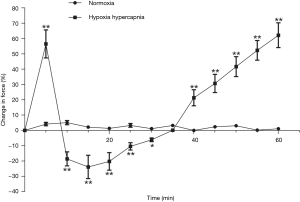
Contractile force variation rate of the second order pulmonary rings exhibited no obvious change when gassed with 95% O2 + 5% CO2, however it presented an inverted parabola curve under the conditions of hypoxia and hypercapnia (92% N2 + 8% CO2). Figure 2 demonstrates a rapid, transient vasoconstriction lasting for about 5 min (phase I vasoconstriction) with vasodilation for 10 min (phase I vasodilation) followed by a slowly developing vasoconstriction that is sustained as long as hypoxia and hypercapnia are present (phase II vasoconstriction). The change of contractile force induced by hypoxia and hypercapnia showed significant differences at the same time points when compared with those induced by normoxia (Figure 3).
The role of ginsenoside Rg1 in pulmonary arterial vasoconstriction induced by hypoxia and hypercapnia and the possible underlying mechanism
After a treatment with DMSO, the pulmonary arterial rings showed no significant difference in the vasoconstriction induced by hypoxia and hypercapnia (Figure 4). Ginsenoside Rg1, at a dosage of 8 mg/L, evidently released phase I vasoconstriction and inverted phase II vasoconstriction, while a higher dosage of ginsenoside Rg1 (100 mg/L) partly reinforced the hypoxia and hypercapnia biphasic vasoconstriction (Figure 5). Whereas, 40 mg/L ginsenoside Rg1 showed no distinct effect on hypoxia and hypercapnia biphasic vasoconstriction (Figure 5). As a result, 8 mg/L was selected as the optimal concentration of ginsenoside Rg1 for attenuating HHPV (Figure 5).
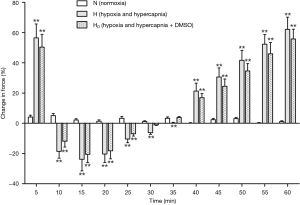
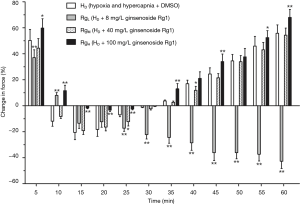
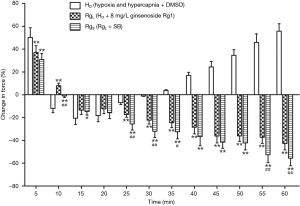
After the combined actions of ginsenoside Rg1 (8 mg/L) and a p38 inhibitor, SB20358 (10−5 mol/L), phase I vasoconstriction was evidently released and phase II vasodilatation was produced. The evident remission of hypoxia and hypercapnia induced pulmonary vasoconstriction showed significant difference when compared with the effect produced from ginsenoside Rg1 only (P>0.05). The combined action of ginsenoside Rg1 (8 mg/L) and SB20358 (10−5 mol/L) evidently released phase I vasoconstriction and even inverted phase II vasoconstriction. This remission of HHPV exhibited reinforcement when compared to the effect of only ginsenoside Rg1 (Figure 6).
Effects of the p38 signaling pathway on pulmonary arterial smooth muscle cells (PASMCs) cultured under conditions of hypoxia and hypercapnia
The phospho-p38 (P-p38) protein expression, as evidenced by western blot analysis, was quite limited in PASMCs under normoxia. On the contrary, the expression of P-p38 protein was apparently higher in a statistically significant manner under hypoxia and hypercapnia (Figures 7 and 8). No significant changes in the expression of P-p38 protein were noted between PASMCs with or without DMSO (Figures 7,8).
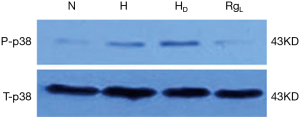
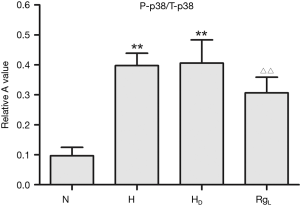
The treatment of PASMC with ginsenoside Rg1 (8 mg/L) resulted in a significant decrease in the expression of P-p38 under hypoxic and hypercapnic conditions (Figures 7,8).
Discussion
Recently, there has been increasing experimental and clinical evidence to prove the statement that pulmonary vasoconstriction, whether induced by only hypoxia or hypoxia and hypercapnia, contributes significantly in the development of pulmonary hypertension. Massive research has been performed to uncover the mechanisms; nevertheless the results still remain inconsistent. Schwenke et al. (19) demonstrated that hypoxic pulmonary vasoconstriction only appeared in pulmonary arterioles with a diameter less than 0.5 mm, while Lu et al. (20) reported that major intra/extra-pulmonary arteries also apparently responded to hypoxia. In our current study, the second order pulmonary rings manifested a biphasic vasoconstrictive under hypoxia and hypercapnia, and the results are in accordance with Tsai’s study (21).
Some researchers presume that pulmonary vasoconstriction possibly results from the release of various vasoactive substances induced by hypoxia, which mainly include nitrogen oxide (NO), calcitonin gene-related peptide (CGRP), endothelin, and agents with dual effects such as bradykinin and histamine (22,23). On the contrary, some studies suggest the direct effects of hypoxia on PASMCs with consequent contraction and ultimate appearance of pulmonary vasoconstriction. The biphasic vasoconstriction as observed in the present study is considered to be associated with an elevation of PASMCs intracellular calcium (Ca2+), which is variously attributed to voltage-dependent and -independent Ca2+ entry, Ca2+ release from ryanodine sensitive or store operated Ca2+ entry (SOCE); moreover, the sustained phase has also been demonstrated to be highly dependent on RhoA/Rho kinase (ROCK)-mediated Ca2+ sensitization (24). It is worth mentioning that the distribution of L-voltage dependent calcium channels in PASMCs differs from the vessel diameter (25). The thinner vascular branches comprise more L-voltage dependent calcium channels. This fact may at least partially explain why pulmonary artery branches of varying diameters respond differently to hypoxia.
It is generally believed that PNS is involved in cell proliferation, differentiation, apoptosis regulation, and Ca2+-overload blocking (26-28). Clinically, PNS was mainly used to treat cardio-cerebrovascular diseases and the central nervous system diseases. In recent years, the role of PNS in modulating pulmonary hypertension and pulmonary heart disease has attracted a large amount of attention. And it is considered as a calcium channel blocker, which may interrupt the calcium influx induced by noradrenalin. We have previously demonstrated that PNS can relax the rat pulmonary rings under hypoxic and hypercapnic conditions (29). In this study, we tested the hypothesis about ginsenoside Rg1 by treating isolated pulmonary arterial rings with ginsenoside Rg1 of different dosage both before and during the conditions of hypoxia and hypercapnia. Our hypothesis was confirmed by the observation that ginsenoside Rg1, at a dosage of 8 mg/L, evidently released phase I vasoconstriction and inverted phase II vasoconstriction compared with other dosage levels. Thus, 8 mg/L ginsenoside Rg1 was considered as the optimal dosage for attenuating HHPV. Consistent with our results, Chen et al. (30) also demonstrated that ginsenoside Rg1 could improve the auricle microcirculation in mice and lengthen clotting time by expanding the caliber of small vessels.
However, the intimate mechanisms of ginsenoside Rg1 and the details of the target cell still require further investigations. Previous research provides evidence on the imperative role of MAPK, including p38 pathway, in the development of pulmonary vasoconstriction (31). Balhara et al. (32) supported the concept that p38 is involved in IgE-mediated upregulation of smooth muscle myosin light chain kinase (smMLCK), which plays a major role in human airway smooth muscle cells (HASM) cell hypercontractility and hypertrophy. Furthermore, Zhang et al. (33) suggested that MIF enhanced vasoconstriction of pulmonary artery elicited by agonist with the involvement of p38. In our study, p38 inhibitor SB20358 induced an increase in the attenuating effect of ginsenoside Rg1 on pulmonary vasoconstriction. In an additional study, we cultured PASMCs under hypoxic and hypercapnic condition and observed the upregulation of p38. All the results obviously indicate that the MAPK signaling pathway may be the key mediators in the pathogenesis of HHPV.
Recent research has demonstrated the antiproliferative effect of ginsenoside Rg1 on SW480 human colorectal cancer cells (34). In fact, increased proliferation of PASMCs, as well as the excess accumulation of the extracellular matrix in the walls of small pulmonary muscularized arteries, are the main pathological processes of hypoxic pulmonary vascular structural remodeling and chronic hypoxia and hypercapnia-induced pulmonary hypertension; however, the exact pathogenesis is not completely uncovered. MAPK is considered as the cross-talk point and common pathway of intracellular signal transmission mediating cell proliferation and hypertrophy. Researchers have also shown the participation of p38 in smooth muscle cell proliferation (35,36), including human PASMC (37). Considering the up-regulation of p38 in our present study, we might as well presume their contribution in the pathogenesis of HHPV by promoting the proliferation of PASMCs, although further experiments are required to confirm the hypothesis.
Nevertheless, ginsenoside Rg1 at low dosage has been proven to attenuate hypoxia and hypercapnia-induced vasoconstriction. At a higher dosage, ginsenoside Rg1 (100 mg/L) was found to partly reinforce the hypoxia and hypercapnia biphasic vasoconstriction. In addition, it has reported that PNS at a low dosage (0.5–3 g/L) promote vascular smooth muscle cells proliferation, while a high dosage (6 g/L) resulted in anti-proliferation (38). In order to identify the detailed mechanism, further investigation will be required. The established evidences along with our observation states that ginsenoside Rg1 possesses the potential to treat pulmonary hypertension and there might be several underlying mechanisms which require further research.
Conclusions
In summary, the present study has proven that isolated pulmonary arterial rings suffer a biphasic vasoconstriction under the conditions of hypoxia and hypercapnia. It is hypothesized that ginsenoside Rg1, a main active ingredient of PNS, may attenuate and even invert vasoconstriction, and the effect can be significantly enhanced by SB20358, the p38 inhibitor. In PASMCs cultured under hypoxic-hypercapnic conditions, MAPKs signal pathway was activated and this activation can be attenuated by ginsenoside Rg1. Our findings strongly support the significant role of ginsenoside Rg1 in the inhibition of hypoxia-hypercapnia induced vasoconstriction by the p38 pathway, and propose that ginsenoside Rg1 can be employed as a novel therapeutic target for patients with COPD and other chronic lung diseases.
Acknowledgements
Funding: This work was supported by the key project of Traditional Chinese Medicine Development Plan of Zhejiang province (2008ZA017, 2013ZZ011) and the Key Construction Academic Subject (Traditional Chinese Medicine) of Zhejiang Province (2012-XK-A28).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All experimental procedures were carried out in accordance with international accepted guidelines on laboratory animal use, the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health.
References
- Boeck L, Tamm M, Grendelmeier P, et al. Acute effects of aerosolized iloprost in COPD related pulmonary hypertension - a randomized controlled crossover trial. PLoS One 2012;7:e52248. [Crossref] [PubMed]
- Calcaianu G, Canuet M, Schuller A, et al. Pulmonary Arterial Hypertension-Specific Drug Therapy in COPD Patients with Severe Pulmonary Hypertension and Mild-to-Moderate Airflow Limitation. Respiration 2016;91:9-17. [Crossref] [PubMed]
- Minai OA, Chaouat A, Adnot S. Pulmonary hypertension in COPD: epidemiology, significance, and management: pulmonary vascular disease: the global perspective. Chest 2010;137:39S-51S. [Crossref] [PubMed]
- Gologanu D, Stanescu C, Bogdan MA. Pulmonary hypertension secondary to chronic obstructive pulmonary disease. Rom J Intern Med 2012;50:259-68. [PubMed]
- Brimioulle S, Lejeune P, Vachiery JL, et al. Effects of acidosis and alkalosis on hypoxic pulmonary vasoconstriction in dogs. Am J Physiol 1990;258:H347-53. [PubMed]
- Barer GR, Shaw JW. Pulmonary vasodilator and vasoconstrictor actions of carbon dioxide. J Physiol 1971;213:633-45. r. [Crossref] [PubMed]
- Xia XD, Xu ZJ, Hu XG, et al. Impaired iNOS-sGC-cGMP signalling contributes to chronic hypoxic and hypercapnic pulmonary hypertension in rat. Cell Biochem Funct 2012;30:279-85. [Crossref] [PubMed]
- Yao Y, Wu WY, Liu AH, et al. Interaction of salvianolic acids and notoginsengnosides in inhibition of ADP-induced platelet aggregation. Am J Chin Med 2008;36:313-28. [Crossref] [PubMed]
- Qiang H, Zhang C, Shi ZB, et al. Protective effects and mechanism of Panax Notoginseng saponins on oxidative stress-induced damage and apoptosis of rabbit bone marrow stromal cells. Chin J Integr Med 2010;16:525-30. [Crossref] [PubMed]
- Pan C, Huo Y, An X, et al. Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vascul Pharmacol 2012;56:150-8. [Crossref] [PubMed]
- Yue QX, Xie FB, Song XY, et al. Proteomic studies on protective effects of salvianolic acids, notoginsengnosides and combination of salvianolic acids and notoginsengnosides against cardiac ischemic-reperfusion injury. J Ethnopharmacol 2012;141:659-67. [Crossref] [PubMed]
- Ma W, Yang Y, Zhou D, et al. The preventive effect of Panax Notoginseng saponins (PNS) on chronic hypoxic pulmonary hypertension in rats. Chinese J Pathophysiol 2004;6:036.
- Gan J, Li P, Wang Z, et al. Rosuvastatin suppresses platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferation and migration via the MAPK signaling pathway. Exp Ther Med 2013;6:899-903. [PubMed]
- Avisetti DR, Babu KS, Kalivendi SV. Activation of p38/JNK pathway is responsible for embelin induced apoptosis in lung cancer cells: transitional role of reactive oxygen species. PLoS One 2014;9:e87050. [Crossref] [PubMed]
- Ma YC, Chen HE, Huang LJ, et al. Role of SB203580 in enhancement of hypoxia hypercapnia-induced pulmonary vasoconstriction by voltage-dependent K+ channel blocker 4-aminopyridione in rats. Chin J Pathophysiol 2013;29:2268-76.
- Guo L, Qiu Z, Zhang L, et al. Hypoxia suppresses Kv 2.1 channel expression through endogenous 15-hydroxyeicosatetraenoic acid in rat pulmonary artery. J Physiol Sci 2010;60:373-81. [Crossref] [PubMed]
- Wang C, Li JF, Zhao L, et al. Inhibition of SOC/Ca2+/NFAT pathway is involved in the anti-proliferative effect of sildenafil on pulmonary artery smooth muscle cells. Respir Res 2009;10:123. [Crossref] [PubMed]
- Ohnishi R, Matsui-Yuasa I, Deguchi Y, et al. 1'-acetoxychavicol acetate inhibits adipogenesis in 3T3-L1 adipocytes and in high fat-fed rats. Am J Chin Med 2012;40:1189-204. [Crossref] [PubMed]
- Schwenke DO, Pearson JT, Umetani K, et al. Imaging of the pulmonary circulation in the closed-chest rat using synchrotron radiation microangiography. J Appl Physiol 1985;2007:787-93. [PubMed]
- Lu W, Wang J, Shimoda LA, et al. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 2008;295:L104-13. [Crossref] [PubMed]
- Tsai BM, Wang M, Pitcher JM, et al. Hypoxic pulmonary vasoconstriction and pulmonary artery tissue cytokine expression are mediated by protein kinase C. Am J Physiol Lung Cell Mol Physiol 2004;287:L1215-9. [Crossref] [PubMed]
- Pichler Hefti J, Sonntag D, Hefti U, et al. Oxidative stress in hypobaric hypoxia and influence on vessel-tone modifying mediators. High Alt Med Biol 2013;14:273-9. [Crossref] [PubMed]
- Baliga RS, Macallister RJ, Hobbs AJ. Vasoactive peptides and the pathogenesis of pulmonary hypertension: role and potential therapeutic application. Handb Exp Pharmacol 2013;218:477-511. [Crossref] [PubMed]
- Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol 2009;9:287-96. [Crossref] [PubMed]
- Franco-Obregón A, López-Barneo J. Differential oxygen sensitivity of calcium channels in rabbit smooth muscle cells of conduit and resistance pulmonary arteries. J Physiol 1996;491:511-8. [Crossref] [PubMed]
- Sun HX, Pan HJ, Pan YJ., et al. Haemolytic activities and immunologic adjuvant effect of Panax notoginseng saponins. Acta Pharmacol Sin 2003;24:1150-4. [PubMed]
- Si YC, Zhang JP, Xie CE, et al. Effects of Panax notoginseng saponins on proliferation and differentiation of rat hippocampal neural stem cells. Am J Chin Med 2011;39:999-1013. [Crossref] [PubMed]
- Xu L, Liu JT, Liu N, et al. Effects of Panax notoginseng saponins on proliferation and apoptosis of vascular smooth muscle cells. J Ethnopharmacol 2011;137:226-30. [Crossref] [PubMed]
- Zhu AN, Lin LN, Wang WT, et al. Effect of Panax notoginoside on the hypoxia hypercapnia induced pulmonary vasoconstriction. J Wenzhou Med Coll 2008;38:29-32.
- Chen C, Su X, Zhang J, et al. The effects of R_ (1) and R_ (d) extracted from panax notoginseng saponins on microcirculation and blood coagulation. J West China Univ Med Sci 2001;33:550-2.
- Mathieson FA, Nixon GF. Sphingolipids differentially regulate mitogen-activated protein kinases and intracellular Ca2+ in vascular smooth muscle: effects on CREB activation. Br J Pharmacol 2006;147:351-9. [Crossref] [PubMed]
- Balhara J, Redhu NS, Shan L, et al. IgE regulates the expression of smMLCK in human airway smooth muscle cells. PLoS One 2014;9:e93946. [Crossref] [PubMed]
- Zhang B, Luo Y, Liu ML, et al. Macrophage migration inhibitory factor contributes to hypoxic pulmonary vasoconstriction in rats. Microvasc Res 2012;83:205-12. [Crossref] [PubMed]
- Wang CZ, Xie JT, Fishbein A, et al. Antiproliferative effects of different plant parts of Panax notoginseng on SW480 human colorectal cancer cells. Phytother Res 2009;23:6-13. [Crossref] [PubMed]
- Jacob T, Ascher E, Alapat D, et al. Activation of p38MAPK signaling cascade in a VSMC injury model: role of p38MAPK inhibitors in limiting VSMC proliferation. Eur J Vasc Endovasc Surg 2005;29:470-8. [Crossref] [PubMed]
- Lee SJ, Park SS, Kim WJ, et al. Gleditsia sinensis thorn extract inhibits proliferation and TNF-α-induced MMP-9 expression in vascular smooth muscle cells. Am J Chin Med 2012;40:373-86. [Crossref] [PubMed]
- Tantini B, Manes A, Fiumana E, et al. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol 2005;100:131-8. [Crossref] [PubMed]
- Liu S, Wang N, Huang J, et al. Effects of total Panax notoginseng saponin on proliferation and JNK phosphorylation of rat vascular smooth muscle cells. J Sun Yat-Sen Univ Med Sci 2008;4:011.