
Surgical approaches for thoracic outlet decompression in the treatment of thoracic outlet syndrome
Introduction
Thoracic outlet syndrome (TOS) is characterized by neurovascular compression in the thoracic outlet. The thoracic outlet extends from the supraclavicular (SC) fossa to the axilla. Structures that may experience compression in the thoracic outlet are the brachial plexus, the subclavian vein (SV), and the subclavian artery. Compression may be caused by bony, muscular, or fibrous structures. Usual places of compression are the scalene triangle, the costoclavicular space, and the pectoral minor space. TOS can be subdivided into three subgroups based on the compressed structure with subsequent complaints. Compression or irritation of the brachial plexus is called neurogenic TOS (NTOS) (1,2). A stenosis or occlusion of the SV at the costoclavicular junction, often with surrounding collaterals, is called venous TOS (VTOS) (1). An objectifiable, persistent abnormality (stenosis or dilatation) of the subclavian artery as a result of extrinsic compression by an anomalous first or cervical rib or other abnormal anatomical structure is called arterial TOS (ATOS) (1).
NTOS is the most common type of TOS, and it is claimed that it makes up 80–90% of all TOS patients (3-5). Common NTOS complaints are pain, paresthesia, and muscle weakness in the neck, shoulder, arm, and/or hand. In general, symptoms often worsen when the brachial plexus is stretched or when arms are reached above the level of the shoulder. Most NTOS complaints can be separated of VTOS and ATOS based on thorough clinical evaluation, however a combination of subtypes is possible. VTOS complaints consist of swelling, pain, heaviness, and distended veins in the upper extremity. Occasionally blue discoloration of the arm can occur. Depending on the underlying condition of the SV (complete occlusion or stenosis), complaints can be present in rest, or provoked by use. In ATOS patients, ischemic manifestations such as pain, coldness, and pallor of the arm due to peripheral micro embolisms can be seen. Although a subset of the TOS patients present with discoloration of the arm, only a minority of patients present with ATOS. Clinicians should be aware that discoloration of the arm, hand and or fingers is more often due to autonomic dysregulation in NTOS cases than as a result of ATOS.
The diagnostic pathway of NTOS differs from both vascular TOS types. Both vascular subtypes, VTOS and ATOS, have an anatomic substrate that can be objectively identified with computed tomography (CT), magnetic resonance imaging (MRI) and/or angiography (venography). These modalities show signs of compression and/or damage of the subclavian artery or vein in patients with ATOS or VTOS. However, NTOS is difficult to diagnose because an objective diagnostic test with sufficient sensitivity and specificity is lacking. Besides, the definition of NTOS in the available literature differs, and outcome parameters of both the diagnostic care pathway and treatment diverge. This makes the diagnosis complex, especially considering that presenting complaints of a patient with possible NTOS overlap with many differential diagnoses. In an attempt to overcome these difficulties, the reporting standards for TOS were constructed in 2016 (1). Based on these standards, we developed a care pathway for the three subtypes of TOS (6).
In this overview article we will discuss the characteristics and diagnosis of NTOS, VTOS and ATOS, and we will discuss treatment of TOS via three approaches used in thoracic outlet decompression (TOD).
TOS
The exact pathophysiology of TOS is still unclear. At least two factors that result in an increased risk for TOS are known. First, there are numerous anatomical variations, both congenital and acquired, such as an anomalous first rib, post-traumatic callus formation on the clavicle and/or first rib, a cervical rib and/or a wide array of abnormal inserted ligaments and muscles (for example a scalene minimus muscle). Secondly, it is thought that a history of trauma or repetitive strain injury of the arm can be the onset of a cycle of scar tissue formation and muscle hypertrophy causing subsequent compression in the thoracic outlet (7).
NTOS
NTOS can be subdivided based on the existence of muscular atrophy. Some patients present with a recognizable pattern of muscular atrophy of the forearm and hand combined with sensory disturbances. NTOS patients with this so-called Gilliatt-Sumner hand (GSH), are rarely seen, as the majority present without evident muscular atrophy. This group of NTOS patients with less evident and more common complaints has a history full of controversy and still remains the most difficult group of TOS patients to diagnose.
NTOS was first described by Stopford and Telford in 1920 (4). Based on their description confusion started that would last for decades. In 1970, ‘true’ NTOS was introduced to describe patients with a GSH by the British neurologist Roger Gilliatt, resulting in NTOS being classified as extremely rare with an incidence of 1:1,000,000 (8). Subsequently, this definition was broadened in literature to include ‘disputed’ NTOS patients. The terms ‘true’ and ‘disputed’ NTOS again resulted in a lot of confusion in the late 20th century. As a result, physicians started to question the mere existence of NTOS. Many NTOS patients nowadays remain undiagnosed and thus untreated.
NTOS-GSH
As described, NTOS patients present rarely with a GSH (9). Common complaints in these patients are numbness, a tingling sensation or pain in the lower arm, stiffness, clumsiness, and loss of strength in the hand (10). In addition, a typical pattern of weakness/atrophy of the M. abductor digiti minimi (Figure 1), abductor pollicis brevis (Figures 1,2) and interossei is seen. This is often combined with hypesthesia of the ulnar nerve and the medial cutaneous nerve of the forearm (10).
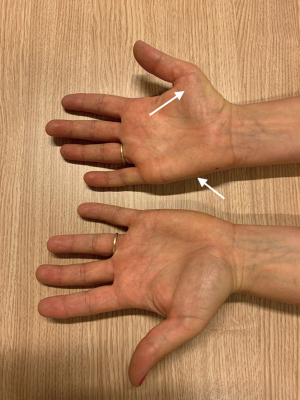
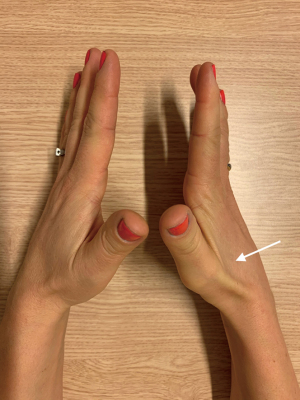
Based on history and physical examination, confirmation of a GSH is done by an electromyogram (EMG) and/or high-resolution ultrasound (HRUS). A typical EMG shows a lowered ‘compound muscle action potential’ (CMAP) over the ulnar and medial nerve and a lowered sensory nerve action potential (SNAP) of the ulnar and cutaneous antebrachia medial nerve in which T1 is more involved than C8, but also deviating patterns of this classic pattern can be seen in GSH patients (11). HRUS of the brachial plexus reveals a so-called wedge sickle sign (WSS). A WSS is caused by a fibromuscular structure at the medial edge of the medial scalene muscle (MSM) that dents the inferior trunk of the brachial plexus. Treatment of a GSH consists of a TOD, as discussed in detail later.
NTOS diagnosis
A large group of patients with NTOS complaints do not have the typical and recognizable GSH complaints, signs, and findings with EMG/HRUS. Patients present with complaints that appear similar to many syndromes of the shoulder, neck, or arm. As a response to a Cochrane Collaboration review of treatment for TOS (12), which concluded a need for generally accepted definitions, and outcome measures, the NTOS reporting standards were constructed in 2016 (13). Based on the reporting standards we constructed a multidisciplinary care pathway in our TOS centers (6).
A correct NTOS diagnosis can be made when three of the following four criteria are present (1):
- Local findings: pain in the chest, shoulder, axilla, neck and/or back, including headache. Local findings include symptoms consistent with inflammation at the scalene triangle.
- Peripheral findings: pain, paresthesia, vasomotor changes and/or weakness of the arm. Peripheral findings include symptoms consistent with nerve compression.
- Every other possible explanation for NTOS complaints should be excluded.
- A positive anterior scalene and/or pectoralis minor test-injection.
Our NTOS care pathway consists of a diagnostic and a therapeutic part. The diagnostic part includes a thorough history taking and physical examination. Anamnesis and physical examination are done separately and independently by a neurologist, a TOS nurse specialist, a TOS-surgeon, and a physical therapist (PT) with extended experience in shoulder and TOS. To diagnose NTOS, the complaints need to exist for at least 12 weeks and symptoms should not be related to a single nerve route (2). Physical examination includes standard neurological examination and 4 provocation tests. The provocation tests are the upper limb tension test (ULTT) (1,4), standardized elevated arm stress test (sEAST) (14), Morley’s compression test and Tinel Sign (at the scalenus anterior/media and/or musculus pectoralis minor) (15). After history taking and physical examination every patient receives an X-ray of the thoracic aperture. This investigation is an anterior to posterior oriented view of the upper thorax and allows for a better view of anatomical abnormalities including cervical ribs, an elongated C7 process and bony abnormalities of the first rib than a conventional X-thorax (16).
In every NTOS diagnosis, a mandatory period of (at least) 3 months of adequate physiotherapy has to be completed. It is one of our TOS physiotherapists that decides whether adequate physiotherapy was provided before referral, based on history taking regarding previous treatment regimens. Every TOS PT has the possibility to postpone or abort further investigation(s) if the physiotherapy requirements are not met.
If a patient is suspected of NTOS and an adequate physiotherapy regimen appeared ineffective, a scalene muscle block (SMB) in the anterior and MSM is performed (17). We define a positive aSMB as an improvement in duration on our sEAST device of at least 50% (6,14). All patients are discussed in a weekly multidisciplinary meeting, involving all specialties participating in the multidisciplinary team.
VTOS
Upper extremity deep vein thrombosis (UEDVT) is uncommon and mostly treated conservatively. Unfortunately, there is some misunderstanding regarding idiopathic or primary UEDVT (Paget-Schroetter Syndrome) and VTOS. We consider VTOS as a secondary cause of UEDVT, and not an idiopathic “primary” condition as it is often classified (18). Due to this misunderstanding, investigation for possible VTOS is often omitted, delayed, or inadequate in the assessment of patients with UEDVT. As a result, patients with VTOS are systematically overlooked and classified as “primary” UEDVT, then typically treated with long-term anticoagulation for varying periods of time (based largely on protocols established for lower extremity DVT) (19,20).
TOS involved health care professionals consider VTOS as a secondary form of UEDVT that can only be diagnosed with contrast-enhanced imaging, usually after catheter-directed thrombolysis (CDT) (21). VTOS should be suspected with the presentation of any otherwise young healthy person with abrupt spontaneous arm swelling, with or without cyanotic discoloration, in the absence of a known central venous catheter, pacemaker, recent arm injury, malignancy, thromboembolic disorder, or history of DVT.
Additional diagnostic tests are needed to diagnose VTOS. Several imaging techniques are proposed [Doppler-ultrasound (Duplex), CT venography (CTV), MR venography (MRV), intravascular ultrasonography (IVUS), and venography]. We consider venography as superior since it is a dynamic investigation which can be performed in provocative maneuvers, providing live footage of the contrast flow in all positions of importance accordingly. The same sheath used for venography can be used to perform CDT in case of UEDVT.
Two different forms of VTOS exist. VTOS caused by thrombosis and VTOS caused by intermittent compression without thrombosis, also called McCleery’s syndrome. The obstruction in all forms is caused by external and intraluminal factors that result in scar tissue generation and stenosis. VTOS caused by thrombosis can be further subdivided in acute (complaints <14 days) and chronic VTOS.
CDT treatment has the highest success rate when applied within 14 days, as fibrosis of the occlusive thrombosis is seen over time. After CDT and lysis of the thrombus a TOD with venolysis and percutaneous transluminal angioplasty (PTA), venoplasty or venous bypass surgery is performed. Patients with chronic VTOS experienced one or multiple UEDVT episodes in the past. As a result, the vein sustained damage due to thrombosis additional on the VTOS induced mechanical trauma of the vessel wall.
ATOS
ATOS is the rarest form of TOS and diagnosis can be made when a diseased subclavian artery (CTA, MRA) causes symptoms due to emboli or stenosis/occlusion or post-stenotic dilatation (aneurysm formation). It is important to consider that 9–53% of the healthy population experience a loss of distal pulsations or a reduced pulse amplitude when holding the arms in a provocative position (22-25). Loss of pulsations at the wrist or fingers or pinging the subclavian artery do not match ATOS. ATOS cases are almost always an indication for surgery as complaints can be progressive with the possibility of acute ischemia (1). ATOS treatment constitutes of a TOD combined with arterial reconstruction if deemed necessary based on the level of damage of the artery.
Treatment
TOD
There are several surgical techniques for the treatment of TOS described in literature. With several other high-volume TOS centers we always perform a complete resection of every possible compressive structure in the thoracic outlet. This encompasses more than a ‘first rib resection’ hence the use of TOD for the surgery performed to emphasize a complete ‘decompression of the thoracic outlet’. The necessity of this is based on our experience with recurrent or persistent TOS, in cases where bony outgrowth of rib remnants and scar tissue from a partially resected scalene muscle (variants) and/or fibrous band appeared to be the cause of recurrent or persistent compression of the brachial plexus (26). Several other authors have warned about recurrence due to regrowth of first ribs or concomitant pectoral minor syndrome (27-34). However, reasonable to good outcomes have been reported with only performing partial first rib resection, only cervical rib resection or scalene muscle transection (1,28,29,35-48).
A primary TOD can be performed through a transaxillary (TA), a SC and a paraclavicular (PC) approach. The infraclavicular (IC) approach is performed by some for VTOS cases but has no role in NTOS or ATOS cases. Recent publications of video- (VATS) and robotic-assisted thoracoscopic (RATS) approach for TOS surgery show promising results (46,49,50). However, in VATS and RATS most authors perform a partial rib resection, which is a known cause of recurrence (as in any approach) as previously stated, transect the scalene muscle (not resect), and do not perform a complete neurolysis of at least the lower brachial plexus. Another difference is that a pectoral minor tenotomy (or tenectomy) is only possible with an extra incision. Albeit promising results in single center reports, we already performed several redo-surgeries for recurrent and persistent NTOS in patients that previously had a VATS or RATS-TOD. To our opinion both approaches might be promising in the hands of skilled thoracoscopic or robotic surgeons, though the clinical advantages and (midterm) results must be validated.
A TA-TOD consists of a complete resection of the first rib (from sternocostal cartilage into and surpassing the costovertebral joint). Any (in-)complete cervical rib is also completely removed. A TA approach makes it impossible to completely remove the scalene muscles. However, a partial resection (1.5–3 cm of the caudal part) is performed, with careful attention for both the phrenic and long thoracic nerve (LTN). If applicable, minimus scalene muscle variants, Sibson’s muscle variants and/or fibrous bands to Sibson’s fascia are completely removed. A thorough neurolysis (resection of the fibrotic tissue) of the lower brachial plexus (inferior trunk, including C8 and T1 root and in most cases C7) is performed routinely.
A SC-TOD allows the surgeon to operate in a readily visualized operative field. A complete anterior and middle scalenectomy can be achieved, which is believed to achieve the best and most durable results. In addition, complete brachial plexus neurolysis, and first rib resection should be performed to minimize recurrence. If possible, pectoralis minor tenotomy has to be considered (28,36,42,51,52).
A PC-TOD allows for optimal exposure via supra- and IC incisions, to allow the surgeon to completely remove the first rib and the associated scalene and subclavius muscles, and to permit SV reconstruction if necessary. PC-TOD is mostly performed when treating VTOS.
We do not favor the IC approach for VTOS, since we favor approaches enabling complete removal of the first rib. However, this is a relatively easy approach giving direct access to the costoclavicular space for focused VTOS treatment and subclavian reconstruction. This approach allows for anterior first rib resection and subclavian venolysis without exposure of the brachial plexus. It remains debatable whether this approach is optimal.
The choice of surgical approach is based on the preferences of the surgical team since no approach has been demonstrated to be superior in literature. However, the TA approach is considered less preferable in patients with a body mass index (BMI) higher as 40. A study performed by Thompson et al. concluded that BMI has no impact on the outcome of surgery via the SC approach (53). The TA approach can be used as a primary approach in TOS surgery for several reasons:
- Through the axilla, the exposure and removal of a complete first rib resection is more easily performed compared to all other approaches.
- The pectoral minor muscle has been frequently (up to 69%) identified as a cause of recurrent or persistent NTOS (27,28). A TA approach enables a perfect exposition to perform a tenectomy of 3–5 cm of the pectoral minor muscle without the need for a separate incision.
- A primary trans-axillary approach leaves the SC region without any scar tissue, making redo-surgery through a SC approach easier to perform. Besides, it prevents damage to the scalene fat pad. This scalene fat pad—if not scarred or otherwise damaged—can be divided at the level of the omohyoid muscle in a deep and a superficial layer and used as a wrap surrounding the completely freed brachial plexus in redo surgery (54).
- The fourth and final argument in favor of a primary TA approach is the fact that it offers by far the best cosmetic result. This should not be underestimated in young patients (1).
The advantages of the SC approach are as follows:
- The ease of exposure to all of the anatomy of the scalene triangle allows the surgeon to perform a more complete decompression than by other approaches. Sc exposure also facilitates recognition and resection of common anatomical variations, such as cervical ribs, the scalene minimus muscle, and fibrofascial bands, that may contribute to nerve compression.
- SC exposure is more readily conducted by vascular, thoracic, and nerve surgeons familiar with this exposure through other areas of practice. The SC approach is also easier to teach to young surgeons due to superior visualization of the relevant anatomy.
- SC exposure allows the surgeon to perform complete anterior and middle scalenectomy, to decompress the entire brachial plexus more thoroughly and to minimize the potential for muscle reattachment as a cause of recurrent nerve compression.
- A complete brachial plexus neurolysis can only be performed through SC exposure, including all five nerve roots (C5-T1) and three trunks (upper, middle, and lower), as well as to allow the surgeon to wrap the nerves upon completion as a means to limit the potential for recurrent nerve compression.
- Vascular control is optimal through SC exposure, either for management of vascular forms of TOS (arterial or venous) or for repair of unexpected vascular injury. When necessary, a parallel IC incision can easily be added, without repositioning of the patient, for open vascular reconstruction. Moreover, the supine position for SC exposure also facilitates percutaneous catheter access (brachial or femoral) for intraoperative fluoroscopy and endovascular procedures, when needed.
- The supine position used for SC decompression makes the addition of pectoralis minor tenotomy, through a small additional deltopectoral groove incision, a straightforward extension of SC decompression. Inclusion of pectoralis minor tenotomy to SC decompression allows the entire path of the brachial plexus to be exposed when necessary.
TA-TOD (including pectoral minor tenectomy)
After successful administration of general anesthesia, the patient is placed in the lateral decubitus position with the operative side up. A Trimano (Arthrex, Naples, FL, USA) arm holder is used to position the arm at the right angle (Figure 3). The head is tilted to the affected side providing relaxation of the brachial plexus.
An incision of 5 centimeters is made at the base of the axillary hair line in between pectoral major and latissimus dorsi muscles. The subcutaneous fat is split onto the less firm axillary fat. We identify the lateral border of the pectoral major muscle and create a tunnel medially towards the coracoid process of the scapula. Care is taken not to damage the medial and especially lateral pectoral nerves that run in between de major and minor pectoral muscles. The pectoral minor muscle attaches to the coracoid process; however, the fibers of the pectoral major can sometimes partially attach to the pectoral minor muscle. With gentle blunt dissection, the correct plane between the two muscles can be found. The pectoral major muscle fibers are placed behind a retractor, creating overview of the anatomy in this region, and isolating the pectoralis minor muscle from all surrounding tissue. Beneath the pectoral minor muscle, the axillary vein and brachial plexus can be in close proximity. When the correct plane is verified a rubber loop is placed around the pectoral minor muscle and pulled caudally. A clamp is placed onto the more cranial fibers of the pectoral minor muscle just below the coracoid process and division of the muscle fibers is performed with a LigasureTM (Maryland 23, Medtronic/Covidien IIc, Mansfield, MA, USA). After the rubber band is released due to this division of the muscle we then pull the clamp caudally and transect the pectoral minor fibers in close proximity to the coracoid process. In this way it is easy to resect up to 4-5 centimeters of pectoral minor muscle fibers, completely freeing the retro pectoral fossa, that is carefully inspected and palpated after resection for fibrous bands.
Dissection and mobilization of the axillary fat
We then return to the axilla and make a new entry, more laterally, and start with mobilization of the axillary fat. We create a tunnel onto the rib cage, below the axillary fat. This can, for the major part, be performed with blunt dissection. During this dissection we look for the intercostal-brachial nerve 2 (ICB2) and maintain great care to identify and preserve this nerve. If this nerve however is completely centrally located in the field of surgery, we transect this nerve below the level of the intercostal muscles (reducing the chance of a neuroma). The nerve is retracted behind either the medial or lateral retractor. Traction of the ICB2 nerve easily leads to allodynia and should therefore be avoided (55).
Dissection of the thoracic outlet
The dissection of the thoracic outlet starts after identification of the SV medially. The thoracic outlet is dissected using a systematic approach going from vein (medially) to T1/inferior trunk (laterally). If small venous or arterial branches are present, we use the LigasureTM to ligate these vessels. With this technique, we completely dissect and free the caudal part of vein, artery, and T1/inferior trunk before moving into the thoracic outlet. This way, the anatomy can be identified ‘with relative ease’ and the risk of creating a tunnel and unclear anatomy is avoided. Using this approach makes it almost impossible to inadvertently resect the 2nd rib, a complication that is still occurring.
Now, the costoclavicular ligament lies medial in the thoracic outlet and forms the medial boundary which can be transected by using monopolar electrocautery. Great care should be taken for the phrenic nerve and SV during this transection. This nerve courses just posteriorly of the costoclavicular ligament and should be protected during transection. We use a forceps with a peanut on the top and place this just behind of the costoclavicular ligament to protect the SV and phrenic nerve.
Now we focus on the subclavian artery (SA). More cranial and posterior than the SV, the SA can be found in the posterior part of the thoracic outlet. There are some side branches that are divided with the LigasureTM. The thyrocervical trunk is often identified and sometimes transected with the aid of the LigasureTM. The mammary artery mostly lies medially and can be seen after the anterior scalene muscle (ASM) is removed. The SA runs posterior of the ASM and dissection of the SA is performed as much as possible from behind the ASM. We then focus on the inferior trunk and identify the T1 and C8 roots posteriorly in the thoracic outlet. When we are certain that the T1 cord has no aberrant position or is covered, we proceed to free the MSM with careful attention for the LTN.
At this part of the operation, we clearly identified from medial to lateral: divided costoclavicular ligament—SV—ASM (phrenic nerve on top/medial border behind SV)—SA—often a scalene minimus muscle or fibrous band coming from transverse process C7, inferior trunk (C8–T1 root) and MSM.
We then use a dissecting forceps to scoop the ASM, whilst paying attention not to harm the SV or phrenic nerve. This forceps is then opened 2–3 centimeters and we use the LigasureTM to transect the ASM as high as possible. The caudal fibers of the ASM on the first rib can then be removed to clarify overview. The same maneuver is performed with the MSM. If we have problems scooping up the complete muscle, we transect the muscle in stages.
First rib resection
By now the first rib is easily identified and the outer-inferior border is dissected free with electrocautery, taking care not to harm the pleura with this maneuver. A small opening underneath the rib is made with the blunt tip of forceps and opened for 6–7 mm. We then push the parietal pleura downwards from the backside of the rib with blunt dissection. This maneuver is strongly facilitated with a collapsed lung. This lowers the upper boarder of the lung and creates more space. Depending on the patient, prolonged periods of apnea (up to 6–8 minutes) are well tolerated.
Once the pleura has been dissected free, the LigasureTM is used to transect the intercostal muscles. Posteriorly, care should be taken not to harm the T1 branch that lies at the medial border of the first rib. Once a major part of the first rib is freed, the overlying tissue (remnants of the ASM, MSM and intercostal muscle) is resected with electrocautery. A Liston bony scissors is used to transect the first rib medially and laterally. An 8 mm straight bone rongeur is used to resect all bony remnants. Again, on the medial side care should be taken not to harm the SV or phrenic nerve, on the lateral side the LTN and T1 root (and in case of a cervical rib the C8/C7 root).
The rib is resected medially until the sternal cartilage is visible. The posterior aspect of the first rib can be a bit more difficult to remove since the first rib lies close to the T1 root (cervical rib C8 root). We often use the suction tube to mildly push the T1 root away from the first rib, whilst performing bone resection with the 8 mm straight bone rongeur. Resection of the posterior part of the first rib is complete when the cartilage of the vertebral body is visible and the bony part of the first rib behind T1 is removed. Going even more medial endangers the sympathetic chain and should be avoided. We then extensively rinse the thoracic outlet with saline fluid to remove all (microscopic) bony fragments.
At the end of the decompression surgery, we look at all reachable roots and trunks of the brachial plexus and resect any overlying tissue that might cause compression. This is performed with scissors. Any overlying fibrous bands or muscle fibers are resected. Orthosympatic (communicating rami) fibers that originate from the brachial plexus roots should not be mistaken for fibrous bands.
SC-TOD
In a SC-TOD, we position the patient in a semi-Fowler position (20/25°) and tilt the head 45° away from the affected side. A transverse incision of 5–7 centimeters is made approximately 2 centimeters above the clavicle, extending from the lateral border of the sternocleidomastoid muscle to the anterior border of the trapezius muscle. During the initial dissection, attention is paid to the SC nerves running from cranio-caudally through the incision. The clavicular head of the sternocleidomastoid muscle is retracted medially but not divided. The scalene fat-pad is mobilized, by detachment along the lateral edge of the internal jugular vein and the superior edge of the clavicle and rotated laterally. The omohyoid muscle is divided and excised. The phrenic nerve is observed descending along the surface of the ASM, from lateral to medial. The scalene fat pad is reflected until the MSM and LTN come in view.
After the scalene fat pad is held in position by retraction sutures a complete resection of the anterior and middle scalene muscle is performed. First the phrenic nerve, subclavian artery, and brachial plexus are isolated. Then the ASM is divided at its insertion, by scissors, at the top of the first rib. The ASM is lifted and mobilized superiorly, and carefully freed of the phrenic nerve by the ‘phrenic flip’ maneuver. The ASM is then dissected to its origin on the C6 transverse processes, divided, and removed. Any anomalous fibrofascial bands passing in front of the lower brachial plexus nerve roots are also resected. The middle scalene muscle is removed next. A small retractor is placed in between the brachial plexus and the middle scalene muscle, and the C5 to T1 nerve roots of the brachial plexus are identified and mobilized. The transverse cervical artery and vein are ligated where they pass through the brachial plexus to prevent traction injury and bleeding. A small retractor is placed in between the LTN and the middle scalene muscle to displace the LTN posterolaterally. The middle scalene muscle is then detached from the surface of the first rib along its insertion, using the electrocautery. It is important to note that if a cervical rib is present, it is typically identified at this point in the dissection. The middle scalene muscle is then excised with scissors, while protecting the LTN. Next the remainder of the intercostal muscle attached to the lateral edge of the first rib is divided with electrocautery. After verifying that the C8 and T1 nerve roots are well protected, the posterior first rib is divided to the level of the costotransverse joint space. To access the anterior first rib, the subclavian artery and brachial plexus are retracted posterolaterally, and the clavicle and SV are elevated with a small retractor. The anterior first rib can now be divided under direct vision immediately medial to the scalene tubercle and the first rib specimen is removed. If a cervical rib was found during middle scalenectomy it is removed in a similar way to the first rib resection, often as a single, combined specimen when a complete cervical rib is present. The final step in a successful SC decompression is brachial plexus neurolysis. Each individual nerve root is freed of investing muscle fibers and perineural fibrous scar tissue. C8 and T1 need to be inspected carefully since there is often a small fibrofascial band overlying these nerve roots.
SC decompression is often combined with pectoralis minor tenotomy. This is performed by a short vertical incision in the deltopectoral groove. The deltoid and pectoralis major muscles are separated and after reaching the axillary space the lateral edge of the pectoralis major is carefully lifted. The pectoralis minor muscle is identified and carefully divided from the coracoid process with the electrocautery, protecting the underlying brachial plexus with a finger placed behind the muscle tendon. At the end of the procedure, the scalene fat pad can be split at the level of the omohyoid muscle into two parts. The deep part can be wrapped underneath the brachial plexus and on the pleura and secured both medial and lateral below the T1 root as well as medially underneath the phrenic nerve, with the superficial part placed over the brachial plexus. An alternative is to wrap the brachial plexus with an absorbable polymer film or with glutaraldehyde-free bovine pericardium, to suppress postoperative adhesions to the brachial plexus nerves as a source of recurrence. A closed-suction drain is placed in the wound at the end of the procedure, often intentionally extending into the upper aspect of the pleura for drainage.
PC-TOD
The PC-TOD used for VTOS involves parallel incisions above and below the clavicle. The start of a PC-TOD is similar to the SC-TOD, with the patient placed in a semi-Fowler position (20/25°) and tilt the head 45° away from the affected side. A transverse incision of 5–7 centimeters is made approximately 2 centimeters above the clavicle, extending from the lateral border of the sternocleidomastoid muscle to the anterior border of the trapezius muscle. First the ASM is removed and then the brachial plexus is mobilized, but in cases of VTOS the brachial plexus is simply mobilized to prevent injury. The middle scalene muscle is removed, after which the posterior first rib can be divided. For removal of the anterior portion of the first rib, a second incision is made one fingerbreadth below the clavicle extending laterally about 6 centimeters from the sternum. The pectoralis major is separated between the upper and middle portions and the first rib is exposed without muscle division, in which identification of the anterior portion of the first rib can be facilitated by putting pressure on the divided posterior portion via the SC incision to better open the costoclavicular space. The subclavius muscle tendon, the muscles of the first intercostal space, and the costoclavicular ligament are all divided from the surface of the first rib. The anterior portion of the first rib is then divided at its attachment to the sternum and the first rib is then removed as a single specimen. The axillary-SV can be identified and well visualized via the lateral portion of the IC incision and carefully separated from the subclavius muscle, and the subclavius muscle is then resected. It is important to check if the SV is obstructed underneath the clavicle, so that it can be freed from surrounding fibrous tissues if necessary. Any remaining residual scar tissue surrounding the SV can then be removed by moving to the SC exposure and tracing the SV to its junction with the internal jugular and innominate veins. After completing the subclavian venolysis, upper extremity venography is performed to determine if axillary-SV patency has been restored. When there is remaining obstruction in the vein, SV reconstruction is performed using a patch angioplasty for focal stenoses or axillary-innominate vein bypass for long-segment occlusions.
Upon completion of the procedure, the pleural apex is opened to eliminate postoperative pneumothorax, and a closed-suction drain is placed via the SC incision with its tip extending in the upper pleura. The scalene fat pad is restored to its anatomic position with sutures. The platysma layer is closed with interrupted sutures and the skin with subcuticular sutures.
Conclusions
TOS has a variety of treatment options. No treatment option is considered best, and it is largely based on the preference of the surgical team and the patients’ characteristics which approach is used for TOD.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Erik de Loos, José Ribas de Campos and Jean Daemen) for the series “Chest Wall Resections and Reconstructions” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-546/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-546/coif). The series “Chest Wall Resections and Reconstructions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Research Ethics Committee United (MEC-U) of the Netherlands (No. W17.004) and informed consent was obtained from all individual participants for publication of this article and accompany images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Illig KA, Donahue D, Duncan A, et al. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome. J Vasc Surg 2016;64:e23-35. [Crossref] [PubMed]
- Balderman J, Holzem K, Field BJ, et al. Associations between clinical diagnostic criteria and pretreatment patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J Vasc Surg 2017;66:533-544.e2. [Crossref] [PubMed]
- Burt BM. Thoracic outlet syndrome for thoracic surgeons. J Thorac Cardiovasc Surg 2018;156:1318-1323.e1. [Crossref] [PubMed]
- Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg 2007;46:601-4. [Crossref] [PubMed]
- Machleder HI. A Brief History of the Thoracic Outlet Compression Syndromes. In: Illig KA, Thompson RW, Freischlag JA, et al., editors. Thoracic Outlet Syndrome. Cham: Springer International Publishing; 2021. p. 7-16.
- Pesser N, Goeteyn J, van der Sanden L, et al. Feasibility and Outcomes of a Multidisciplinary Care Pathway for Neurogenic Thoracic Outlet Syndrome: A Prospective Observational Cohort Study. Eur J Vasc Endovasc Surg 2021;61:1017-24. [Crossref] [PubMed]
- Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg 1992;16:534-42; discussion 542-5.
- Roos DB. Congenital anomalies associated with thoracic outlet syndrome. Anatomy, symptoms, diagnosis, and treatment. Am J Surg 1976;132:771-8. [Crossref] [PubMed]
- Gilliatt RW. Thoracic outlet syndrome. Br Med J (Clin Res Ed) 1983;287:764. [Crossref] [PubMed]
- Gilliatt RW, Le Quesne PM, Logue V, et al. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry 1970;33:615-24. [Crossref] [PubMed]
- Mul K, Pesser N, Vervaart K, et al. Variability in electrodiagnostic findings associated with neurogenic thoracic outlet syndrome. Muscle Nerve 2022;65:34-42. [Crossref] [PubMed]
- Povlsen B, Belzberg A, Hansson T, et al. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2010;CD007218. [Crossref] [PubMed]
- Illig KA, Donahue D, Duncan A, et al. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome: Executive summary. J Vasc Surg 2016;64:797-802. [Crossref] [PubMed]
- Pesser N, de Bruijn BI, Goeteyn J, et al. Reliability and validity of the standardized elevated arm stress test in the diagnosis of neurogenic thoracic outlet syndrome. J Vasc Surg 2022;76:821-829.e1. [Crossref] [PubMed]
- Ho T, Braza ME. Hoffmann Tinel Sign. 2023. Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Raptis CA, Sridhar S, Thompson RW, et al. Imaging of the Patient with Thoracic Outlet Syndrome. Radiographics 2016;36:984-1000. [Crossref] [PubMed]
- Lum YW, Brooke BS, Likes K, et al. Impact of anterior scalene lidocaine blocks on predicting surgical success in older patients with neurogenic thoracic outlet syndrome. J Vasc Surg 2012;55:1370-5. [Crossref] [PubMed]
- Illig KA, Gober L. Optimal management of upper extremity deep vein thrombosis: Is venous thoracic outlet syndrome underrecognized? J Vasc Surg Venous Lymphat Disord 2022;10:514-26. [Crossref] [PubMed]
- Noyes AM, Dickey J. The Arm is Not the Leg: Pathophysiology, Diagnosis, and Management of Upper Extremity Deep Vein Thrombosis. R I Med J (2013) 2017;100:33-6.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep vein thrombosis: A review of the literature. Thromb Res 2017;156:54-9. [Crossref] [PubMed]
- Lee JA, Zierler BK, Zierler RE. The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg 2012;46:139-44. [Crossref] [PubMed]
- Gergoudis R, Barnes RW. Thoracic outlet arterial compression: prevalence in normal persons. Angiology 1980;31:538-41. [Crossref] [PubMed]
- Warrens AN, Heaton JM. Thoracic outlet compression syndrome: the lack of reliability of its clinical assessment. Ann R Coll Surg Engl 1987;69:203-4.
- Colon E, Westdorp R. Vascular compression in the thoracic outlet. Age dependent normative values in noninvasive testing. J Cardiovasc Surg (Torino) 1988;29:166-71.
- Plewa MC, Delinger M. The false-positive rate of thoracic outlet syndrome shoulder maneuvers in healthy subjects. Acad Emerg Med 1998;5:337-42. [Crossref] [PubMed]
- Goeteyn J, Van Der Sanden L, Pesser N, et al. Redo surgery for neurogenic thoracic outlet syndrome is useful. J Vasc Surg 2022;76:531-537.e1. [Crossref] [PubMed]
- Sanders RJ, Rao NM. The forgotten pectoralis minor syndrome: 100 operations for pectoralis minor syndrome alone or accompanied by neurogenic thoracic outlet syndrome. Ann Vasc Surg 2010;24:701-8. [Crossref] [PubMed]
- Sanders RJ. Recurrent neurogenic thoracic outlet syndrome stressing the importance of pectoralis minor syndrome. Vasc Endovascular Surg 2011;45:33-8. [Crossref] [PubMed]
- Urschel HC Jr, Razzuk MA, Albers JE, et al. Reoperation for recurrent thoracic outlet syndrome. Ann Thorac Surg 1976;21:19-25. [Crossref] [PubMed]
- Roos DB. Recurrent thoracic outlet syndrome after first rib resection. Acta Chir Belg 1980;79:363-72.
- Urschel HC Jr, Razzuk MA. The failed operation for thoracic outlet syndrome: the difficulty of diagnosis and management. Ann Thorac Surg 1986;42:523-8. [Crossref] [PubMed]
- Mingoli A, Sapienza P, di Marzo L, et al. Role of first rib stump length in recurrent neurogenic thoracic outlet syndrome. Am J Surg 2005;190:156. [Crossref] [PubMed]
- Jammeh ML, Ohman JW, Vemuri C, et al. Anatomically Complete Supraclavicular Reoperation for Recurrent Neurogenic Thoracic Outlet Syndrome: Clinical Characteristics, Operative Findings, and Long-term Outcomes. Hand (N Y) 2022;17:1055-64. [Crossref] [PubMed]
- Lassner F, Becker M, Prescher A. Relevance of Costovertebral Exarticulation of the First Rib in Neurogenic Thoracic Outlet Syndrome: A Retrospective Clinical Study. J Pers Med 2023;13:144. [Crossref] [PubMed]
- Abdel Ghany W, Nada MA, Toubar AF, et al. Modified Interscalene Approach for Resection of Symptomatic Cervical Rib: Anatomic Review and Clinical Study. World Neurosurg 2017;98:124-31. [Crossref] [PubMed]
- Ambrad-Chalela E, Thomas GI, Johansen KH. Recurrent neurogenic thoracic outlet syndrome. Am J Surg 2004;187:505-10. [Crossref] [PubMed]
- Ann Freischlag J. A Decade of excellent outcomes after surgical intervention: 538 patients with thoracic outlet syndrome. Trans Am Clin Climatol Assoc 2018;129:88-94.
- Caputo FJ, Wittenberg AM, Vemuri C, et al. Supraclavicular decompression for neurogenic thoracic outlet syndrome in adolescent and adult populations. J Vasc Surg 2013;57:149-57. [Crossref] [PubMed]
- Cheng SW, Stoney RJ. Supraclavicular reoperation for neurogenic thoracic outlet syndrome. J Vasc Surg 1994;19:565-72. [Crossref] [PubMed]
- Desai SS, Toliyat M, Dua A, et al. Outcomes of surgical paraclavicular thoracic outlet decompression. Ann Vasc Surg 2014;28:457-64. [Crossref] [PubMed]
- Lafosse T, Le Hanneur M, Lafosse L. All-Endoscopic Brachial Plexus Complete Neurolysis for Idiopathic Neurogenic Thoracic Outlet Syndrome: A Prospective Case Series. Arthroscopy 2017;33:1449-57. [Crossref] [PubMed]
- Likes K, Dapash T, Rochlin DH, et al. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg 2014;28:939-45. [Crossref] [PubMed]
- Lindgren KA, Leino E, Lepäntalo M, et al. Recurrent thoracic outlet syndrome after first rib resection. Arch Phys Med Rehabil 1991;72:208-10.
- Lindgren KA, Oksala I. Long-term outcome of surgery for thoracic outlet syndrome. Am J Surg 1995;169:358-60. [Crossref] [PubMed]
- Longo MF, Clagett OT, Fairbairn JF 2nd. Surgical treatment of thoracic outlet compression syndrome. Ann Surg 1970;171:538-42. [Crossref] [PubMed]
- Peek J, Vos CG, Ünlü Ç, et al. Outcome of Surgical Treatment for Thoracic Outlet Syndrome: Systematic Review and Meta-Analysis. Ann Vasc Surg 2017;40:303-26. [Crossref] [PubMed]
- Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg 1989;10:626-34. [Crossref] [PubMed]
- Yoshizumi T, Murata H, Kanno H, et al. Efficacy of Supraclavicular Scalenotomy Followed by External Neurolysis without Rib Resection for Post-traumatic Neurogenic Thoracic Outlet Syndrome. Spine (Phila Pa 1976) 2021;46:E632-8. [Crossref] [PubMed]
- Gharagozloo F, Meyer M, Tempesta B, et al. Robotic First Rib Resection for Thoracic Outlet Syndrome. Surg Technol Int 2020;36:239-44.
- Burt BM, Palivela N, Cekmecelioglu D, et al. Safety of robotic first rib resection for thoracic outlet syndrome. J Thorac Cardiovasc Surg 2021;162:1297-1305.e1. [Crossref] [PubMed]
- Altobelli GG, Kudo T, Haas BT, et al. Thoracic outlet syndrome: pattern of clinical success after operative decompression. J Vasc Surg 2005;42:122-8. [Crossref] [PubMed]
- Gelabert HA, Jabori S, Barleben A, et al. Regrown first rib in patients with recurrent thoracic outlet syndrome. Ann Vasc Surg 2014;28:933-8. [Crossref] [PubMed]
- Ohman JW, Abuirqeba AA, Jayarajan SN, et al. Influence of Body Weight on Surgical Treatment for Neurogenic Thoracic Outlet Syndrome. Ann Vasc Surg 2018;49:80-90. [Crossref] [PubMed]
- Teijink JAW. Use of Scalene Fat Pad Wrap to Protect the Brachial Plexus After Supraclavicular Thoracic Outlet Decompression for Neurogenic Thoracic Outlet Syndrome. Eur J Vasc Endovasc Surg 2022;63:665. [Crossref] [PubMed]
- Goeteyn J, Pesser N, Houterman S, et al. Surgery Versus Continued Conservative Treatment for Neurogenic Thoracic Outlet Syndrome: the First Randomised Clinical Trial (STOPNTOS Trial). Eur J Vasc Endovasc Surg 2022;64:119-27. [Crossref] [PubMed]



