
Dual-portal robotic-assisted thoracic surgery (DRATS) as a reduced port RATS: early experiences in three institutions in Japan
Highlight box
Key findings
• Dual-portal robotic-assisted thoracic surgery (DRATS) for early-stage non-small cell lung cancer seems to be safe, feasible, and less invasive than multiportal robotic-assisted thoracic surgery.
What is known and what is new?
• RATS for early-stage lung cancer is conventionally performed via four to five incisions.
• Uniportal RATS (URATS) is less invasive than conventional RATS but requires specific techniques and surgical skills.
• We introduced DRATS as an alternative to URATS that is less invasive than conventional multiportal RATS.
What is the implication, and what should change now?
• DRATS is safe and feasible, with excellent perioperative outcomes.
• DRATS seems to be easier to perform than URATS and should be considered as a step toward the transition from multiportal RATS to URATS.
• Future advances in robotic technology will aid in the transition from DRATS to URATS.
Introduction
Video-assisted thoracoscopic surgery (VATS) lobectomy was introduced to treat non-small cell lung cancer in 1993 (1), and early VATS lobectomy was performed using three or four ports, including a 4–6 cm utility incision. With increasing experience and the development of thoracoscopic instruments, the number and size of ports have decreased, resulting in single-port lobectomy referred to as uniportal VATS (2). VATS techniques with fewer ports are less invasive than those with multiple ports and are associated with improved cosmetic outcomes, decreased postoperative pain, faster rehabilitation, and improved patient satisfaction (3-5).
In 1999, a robotic surgical system was introduced that provided surgeons with three-dimensional vision and angulated movements that were not possible with ordinary VATS instruments (6). Almost two decades have passed since the first robotic pulmonary lobectomy was performed (7), and many thoracic surgeons have improved the surgical techniques of robotic procedures based on their experiences. However, as robotic platforms are designed for four robotic arms, four to five incisions have been considered necessary for most thoracic approaches, which contrasts with the concept of minimal invasiveness. The blending of the uniportal approach with robotic technology would bring enormous improvements in feasibility, safety, oncological outcomes, and postoperative recovery. Recent studies have described the technique of uniportal robotic-assisted thoracic surgery (URATS) (8-10). However, before performing URATS, it is recommended that surgeons perform biportal robotic-assisted thoracic surgery (RATS), which has one port in addition to the main access port, considering the characteristics of the robotic stapler and the risk of arm-to-arm interference (10). Given the surgical safety of reduced-port RATS, the implementation of biportal RATS could be beneficial.
Considering our experience with uniportal VATS and standard robotic techniques, we recently started performing biportal RATS, hereafter referred to as dual-portal RATS (DRATS). Herein, we present our preliminary series of DRATS for early-stage lung cancer, focusing on feasibility, safety, surgical technique, and early postoperative outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1141/rc).
Methods
Study design and participants
Based on our experiences with uniportal VATS and multiportal RATS, the DRATS concept was conceived and initiated in December 2022. Twenty consecutive patients with lung cancer (7 men and 13 women; mean age 72.5±9.6 years, range 44 to 85 years) underwent anatomical lung resections via DRATS from December 2022 to May 2023 at three Japanese institutions (Nihonkai General Hospital, Yamagata University Hospital, and Tokyo Metropolitan Bokutou Hospital). The data of 20 patients were retrospectively evaluated in the present study. All patients underwent preoperative screening consisting of blood biochemistry, chest radiography, chest computed tomography, positron emission tomography-computed tomography, pulmonary function testing, echocardiography, and brain magnetic resonance imaging. A preoperative definitive histological diagnosis was not mandatory. For the patients who were not diagnosed preoperatively, intraoperative frozen section diagnosis was performed through lung wedge resection. Patients with suspected mediastinal lymph node metastasis underwent bronchoscopy/endobronchial ultrasound transbronchial needle aspiration for diagnosis and staging. The patients were followed up until they were discharged from hospital. Data were retrospectively collected from the surgical and medical records. The assessed outcomes were the surgery time, console time, intraoperative blood loss volume, need for blood transfusion, number of harvested lymph nodes, postoperative blood test results, lung expansion on radiographs, numerical rating scale for pain in the early postoperative period, duration of chest tube drainage, length of hospital stay, need for additional non-steroidal anti-inflammatory drugs or opioids to control postoperative pain, early postoperative complications, and deaths. The surgical and postoperative outcomes were assessed using descriptive statistics. There was no loss to follow-up because the patients were only followed up until they were discharged from hospital.
Each hospital surgical team consisted of three surgeons: one main surgeon at the console, and two as bedside assistants. One surgeon from Yamagata University Hospital was always present at each surgery to share information. Our surgical team consists of a total of six skillful thoracic surgeons. Before the introduction of DRATS, each surgeon performed multiportal RATS or uniportal VATS.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Ethics Committee of the Nihonkai General Hospital (No. 005-2-5) on behalf of all participating institutions and individual consent for this retrospective analysis and the publication was waived. Informed consent for surgery was obtained from all patients.
Surgical preparation and techniques
Typical images of the body surface during DRATS are shown in Figure 1. Under general anesthesia with single-lung ventilation, the patients were placed in the lateral decubitus position. The da Vinci Xi Surgical System® (Intuitive Inc., Sunnyvale, CA, USA) was positioned at the patient’s posterior side, and the boom of the patient cart was rotated 90° toward the patient’s head. Conventional targeting was not necessary, and the cross of the laser was placed in the upper part of the skin incision posteriorly, parallel to the spine. To avoid collisions, we did not use all four arms for lung deployment. Arm 1 was canceled when operating on the right lung (arm 2 was used for the camera, arm 3 for the left hand, and arm 4 for the right hand) (Figure 2). Arm 4 was canceled when operating on the left side (arm 3 was used for the camera, arm 1 for the left hand, and arm 2 for the right hand). These techniques were identical to those used in URATS (9).
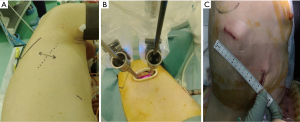

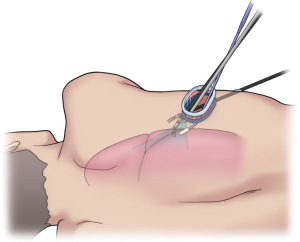
In the DRATS approach, a 3–4-cm working port using a wound protector (Alexis wound retractor XS®, Applied Medical, Rancho Santa Margarita, CA, USA) was created in the fifth intercostal space (ICS) for upper lobe resection and the sixth ICS for middle and inferior lobe resection along the posterior axillary line as a main port. A 1.2–1.5-cm second port using a wound protector (Lap-Protector Mini®, Hakko Co., Nagano, Japan) was created on the sixth or seventh ICS along the anterior axillary line. However, the working port was adjusted in accordance with the patient body shape. We always used a 30-stapler with a curved tip (Endo wrist stapler®, Intuitive Surgical Inc., Sunnyvale, California, USA) and a 45-stapler with a curved tip (Sureform®, Intuitive Surgical Inc., Sunnyvale, California, USA). The 60-stapler was not used because it has restricted internal angulation. One of the reasons for placing another incision in an inferior location was to allow for the correct articulation of the staplers internally.
A 30° camera was used, usually with a downward orientation to reduce forceps interference. However, when dissecting blood vessels and adhesions or using staplers, it was sometimes necessary to change the angle of the camera upward and work with the instrument above the camera. By changing the angle of the camera and adjusting the arm placement according to the vertical position, it was possible to reach any position in the thoracic cavity without restriction.
The surgical field was created using long curved suction forceps and cotton forceps (Delta forceps®; Sugai Corporation, Japan) with the help of the side assistant. This method removes the need for CO2 insufflation. We also used other forceps created for use in uniportal VATS. These additional devices were inserted inferior to the trocar used for the camera to minimize interference with the robotic instruments (Figure 3). At the end of the surgery, the assistant surgeon placed a single chest drain toward the apex. An intraoperative video of a right upper lobectomy using the DRATS approach is provided (11) (Video 1). An intraoperative video of superior mediastinal lymph node dissection using the DRATS approach is provided (Video 2).
Statistical analysis
Clinical and surgical data for all patients were collected from the clinical database of three institution by each institution doctors. All data collected was tabulated using Microsoft Excel for further analysis. Statistical analysis was undertaken using JMP ver.11 (SAS Institute Inc., Cary, NC, USA).
Results
The patient characteristics are shown in Table 1. The surgical details are shown in Table 2. All procedures were completed with the DRATS approach. The main procedure of anatomical resection was lobectomy (16/20; 80%), while four patients (20%) underwent segmentectomies. The mean surgery time was 121±60 minutes (range 60 to 290 minutes) and mean console time was 91±47 minutes (range, 41 to 224 minutes). The mean intraoperative blood loss volume was 9.6±12.1 g (range, 0 to 46 g). No patient required blood transfusion. The mean number of harvested lymph nodes was 9±9 (range, 0 to 34).
Table 1
| Variables | DRATS (n=20) |
|---|---|
| Age (years) | 72.5±9.6 [44–85] |
| Gender | |
| Male | 7 [35] |
| Female | 13 [65] |
| Body size | |
| Height (cm) | 155.1±10.5 [143.7–176.9] |
| Weight (kg) | 59.9±13.2 [42.1–87.2] |
| BMI | 24.0±4.1 [18.7–33.8] |
| Smoking habit | |
| Smoking index | 233±494 [0–1,800] |
| Current/ex-smoker/never smoker | 2 [10]/4 [20]/14 [70] |
| Respiratory function | |
| FVC (mL) | 3,090±936 [2,150–5,020] |
| FEV1 (mL) | 2,341±620 [1,670–3,870] |
| FEV1% | 76.3±6.4 [63.7–85.9] |
| Preoperative diagnosis | 5 [25] |
| Histology | |
| Adenocarcinoma | 19 [95] |
| Squamous cell carcinoma | 1 [5] |
| Clinical tumor size (cm) | 2.1±0.8 [0.8–3.6] |
| c-stage (8th edition) | |
| 0 | 0 [0] |
| IA1 | 6 [30] |
| IA2 | 5 [25] |
| IA3 | 6 [30] |
| IB | 2 [10] |
| IIA | 1 [5] |
Values are presented as n [%] or mean ± standard deviation [range], as appropriate. DRATS, dual-portal robotic-assisted thoracic surgery; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; FEV1%, FEV1/FVC.
Table 2
| Variables | DRATS (n=20) |
|---|---|
| Laterality (right/left) | 15 [75]/5 [25] |
| Lobectomy | 16 [80] |
| RUL | 5 [25] |
| RML | 2 [10] |
| RLL | 7 [35] |
| LUL | 0 [0] |
| LLL | 2 [10] |
| Segmentectomy | 4 [20] |
| Lingular | 1 [5] |
| Left superior segment of inferior lobe | 2 [10] |
| Right basal | 1 [5] |
| Surgery time (min) | 121±60 [60–290] |
| Console time (min) | 91±47 [41–224] |
| Number of harvested lymph nodes | 9±9 [0–34] |
| Blood loss (g) | 9.6±12.1 [0–46] |
| Duration of chest tube drainage (day) | 2±1 [0–6] |
| Length of hospital stay (day) | 5±2 [3–12] |
| Conversion to thoracotomy | 0 |
| Morbidity | 0 |
| Mortality | 0 |
Values are presented as n [%] or mean ± standard deviation [range], as appropriate. DRATS, dual-portal robotic-assisted thoracic surgery; RUL, right upper lobectomy; RML, right middle lobectomy; RLL, right lower lobectomy; LUL, left upper left upper; LLL, left lower lobectomy.
The postoperative management followed the enhanced recovery after surgery protocol (12). Based on this protocol, the chest tube was removed 4 hours after surgery or the next morning. Patients were allowed to drink water from 2 hours after surgery and started eating on the day of surgery. Analgesia was achieved by administering non-steroidal anti-inflammatory drugs three times daily starting on the day of surgery. After chest tube removal, all patients were discharged following the confirmation of no abnormal findings on blood tests and normal lung expansion on radiographs.
The mean numerical rating scale for pain was 2±1 (range, 0 to 5) on the first postoperative day, 1±1 (range, 0 to 3) on the third day, and 1±1 (range, 0 to 3) at discharge. The mean duration of chest tube drainage was 2±1 days (range, 0 to 6 days). The mean hospital stay (and therefore the follow-up time) was 5±2 days (range, 3 to 12 days). No patient required additional adjunctive administration of drugs to control postoperative pain and no opioid drugs were administered. There were no complications and no perioperative deaths.
Discussion
Minimally invasive surgery has become the standard in lung cancer surgery. The National Comprehensive Cancer Network Guidelines version 3.2023 state that VATS or minimally invasive surgery (including robotic-assisted approaches) should be strongly considered for patients without anatomic or surgical contraindications, as long as the standard oncologic and anatomic principles of thoracic surgery are not compromised (13). Additionally, in high-volume centers with significant VATS experience, VATS lobectomy in selected patients results in improved early outcomes (i.e., decreased pain, reduced hospital stay, more rapid return to function, and fewer complications) without compromising cancer outcomes (13). The use of robotic-assisted surgery has recently increased with the advent of innovations and technologies including three-dimensional vision, removal of physiological tremors, camera stability, and a shorter learning curve compared with traditional endoscopic surgery.
In the field of thoracic surgery, uniportal VATS is reported to be the least invasive approach available for not only major lung resections such as wedge resection, lobectomy, and segmentectomy, but also for advanced surgeries such as bronchovascular resection and reconstruction (14,15). However, RATS uses three or four incisions plus a utility incision of 4 cm (16), which makes it more invasive than uniportal VATS with a single incision (17). In other words, there is a contradiction in that VATS can be done with a single incision, while RATS, with its technological innovations, requires numerous skin incisions. Thus, the traditional RATS evolved into URATS based on the uniportal VATS techniques, and is now spreading worldwide (8-10).
Compared with uniportal VATS, URATS has several problems. First, as three 8.0-mm-diameter arms are inserted through a single incision, there is a risk of interference and collision between the arms. Second, the third arm is not used and specific techniques are required to create a good surgical field. Third, as the instrumentation used for URATS differs from that used for traditional RATS, it is essential for assistants to be familiar with the techniques of uniportal VATS and surgeons must practice avoiding instrument collisions (10). Thus, the role of the assistant is very important in URATS. Fourth, in patients with a small chest cavity, the insertion and angulation of the stapler may be compromised by the limited space (9). The da Vinci stapler is particularly difficult to maneuver when performing URATS in small-bodied Japanese patients. Therefore, we devised DRATS, which adds a stapler port on the caudal side in addition to the main surgical incision.
DRATS has some advantages over URATS. First, the use of a second port in DRATS allows the da Vinci stapler and forceps to be maneuvered around all parts of the thoracic cavity without interference. In addition, at the end of the operation, the second port can be used as an incision for inserting a thoracic drain. Second, in contrast to conventional RATS, the main port of DRATS is placed on the cranial side, making it easier to deal with emergencies such as massive bleeding and calcified lymph nodes (18). Third, DRATS results in less pain and better postoperative outcomes than three-port RATS lobectomy (19), which is consistent with the early results of the present study. Fourth, as DRATS only uses three arms (i.e., 2 instruments instead of 3) and does not require CO2 insufflation, the procedure is cheaper than traditional RATS. The high cost of the robotic platform is one of the main limitations of the introduction and maintenance of RATS in many hospitals worldwide.
The present study has several limitations. First, assistants in DRATS must have the skills required for uniportal VATS. Second, as this study reports our experiences during the initial introductory period of DRATS, the cohort may comprise a high number of patients with relatively good conditions. Therefore, there were no cases of left upper lobectomy. However, it will be necessary to find the efficacy of DRATS and accumulate cases including left upper lobectomy. Third, this retrospective study had a small sample size. Fourth, as this study focused on evaluating the effect of a reduced number of ports in robotic pulmonary resection, we did not evaluate the oncological outcomes, including survival or recurrence. Further study is required to evaluate the oncological outcomes of patients who have undergone DRATS.
Based on our experience, we believe that DRATS is an improvement over the current URATS procedure with its associated difficulties. However, a retrospective cohort study of VATS reported that the uniportal approach is less invasive with lower pain levels than the dual-portal approach (20). The present study is one of the first steps toward the implementation of URATS.
Conclusions
Our early results suggest that DRATS is safe, feasible, and comparable to conventional multiportal RATS, offering excellent perioperative outcomes. We believe that it is very important to master DRATS before introducing URATS, and that future advances in robotic technology will aid in the transition from DRATS to URATS.
Acknowledgments
We thank Kelly Zammit, BVSc, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1141/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1141/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1141/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1141/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Ethics Committee of Nihonkai General Hospital, Yamagata, Japan, on behalf of all other institutions (No. 005-2-5) and individual consent for this retrospective analysis and the publication was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg 1993;56:1248-53. [Crossref] [PubMed]
- Bertolaccini L, Batirel H, Brunelli A, et al. Uniportal video-assisted thoracic surgery lobectomy: a consensus report from the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;56:224-9. [Crossref] [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [Crossref] [PubMed]
- Park SY, Kim HK, Jang DS, et al. Initial Experiences With Robotic Single-Site Thoracic Surgery for Mediastinal Masses. Ann Thorac Surg 2019;107:242-7. [Crossref] [PubMed]
- Wang L, Liu D, Lu J, et al. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer 2017;17:75. [Crossref] [PubMed]
- Cadiere GB, Himpens J, Vertruyen M, et al. The world's first obesity surgery performed by a surgeon at a distance. Obes Surg 1999;9:206-9. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Motas N, et al. Uniportal robotic-assisted thoracic surgery for lung resections. Eur J Cardiothorac Surg 2022;62:ezac410. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Manolache V, et al. Uniportal fully robotic-assisted major pulmonary resections. Ann Cardiothorac Surg 2023;12:52-61. [Crossref] [PubMed]
- Manolache V, Motas N, Bosinceanu ML, et al. Comparison of uniportal robotic-assisted thoracic surgery pulmonary anatomic resections with multiport robotic-assisted thoracic surgery: a multicenter study of the European experience. Ann Cardiothorac Surg 2023;12:102-9. [Crossref] [PubMed]
- Hiroki E. DRATS; Dual portal RATS, right lower lobectomy. Uploaded to YouTube by Hiroki Ebana MD, PhD, 2023 Apr 8. Available online: https://youtu.be/FBjvk_Tx_eI.
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Ng CSH, MacDonald JK, Gilbert S, et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innovations (Phila) 2019;14:90-116. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Soultanis KM, Chen Chao M, Chen J, et al. Technique and outcomes of 79 consecutive uniportal video-assisted sleeve lobectomies. Eur J Cardiothorac Surg 2019;56:876-82. [Crossref] [PubMed]
- Aprile V, Ceccarelli I, Korasidis S, et al. A narrative review on lymphadenectomy: from open to minimally invasive surgery. Video-assist Thorac Surg 2022;7:6.
- Han KN, Lee JH, Hong JI, et al. Comparison of Two-Port and Three-Port Approaches in Robotic Lobectomy for Non-Small Cell Lung Cancer. World J Surg 2022;46:2517-25. [Crossref] [PubMed]
- Hu CG, Zheng K, Liu GH, et al. Effectiveness and postoperative pain level of single-port versus two-port thoracoscopic lobectomy for lung cancer: a retrospective cohort study. Gen Thorac Cardiovasc Surg 2021;69:318-25. [Crossref] [PubMed]


