Protective effect of angulated aorta for saving coronary artery during endovascular repair for ascending aortic pseudoaneurysm
Introduction
Ascending aortic pseudoaneurysm is a rare complication which occurs in less than 0.5% of all cardiothoracic surgical cases (1). Open surgical repair is most frequently advised for treatment of pseudoaneurysm. However, the open surgical repair of a pseudoaneurysm is challenging because of high risk of bleeding (2-4). We reported on a patient who developed an ascending aortic pseudoaneurysm after aortic valve replacement (AVR), which was treated successfully with the implantation of a stent graft.
Case presentation
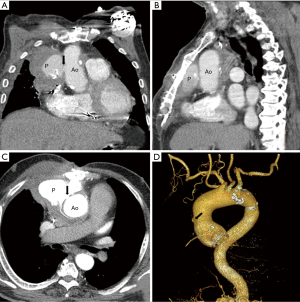
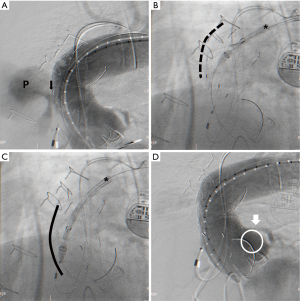
We report the case of a 74-year-old male patient had undergone AVR due to severe aortic stenosis 3 years ago. Immediate after the surgery, massive bleeding occurred at the cystostomy site of the ascending aorta, which was surgically repaired with rubber band. He did well after the surgery for a while. However he was recently readmitted due to mild hemoptysis for 1 week. The chest X-ray showed widening of the mediastinal shadow and chest CT angiography showed an anterior para-aortic hematoma with a pseudoaneurysm (6 cm × 6 cm) communicating with the ascending thoracic aorta (Figure 1). The hematoma extended to the right anterior chest wall with soft tissue swelling. However, the cardiac surgeon hesitated to perform open surgical repair because of the risk of massive bleeding and the patient also refused open surgical repair. CT angiography was reviewed carefully for endovascular repair. Although the length of the distal landing zone was adequate, the distance between the entry point of the pseudoaneurysm and left coronary artery (LCA) was short (less than 10 mm). However, endovascular repair was the only option. From the right CFA, a custom-made 22-Fr delivery SEAL thoracic stent graft (48 mm × 60 mm, S&G Biotech, Seoul, Korea) was deployed in the ascending aorta during temporarily rapid ventricular pacing, but the stent graft migrated proximally slightly. There was a risk of LCA occlusion due to migration of the stent graft. Fortunately, the patient was hemodynamically stable and there was no electrocardiographic change. Follow-up angiography showed good flow to LCA but unusual expansion of the stent graft was noted. However, consistent flow to the pseudoaneurysm through the entry point was still noted (Figure 2A). Due to the severely angulated distal part of the ascending aorta, the proximal part of the stent graft might not have been fully expanded, causing unusual expansion of the stent graft. At this point, we discussed the utility of an additional stent graft to occlude the entry point of the pseudoaneurysm at the proximal ascending aorta. Our idea was, even though we had placed an additional stent graft at the proximal part of the first stent graft, the LCA might not be blocked by the second stent graft due to severe angulation of the proximal ascending aorta. This angulation might create space between stent graft and LCA, which might allow maintenance of flow to LCA. Therefore we prepared another stent graft (46 mm × 60 mm, S&G Biotech, Seoul, Korea) and delivered it to the ascending aorta. However, during delivery of the second stent graft, the first stent graft migrated to the proximal part of the ascending aorta by pushing force of the second stent graft (Figure 2B,C). But the patient’s vital signs were stable and there was no electrocardiographic change. Immediate angiography after removal of the second stent graft showed complete exclusion of the pseudoanerysm without blockage of LCA (Figure 2D). For stabilization of the first stent graft, the second stent graft was delivered again at the distal part of the first stent graft with overlapping technique. Final angiography showed no flow to the pseudoaneurysm (Figure 3A). The patient was discharged without additional complications 2 weeks after the procedure. Follow-up CT angiography performed 1 month after the procedure showed no migration of stent graft and no additional extravasation of contrast material toward the pseudoaneurysm, suggesting its occlusion (Figure 3B-D).

Discussion
In some cases, the endovascular repair of the pseudoaneurysm might be performed safely in the presence of special anatomical circumstances allowing an adequate landing zone for the stent graft. In our case, based on CT angiography, the endovascular repair was not feasible. The distance between LCA and entry point of the pseudoaneurysm was less than 10 mm, which was not adequate for position of the stent graft. Fortunately the length of the distal landing zone was adequate. During the procedure, the first stent graft was positioned with an unexpected pattern. At that time, we needed to decide on whether to perform an additional procedure, but that procedure could cover LCA ostium. We thought, because there was space between stent graft and ostium of LCA created by radial force of the stent graft to the greater curvature and severe angulation of the proximal aorta, even with an additional stent graft at the proximal part of the first stent graft, LCA might be safe. In this case, several complications during follow-up period might be noted. First, thrombotic complication is possible due to local turbulence at the space between stent graft and left aortic cusp. To avoid this complication, antithrombotic agents should be considered. Second, there is a risk of stent graft migration and occlusion of coronary artery ostium. For stabilization of the stent graft, we placed the additional stent graft at the distal part of the stent graft with overlapping technique. However, if the aortic anatomy is transformed in the future, the stent graft can migrate. Currently, there is no evidence for use of endovascular therapy for ascending aortic psuedoaneurysm and there are many limitations, including landing zone, and risk of injury of coronary artery or aortic valve. Our case, however, showed that endovascular therapy might be an option for management of ascending aortic pathology in patients with high surgical risk, particularly patients with a severely angulated proximal ascending aorta.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Katsumata T, Moorjani N, Vaccari G, et al. Mediastinal false aneurysm after thoracic aortic surgery. Ann Thorac Surg 2000;70:547-52. [Crossref] [PubMed]
- Dumont E, Carrier M, Cartier R, et al. Repair of aortic false aneurysm using deep hypothermia and circulatory arrest. Ann Thorac Surg 2004;78:117-20; discussion 120-1. [Crossref] [PubMed]
- Emaminia A, Amirghofran AA, Shafa M, et al. Ascending aortic pseudoaneurysm after aortic valve replacement: Watch the tip of the cardioplegia cannula! J Thorac Cardiovasc Surg 2009;137:1285-6. [Crossref] [PubMed]
- Navaravong L, Saab F, Cook JR, et al. Ascending aortic pseudoaneurysm, a ticking bomb after cardiac surgery. Cardiovasc Revasc Med 2011;12:177-80. [Crossref] [PubMed]


