
Survival following acute type A aortic dissection: a multicenter study
Highlight box
Key findings
• Acute type A aortic dissection (ATAAD) repair using ascending aorta replacement with hemiarch or root-sparing repair leads to favorable early outcomes in patients with urgent and emergency 1 clinical status at presentation.
What is known and what is new?
• Ascending aorta replacement with hemiarch techniques or root replacement and more extensive total arch repair lead to consistently less operative mortality in urgent and emergency 1 patients.
• A conservative approach is recommended in grade 2 emergency and salvage patients due to the increased risk of perioperative mortality.
What is the implication, and what should change now?
• Mortality rates for ATAAD remain high, with a strong association with presentation acuity. The surgeon and centre experience should determine the extent of repair weighing in the additional risks associated with more extensive surgery against the need for further reintervention.
Introduction
Acute aortic syndrome (AAS) is caused by a life-threatening clinicopathological entity involving the aortic wall and should be clinically suspected in patients with a history of hypertension presenting with severe chest pain. Approximately 90% of patients with AAS have an aortic dissection while an intramural hematoma occurs in the remainder (1). AAS can be aggravated by poor multi-visceral perfusion, uncontrollable pain, and hypertension. In some cases, an aneurysm of the aorta coexists. Although AAS is an infrequent entity reaching roughly 3.5–6.0 per 100,000 patient-years, timely diagnosis and treatment is nevertheless essential as an emergency procedure addressing surgery must be considered in most patients (1).
Clinically, AAS may present with the features of acute type A aortic dissection (ATAAD) necessitating emergency surgery. Despite recent progress achieved in surgical techniques and improved management, ATAAD is still linked with significant morbidity and mortality (2-5). Two objectives are essential during the surgery of ATAAD. The first is to save the life of the patient, treating and preventing life-threatening complications e.g., aortic rupture, cardiac tamponade, aortic valve regurgitation, and malperfusion syndrome. The secondary objective is to resect the primary entrance or tear to decrease the flow of blood to the false lumen and to obtain the most complete and stable aortic repair possible to avoid the evolution of the aneurysm, the appearance of secondary aortic regurgitation and reduce the risk of long-term aortic reoperation.
In the majority of cases, the main tear is identified within the ascending aorta, therefore ascending aorta replacement (AAR) is the most frequent surgical technique of repair for ATAAD (6). Usually, an extensive aortic replacement is justifiable if the primary tear is in the aortic root or beyond the ascending aorta. However, resection of the primary tear alone is called into question because even after its resection, residual perfused false lumens are noted at follow-up, with an increased risk for late aortic events (7).
The use of extensive aortic replacement of the aortic root (Ao-R), aortic arch, and descending aorta is constantly debated. It is mandatory to balance the benefits of decreased long-term distal aortic events and the risk of increased early mortality. Recent studies appear to show that a more aggressive approach is associated with better long-term survival and fewer aortic distal events and reinterventions compared with the conservative approach (8-11).
In this study, we investigated the outcome after surgery for ATAAD and assessed the prognostic impact of surgical strategy with and without aortic arch replacement. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1137/rc).
Methods
Study design and oversight
The data consists of patients operated on between 1 January 2005 and 31 December 2021 from three heart surgery institutions (Centre Cardiologique du Nord, Hôpital Henri-Mondor AP-HP, and University of Genoa) and were retrospectively analyzed. The protocol mandated continuous monitoring and checking of the database by analysts, and clinical information within each unit was systematically validated with internal and external audits. During the hospital stay, preoperative and postoperative variables were recorded. For the survival follow-up analysis, there were no missing data. Data integrity was updated annually through an accurate review process of the correspondence of the electronic medical records of the patients by the referring units. Alternatively, follow-up was obtained by directly contacting the referring doctors, the patients themselves, or the family members. This study complied with the Declaration of Helsinki (as revised in 2013). Patient consent was obtained after the assigned ethics approval from the institutional review board of Montpellier University Hospital (IRB approval No. 202201173) in accordance with the research guidance. The other institutions are informed and agreed with the study.
Patients
A total of 601 patients were operated on during the study period and included in the study. Baseline characteristics, demographics, and follow-up data were evaluated. The target population was adults (aged >18 years) with ATAAD or intramural hematoma. Specific inclusion criteria included the presence of the lesion involving the ascending aorta, symptoms within 7 days of surgery, and patients referred for primary surgical repair of ATAAD based on recommendations. In addition, any other major cardiac surgical procedures needed during ATAAD and retrograde extension of ATAAD were considered. Patients who were <18 years old, had previous ATAAD procedures or who had delayed presentation (AAS >7 days prior), traumatic aortic dissections, and endocarditis were also excluded.
The patients were segregated into five categories of increasing hemodynamic severity. In patients requiring “urgent” intervention, the procedure was performed within 24 hours of hospital admission during the initial hospitalization. Patients in this category were paucisymptomatic with stable hemodynamics and did not exhibit any signs of malperfusion. Patients in the “emergency 1” category were symptomatic but stable from a hemodynamic standpoint. They had no signs of malperfusion and/or rupture and surgery was recommended within hours after admission. The “emergency 2” category included patients who required prompt intervention immediately after hospitalization or those who rapidly deteriorated clinically. These patients were characterized by hemodynamic instability refractory to the administration of inotropes and/or malperfusion. These categories of patients require cardiopulmonary resuscitation. “Salvage 1” and “salvage 2” categories included hospitalized patients requiring cardiopulmonary resuscitation by means of either external chest compressions and/or open cardiac massage. These patients were induced and initiated on cardiopulmonary bypass in critical condition. “Salvage 1” patients underwent the surgical procedure with timely initiation of cardiopulmonary bypass due to worsening clinical conditions after anesthesia induction. They either had a cardiac tamponade, acute heart failure, and/or sudden rupture of the aorta. “Salvage 2” patients required cardiopulmonary resuscitation with external chest compressions during transport to the operating room or before anesthesia induction. For these, prompt initiation of cardiopulmonary bypass was required, which was often preceded by cardiac massage after median sternotomy. The surgical procedure was performed without knowledge of genuine aortic rupture or severe organ damage (12).
Endpoints
The primary endpoint of the study was operative mortality (OM) reported as both 30-day and in-hospital mortality. Secondary endpoints included cerebrovascular events including strokes, resulting in permanent neurological deficit (PND), spinal cord injury (SCI), post-operative dialysis dependency, intensive care unit (ICU) stay, respiratory failure requiring tracheostomy, a combination of major adverse events (MAE) including surgical mortality, cerebrovascular accident, new dialysis requirement or tracheostomy initiation and late survival. The primary and secondary endpoints were evaluated alongside the type of surgery performed and the portion of the aorta restored. In addition, the disabling status of the patients at the time of hospital admission was considered.
Surgical technique
Sternotomy was performed for all the patients. The lead surgeon of each institution directed the operative strategy regarding the preferred cannulation site. Arterial cannulation was performed in either the right common femoral artery, brachiocephalic trunk, axillary artery, or centrally via the aortic lumen. Surgical preference also took priority in dictating the degree of systemic cooling. Cardiopulmonary bypass was initiated alongside systemic cooling. Potassium-rich antegrade cardioplegia solution was directly injected into the coronary ostium to achieve diastolic arrest. Alternatively, when ATAAD caused aortic regurgitation or when patients required more radical aortic root replacement (ARR) procedures or extensive repairs involving the aortic arch, the use of a coronary sinus perfusion cannula was placed to deliver the cardioplegic solution retrogradely. The sinotubular junction was the landmark for the resection of the ascending aorta. Thrombus was normally found occupying the false lumen of the Ao-R and/or of the ascending aorta and evacuated to appreciate the extent of the aortic injury. Subsequently, the anatomical integrity of the aortic root and aortic leaflets was evaluated with careful inspection. 4-0 or 5-0 sutures with Teflon pledgets were used to approximate the tunica intima of the commissures to the adventitia to reinforce each commissure for resuspension. This technique has been used both in patients undergoing root sparing-AAR and in those receiving root replacement—AAR if the intimal detachment extended into the sinuses of Valsalva resulting in commissural collapse. Normally neo-media biological glue was used during the reconstruction of the aorta, while the use of felt was driven by surgical preference. A 4-0 or 5-0 polypropylene suture may be used to seal the proximal suture line and this anastomosis also buttressed the two intimal and adventitial walls to ensure solid continuity. Some surgeons used felt as the neo-media or utilized horizontal felt-mattress sutures arranged circumferentially to obtain a continuous outer ring of felt reinforcement. ARR was performed using either biological or mechanical composite valve graft or performing a valve sparing-ARR procedure dictated by the sinuses of Valsalva on computed tomography using 4.5 cm as the cut-off, or if intimal tears were noted in the sinus of Valsalva and patients with connective tissue disease (CTD). Conversely, the preferred surgical option in patients presenting with non-dilated aortic roots but poor-quality valve leaflets was the concomitant replacement of the aortic valve with a bioprosthetic or mechanical prosthesis with the interposition of a Dacron graft.
In patients requiring total arch replacement procedure (TARP), deep hypothermic circulatory arrest (DHCA) was used with continuous antegrade cerebral perfusion or retrograde cerebral perfusion with systemic cooling to a temperature of 19 and 25 ℃ dictated by surgical preference. Symmetrical cooling and re-warming of the brain were continuously monitored using near-infrared spectroscopy. And, 80.5% of patients who had TARP required a protocol for antegrade brain protection which was ensured either by endoluminal technique or with direct insertion of the cannula into the brachiocephalic trunk or left common carotid artery or right axillary artery. A different flow rate was delivered ranging from 800 to 1,000 mL/min when the temperature was fixed at 28 or 36 ℃ and the systemic arterial pressure was maintained between 40–60 mmHg. The remaining TARP (19.5%) utilized DHCA by retrograde cerebral perfusion via a superior vena cava cannula. The delivery of the solution was set at 200–350 mL/min at 18 ℃ and a target central venous pressure of between 25–35 mmHg.
TARPs included partial or total resections. 1- and 4-branch grafts were used both in total excision of the aortic tissue up to zone 2—including the left common carotid artery (total hemiarch) and in less extensive excisions (partial hemiarch) in patients receiving reimplantation of the brachiocephalic trunk only. TARP with reimplantation of the entire great vessel block was the primary surgical option in patients with extensive arch aneurysms or a large intimal lesion involving the inner arch. Patients with CTD or significant dislocation of the great vessels underwent debranching and selective implantation. The same category of subjects were ideal recipients of the frozen elephant trunk (FET) option and received selective debranching/vessel implantation or insular reimplantation. The advantage offered by the 4-branch grafts was the possibility of restoring antegrade cardiopulmonary bypass through the reperfusion arm (lateral branch) of the graft used. Finally, systemic warming was ensured by maintaining a temperature gradient (core blood and internal temperature) of 10 ℃ while performing surgical hemostasis. In this phase, the residual suture line was made and strengthened according to the formerly described procedure. After reaching a body temperature of 36 ℃, cardiopulmonary bypass was discontinued.
Statistical analysis
Descriptive statistics
Categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test as appropriate. Non-parametric continuous variables were compared using the Mann-Whitney U test. Operative (pre, intra and post) variables were compared across the subgroups, to establish an association with the extent of aortic replacement and with the urgency at presentation (as described earlier) (11). Kaplan-Meier curves with log-rank tests were used to assess survival between the groups. P value <0.05 was accepted as significant without adjustment for multiple testing.
Risk adjustment
Risk factors for mortality, during initial hospitalization and subsequent follow-up, were initially evaluated by univariate regression. Continuous variables were factorized according to recognized classification systems. Anemia was classified according to hemoglobin (Hb) levels as per World Health Organization (WHO) guidelines: Hb >110 g/L, grade 0; Hb 95–109 g/L, grade I; Hb 80–94 g/L, grade II; Hb 65–79 g/L, grade III; Hb <65 g/L, grade IV (13). The renal function was classified using the WHO classification for chronic kidney disease because the baseline levels of estimated glomerular filtration rate (eGFR) (i.e. before the dissection occurred) were not captured by the database: eGFR >90, normal; eGFR 80–90, stage 1; eGFR 60–79, stage 2; eGFR 30–59, stage 3; eGFR 15–29, stage 4; eGFR <15, stage 5 (14). Thrombocytopenia was classified: >150,000/mcL, normal; 100,000–150,000 mcL, mild; 50,000–100,000 mcL, moderate; <50,000/mcL, severe (15). Lactate levels were classified as: <2 mmol/L, normal; 2–4 mmol/L, lactic acidosis; >4 mmol/L, severe lactic acidosis (16). Variables with an association of P<0.2 with in-hospital or follow-up mortality, or with recognized clinical significance were retained respectively for the multivariable logistic and the Cox regression models. Multi-collinearity was evaluated with variance inflation factor analysis, and excluded by a value ≤3.
Statistical software
R studio, with appropriate packages, was utilized for the statistical analysis.
Results
Our analysis is summarized in this section, but detailed in the referenced tables and figures to avoid duplication of information.
Overall sample
During the study period, 601 patients received ATAAD repair across the recruiting centers. Clinical variables are detailed in Table 1. Males in their 60 s were the most common presenting demographic. The women in our cohorts were significantly elder, anaemic (P<0.001), and with reduced eGFR compared to men (P<0.001). About half of the patients were operated on in an urgent setting and the remaining half as emergency or salvage cases. The aortic valve was moderate to severely regurgitant in more than a third of cases. Evidence of malperfusion was present in one-quarter of patients, with the brain being the most affected site. AAR (367, 61.1%) was the most commonly performed procedure. Proximal (i.e., root) and distal (i.e., arch) extension of the repair occurred in an equal number of cases (both 105, 17.5%). A root-to-arch extension was used in 24 patients (4.0%). Male patients were more likely to receive AAR and partial or total arch repair compared to women who were more likely to receive a ‘conservative’ procedure undergoing ascending aorta, replacement using an interposed Dacron graft (34,9% P<0.01). The yearly volume by type of repair is detailed in Figure 1. The OM was 146 patients (24.3%) with a steady decrement from 34.3% in 2010 to 27.8% in 2021, despite a substantial correlation with the severity of clinical conditions at hospital admission observed. Stroke was the most common morbidity (75, 12.6%). Table 1 survival at 1-, 5-, and 10-year was 73.3%, 68.2%, and 53.5%, respectively.
Table 1
| Variables | Data |
|---|---|
| Demographics | |
| Age (years) | 64.4 (20.1) |
| BMI (kg/m2) | 25.8 (5.2) |
| Female | 180 (30.0) |
| Biochemistry | |
| Creatinine (mg/dL) | 88.4 (29.1) |
| Hemoglobin (g/dL) | 121.0 (28.5) |
| Platelet count (×109L) | 220.0 (194.5) |
| Arterial lactate (mmol/L) | 2.2 (2.3) |
| Cardiac biomarkers increase | 150 (25.0) |
| Comorbidities and presentation | |
| Diabetes | 36 (6.0) |
| Cerebro-vascular accident | 32 (5.3) |
| Pulmonary disease | 33 (5.5) |
| Extracardiac arteriopathy | 21 (3.5) |
| Poor mobility | 49 (8.2) |
| Moderate-to-severe frailty | 3 (0.5) |
| Recent myocardial infarction | 19 (3.2) |
| Preoperative cardiac massage | 26 (4.3) |
| Status | |
| Urgent | 304 (50.6) |
| Emergency 1 | 107 (17.8) |
| Emergency 2 | 161 (26.8) |
| Salvage 1 | 24 (4.0) |
| Salvage 2 | 5 (0.8) |
| Aortic valve | |
| Bicuspid | 12 (2.0) |
| Moderate or severe regurgitation | 212 (35.2) |
| No trace | 203 (33.8) |
| Malperfusion | 147 (24.5) |
| Cerebral | 80 (13.3) |
| Spinal | 12 (2.0) |
| Renal | 61 (10.1) |
| Mesenteric | 33 (5.5) |
| Peripheral | 32 (5.3) |
| Aortic segments replaced | |
| Ascending only | 367 (61.1) |
| Ascending + root | 105 (17.5) |
| Ascending + arch | 105 (17.5) |
| Ascending + root & arch | 24 (4.0) |
| Type of root procedure | |
| Modified Bentall | 121 (20.1) |
| David procedure | 5 (0.8) |
| Yacoub procedure | 3 (0.5) |
| Type of arch procedure | |
| Hemi-arch | 26 (4.3) |
| Total arch | 52 (8.7) |
| Total arch + FET | 51 (8.5) |
| Type of cerebroplegia | |
| Antegrade | 248 (41.3) |
| Retrograde | 117 (19.5) |
| CPB time (minutes), median (range) | P value <0.1 |
| Overall (N=601): 143.00 (127.00–167.00) | |
| Conservative (n=367): 135.00 (124.00–153.50) | |
| Extensive (n=234): 164.00 (143.00–179.50) | |
| Cardiac ischemia time (minutes), median (range) | P value <0.1 |
| Overall (N=601): 88.00 (73.00–110.50) | |
| Conservative (n=367): 80.00 (70.75–94.00) | |
| Extensive (n=234): 115.00 (94.00–139.50) | |
| Adverse events | |
| Stroke | 76 (12.6) |
| Spinal cord injury | 25 (4.2) |
| Tracheostomy | 27 (4.5) |
| Hemodialysis | 63 (10.5) |
| In-hospital mortality | 146 (24.3) |
| Major adverse events | 240 (39.9) |
| ICU stay (days) | 9.0 (17.0) |
Data are presented as median (IQR) or n (%) unless otherwise stated. Pre/intra/post-operative variables after type A aortic dissection repair. Major adverse events include composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis. BMI, body mass index; FET, frozen elephant trunk; CPB, cardiopulmonary bypass; ICU, intensive care unit; IQR, interquartile range.

Subgroup analysis I: aortic segments
Patients who received less invasive procedures based on root sparing with replacement of ascending aorta, were on average 8 years older than patients receiving extensive repairs (root and/or arch repair). Preoperative clinical and biochemical risk factors were similar across the subgroups. Congenital bicuspid and regurgitant/insufficient aortic valves were more common in patients who underwent root replacement/repair. The extent of repair did not affect OM and MAE, with the exception of a stroke rate that was lowest in the “+ root” group (7.6%) compared to the “+ arch” group (21.9%) (P=0.01). The incidence of stroke (P=0.01), chordal injury (P=0.57), tracheostomy (P=0.67), renal failure requiring dialysis (P=0.35), and MAE (P=0.11) were not significant (Tables 2,3 and Figure 2).
Table 2
| Variables | Ascending only (N=367) | + Root (N=105) | + Arch (N=105) | + Root & arch (N=24) | P value |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age (years) | 67.4 (20.6) | 61.7 (19.8) | 61.1 (16.5) | 59.7 (11.4) | <0.01 |
| BMI (kg/m2) | 25.9 (5.4) | 25.6 (4.3) | 25.6 (4.5) | 25.4 (4.0) | 0.64 |
| Female | 128 (34.9) | 23 (21.9) | 25 (23.8) | 4 (16.7) | <0.01 |
| Biochemistry | |||||
| Cr (mg/dL) | 88.5 (32.4) | 88.0 (19.9) | 88.4 (33.0) | 81.0 (7.0) | 0.50 |
| Hb (g/dL) | 122.0 (28.5) | 122.0 (31.0) | 120.0 (28.0) | 112.5 (29.5) | 0.16 |
| PLT (×109L) | 211.0 (166.0) | 250.0 (182.5) | 199.0 (224.0) | 281.0 (186.2) | 0.05 |
| Lactate (mmol/L) | 2.2 (2.4) | 2.1 (2.0) | 2.0 (2.4) | 2.5 (1.9) | 0.80 |
| Enzymes increase | 90 (24.5) | 81 (77.1) | 32 (30.5) | 20 (83.3) | 0.41 |
| Comorbidities and presentation | |||||
| Diabetes | 21 (5.7) | 6 (5.7) | 9 (8.6) | 0 (0.0) | 0.42 |
| Cerebro-vascular accident | 19 (5.2) | 7 (6.7) | 5 (4.8) | 1 (4.2) | 0.91 |
| Pulmonary disease | 25 (6.8) | 5 (4.8) | 1 (1.0) | 2 (8.3) | 0.12 |
| Extracardiac arteriopathy | 18 (4.9) | 1 (1.0) | 2 (1.9) | 0 (0.0) | 0.12 |
| Poor mobility | 37 (10.1) | 7 (6.7) | 5 (4.8) | 0 (0.0) | 0.12 |
| Moderate-to-severe frailty | 3 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.) | 0.59 |
| Recent myocardial infarction | 9 (2.5) | 6 (5.7) | 3 (2.9) | 1 (4.2) | 0.40 |
| Cardiac massage | 14 (3.8) | 6 5.7) | 6 (5.7) | 0 (0.0) | 0.51 |
| Intubated/sedated | 100 (27.2) | 32 (30.5) | 37 (35.2) | 10 (41.7) | 0.23 |
| Status | 0.06 | ||||
| Emergency 1 | 77 (21.0) | 18 (17.1) | 12 (11.4) | 0 (0.0) | |
| Emergency 2 | 98 (26.7) | 29 (27.6) | 30 (28.6) | 4 (16.7) | |
| Salvage 1 | 12 (3.3) | 6 (5.7) | 6 (5.7) | 0 (0.0) | |
| Salvage 2 | 4 (1.1) | 1 (1.0) | 0 (0.0) | 0 (0.0) | |
| Urgent | 176 (48.0) | 51 (48.6) | 57 (54.3) | 20 (83.3) | |
| Aortic valve | |||||
| Bicuspid | 4 (1.1) | 6 (5.7) | 1 (1.0) | 1 (4.2) | 0.02 |
| Regurgitation | <0.01 | ||||
| No trace | 135 (36.9) | 10 (9.5) | 57 (54.3) | 1 (4.2) | |
| Mild | 147 (40.2) | 8 (7.6) | 29 (27.6) | 1 (4.2) | |
| Moderate | 59 (16.1) | 17 (16.2) | 12 (11.4) | 7 (29.2) | |
| Severe | 25 (6.8) | 70 (66.7) | 7 (6.7) | 15 (62.5) | |
| Malperfusion | 89 (24.3) | 27 (25.7) | 27 (25.7) | 4 (16.7) | 0.81 |
| Cerebral | 47 (12.8) | 15 (14.3) | 16 (15.2) | 2 (8.3) | |
| Spinal | 7 (1.9) | 3 (2.9) | 2 (1.9) | 0 (0.0) | |
| Renal | 37 (10.1) | 11 (10.5) | 12 (11.4) | 1 (4.2) | |
| Mesenteric | 25 (6.8) | 4 (3.8) | 3 (2.9) | 1 (4.2) | |
| Peripheral | 18 (4.9) | 5 (4.8) | 7 (6.7) | 2 (8.3) | |
| Outcomes | |||||
| Stroke | 43 (11.7) | 8 (7.6) | 23 (21.9) | 2 (8.3) | 0.01 |
| Spinal cord injury | 12 (3.3) | 6 (5.7) | 6 (5.7) | 1 (4.2) | 0.57 |
| Tracheostomy | 16 (4.4) | 6 (5.7) | 5 (4.8) | 0 (0.0) | 0.67 |
| Dialysis | 33 (9.0) | 11 (10.5) | 15 (14.3) | 4 (16.7) | 0.35 |
| In-hospital mortality | 89 (24.3) | 20 (19.0) | 31 (29.5) | 6 (25.0) | 0.37 |
| Major adverse events | 130 (35.4) | 39 (37.1) | 51 (48.6) | 10 (41.7) | 0.11 |
| ICU stay (days) | 7.0 (15.0) | 10.0 (22.0) | 11.0 (17.0) | 15.5 (13.2) | <0.01 |
Data are presented as median (IQR) or n (%). Pre/intra/post-operative variables after type A aortic dissection repair. Major adverse events include composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis. BMI, body mass index; Cr, creatinine; Hb, hemoglobin; PLT, platelets count; ICU, intensive care unit; IQR, interquartile range.
Table 3
| Variables | Conservative (N=393) | Extensive (N=208) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 66.9 (20.4) | 61.1 (17.4) | <0.01 |
| BMI (kg/m2) | 25.9 (5.6) | 25.7 (4.3) | 0.53 |
| Female | 137 (34.9) | 43 (20.7) | <0.01 |
| Biochemistry | |||
| Cr (mg/dL) | 88.4 (31.6) | 88.0 (25.7) | 0.86 |
| Hb (g/dL) | 122.0 (29.0) | 119.0 (27.0) | 0.22 |
| PLT (×109L) | 212.5 (168.0) | 230.0 (204.7) | 0.12 |
| Lactate (mmol/L) | 2.2 (2.3) | 2.3 (2.2) | 0.85 |
| Enzymes increase) | 95 (24.2) | 55 (26.4) | 0.61 |
| Comorbidities and presentation | |||
| Diabetes | 22 (5.6) | 14 (6.7) | 0.71 |
| Stroke | 7 (1.8) | 7 (3.4) | 0.35 |
| Pulmonary disease | 26 (6.6) | 7 (3.4) | 0.14 |
| Extracardiac arteriopathy | 18 (4.6) | 3 (1.4) | 0.08 |
| Poor mobility | 39 (9.9) | 10 (4.8) | 0.04 |
| Moderate-to-severe frailty | 3 (0.8) | 0 (0.0) | 0.59 |
| Recent myocardial infarction | 10 (2.5) | 9 (4.3) | 0.34 |
| Cardiac massage | 14 (3.6) | 12 (5.8) | 0.29 |
| Intubated/sedated | 107 (27.2) | 72 (34.6) | 0.07 |
| Status | 0.10 | ||
| Emergency 1 | 79 (20.1) | 28 (13.5) | |
| Emergency 2 | 108 (27.5) | 53 (25.5) | |
| Salvage 1 | 12 (3.1) | 12 (5.8) | |
| Salvage 2 | 4 (1.0) | 1 (0.5) | |
| Urgent | 190 (48.3) | 114 (54.8) | |
| Aortic valve | 0.04 | ||
| Bicuspid | 4 (1.0) | 8 (3.8) | |
| Regurgitation | <0.01 | ||
| No/trace | 143 (36.5) | 60 (28.8) | |
| Mild | 153 (39.0) | 32 (15.4) | |
| Moderate | 69 (17.6) | 26 (12.5) | |
| Severe | 27 (6.9) | 90 (43.3) | |
| Malperfusion | 98 (24.9) | 49 (23.6) | 0.78 |
| Cerebral | 52 (13.2) | 28 (13.5) | |
| Spinal | 8 (2.0) | 4 (1.9) | |
| Renal | 41 (10.4) | 20 (9.6) | |
| Mesenteric | 26 (6.6) | 7 (3.4) | |
| Peripheral | 22 (5.6) | 10 (4.8) | |
| Outcomes | |||
| Stroke | 48 (24.9) | 28 (23.6) | 0.75 |
| Spinal cord injury | 13 (3.3) | 12 (5.8) | 0.22 |
| Tracheostomy | 18 (4.6) | 9 (4.3) | >0.99 |
| Dialysis | 37 (9.4) | 26 (12.5) | 0.30 |
| In-hospital mortality | 97 (24.7) | 49 (23.6) | 0.84 |
| Major adverse events | 148 (37.7) | 92 (44.2) | 0.14 |
| ICU stay (days) | 8.0 (15.0) | 11.0 (22.0) | 0.03 |
Data are presented as median (IQR) or n (%). Major adverse events include composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis. BMI, body mass index; Cr, creatinine; Hb, hemoglobin; ICU, intensive care unit; IQR, interquartile range; lactate, arterial lactate; PLT, platelets count; enzymes, cardiac enzymes.
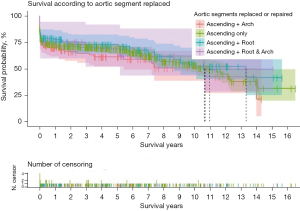
Subgroup analysis II: urgency status
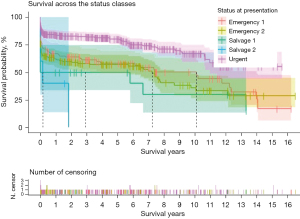
No substantial differences were found in demographics between groups. However, biochemical markers were considerably affected by urgency status. For example, Hb and creatinine levels reflected the worsening of presentation, from “urgent” (Hb 116 g/dL, Cr 82 mg/dL) to “salvage 2” (Hb 99 g/dL, Cr 145 mg/dL) (P<0.01). Other preoperative factors and the aortic valve status were similar across groups. The incidence of stroke, cord injury, and tracheostomy was not statistically significant between groups, whilst renal failure requiring dialysis significantly correlated with “emergency 2” 18.6% and “salvage 1” 12.5% (P<0.01). Malperfusion was associated with worsening hemodynamic status classification (urgency: “urgent” and “emergency 1” groups (0.0%) compared to “emergency 2” group (79.5%) (P<0.01). OM and MAE were significantly correlated with the urgency status, rising from urgent to salvage 2 (P<0.01) (Table 4).
Table 4
| Variables | Urgent (N=304) | Emergency 1 (N=107) | Emergency 2 (N=161) | Salvage 1 (N=24) | Salvage 2 (N=5) | P value |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (years) | 64.2 (18.2) | 64.0 (21.2) | 63.4 (21.2) | 67.1 (13.1) | 73.2 (13.2) | 0.41 |
| BMI (kg/m2) | 25.8 (5.4) | 26.8 (5.0) | 25.7 (4.7) | 25.3 (3.2) | 24.2 (2.1) | 0.41 |
| Female | 104 (34.2) | 26 (24.3) | 43 (26.7) | 6 (25.0) | 1 (20.0) | 0.23 |
| Biochemical analysis | ||||||
| Cr (mg/dL) | 82.0 (23.0) | 94.1 (40.2) | 96.0 (40.5) | 88.0 (27.0) | 145.0 (34.5) | <0.01 |
| Hb (g/dL) | 116.0 (25.0) | 130.0 (26.0) | 125.5 (28.0) | 114.0 (18.0) | 99.0 (28.0) | <0.01 |
| PLT (×109/L) | 254.0 (187.0) | 198.0 (67.5) | 207.5 (182.2) | 166.0 (208.0) | 128.0 (23.0) | <0.01 |
| Lactate (mmol/L) | 2.5 (2.5) | 1.2 (1.3) | 2.3 (2.0) | 2.3 (4.5) | 3.3 (6.0) | <0.01 |
| Enzymes increase | 82 (27.0) | 14 (13.1) | 41 (25.5) | 12 (50.0) | 1 (20.0) | <0.01 |
| Patients status before surgery | ||||||
| Diabetes | 23 (7.6) | 4 (3.7) | 7 (4.3) | 2 (8.3) | 0 (0.0) | 0.46 |
| Cerebro-vascular accident | 13 (4.3) | 6 (5.6) | 11 (6.8) | 1 (4.2) | 1 (20.0) | 0.46 |
| Pulmonary disease | 17 (5.6) | 8 (7.5) | 6 (3.7) | 1 (4.2) | 1 (20.) | 0.42 |
| Extracardiac arteriopathy | 6 (2.0) | 8 (7.5) | 6 (3.7) | 1 (4.2) | 0 (0.0) | 0.12 |
| Poor mobility | 33 (10.9) | 5 (4.7) | 9 (5.6) | 2 (8.3) | 0 (0.0) | 0.16 |
| Moderate-to-severe frailty | 2 (0.7) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0.93 |
| Recent myocardial infarction | 7 (2.3) | 3 (2.8) | 7 (4.3) | 2 (8.3) | 0 (0.0) | 0.44 |
| Cardiac massage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 21 (87.5) | 5 (100) | <0.01 |
| Intubated/sedated | 95 (31.2) | 15 (14.0) | 59 (36.6) | 9 (37.5) | 1 (20.0) | <0.01 |
| Aortic valve | ||||||
| Bicuspid | 3 (1.0) | 4 (3.7) | 5 (3.1) | 0 (0.0) | 0 (0.0) | 0.30 |
| Regurgitation | 0.14 | |||||
| No trace | 110 (6.2) | 27 (25.2) | 55 (34.4) | 10 (41.7) | 1 (20.0) | |
| Mild | 96 (31.6) | 36 (33.6) | 47 (29.4) | 5 (20.8) | 1 (20.0) | |
| Moderate | 36 (11.8) | 27 (25.2) | 27 16.9) | 3(12.5) | 1 (40.0) | |
| Severe | 62 (20.4) | 17 (15.9) | 31 (19.4) | 6 (25.0) | 1 (20.0) | |
| Malperfusion | 0 (0.0) | 0 (0.0) | 128 (79.5) | 16 (66.7) | 3 (60.0) | <0.01 |
| Cerebral | 0 (0.0) | 0 (0.0) | 66 (41.0) | 47 (50.0) | 1 (40.0) | |
| Spinal | 0 (0.0) | 0 (0.0) | 8 (5.0) | 4 (16.7) | 0 (0.0) | |
| Renal | 0 (0.0) | 0 (0.0) | 55 (34.2) | 4 (16.7) | 2 (40.0) | |
| Mesenteric | 0 (0.0) | 0 (0.0) | 25 (15.5) | 5 (20.8) | 3 (60.0) | |
| Peripheral | 0 (0.0) | 0 (0.0) | 30 (18.6) | 2 (8.3) | 0 (0.0) | |
| Aortic segments replaced | 0.06 | |||||
| Ascending only | 176 (57.9) | 77 (72.0) | 98 (60.9) | 12 (50.0) | 4 (40.0) | |
| + Root | 51 (16.8) | 18 (16.8) | 29 (18.0) | 6 (25.0) | 1 (20.0) | |
| + Arch | 57 (18.8) | 12 (11.2) | 30 (18.6) | 6 (25.0) | 0 (0.0) | |
| +Root & arch | 20 (6.6) | 0 (0.0) | 4 (2.5) | 0 (0.0) | 0 (0.0) | |
| Adverse events | ||||||
| Stroke | 41 (13.5) | 11 (10.3) | 18 (11.2) | 6 (25.0) | 0 (0.0) | 0.28 |
| Spinal cord injury | 8 (2.6) | 6 (5.6) | 9 (5.6) | 2 (8.3) | 0 (0.0) | 0.35 |
| Tracheostomy | 16 (5.3) | 3 (2.8) | 8 (5.0) | 0 (0.0) | 0 (0.0) | 0.63 |
| Dialysis | 24 (7.9) | 6 (5.6) | 30 (18.6) | 3 (12.5) | 0 (0.0) | <0.01 |
| In-hospital mortality | 48 (15.8) | 27 (25.2) | 56 (34.8) | 12 (50.0) | 3 (60.0) | <0.01 |
| Major adverse events | 99 (32.6) | 35 (32.7) | 79 (49.1) | 14 (58.3) | 3 (60.0) | <0.01 |
| ICU stay (days) | 11.0 (20.0) | 5.0 (10.0) | 8.0 (16.0) | 3 (13.5) | 1.0 (14.0) | <0.01 |
Data are presented as median (IQR) or n (%). Pre/intra/post-operative variables after type A aortic dissection repair. Major adverse events include composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis. BMI, body mass index; Cr, creatinine; Hb, hemoglobin; PLT, platelets count; ICU, intensive care unit; IQR, interquartile range.
Survival
In the overall sample, the rate of survival was 73.3% at 1-year, 68.2% at 5-year, and 53.5% at 10-year follow-up (Table 5). Median follow-up period was 2.5 years (IQR, 6.6 years). The extent of the surgical repair had no bearing on survival (Table 5, Figure 2). Survival was strongly affected by status at presentation (P<0.01). At the 1-year, the rate of survival of the “urgent” group was double the rate of survival of the “salvage 2” group (P<0.01). At the 5-year mark, the rate of survival was 80.2%±2.4% and 50%±10.2% respectively for the “urgent” and “salvage 1” groups. At the 10-year mark, the rate of survival was 66.8%±3.9% and 35.9%±5.6% respectively for the “urgent” and “emergency 2” groups (Table 5, Figure 3).
Table 5
| Survival | 1-year | 5-year | 10-year |
|---|---|---|---|
| Survival: overall sample | |||
| At risk | 370 | 225 | 69 |
| Events | 157 | 22 | 33 |
| Survival, % | 73.3 | 68.2 | 53.5 |
| SE, % | 1.8 | 2 | 2.8 |
| Survival according to aortic segments repaired | |||
| Ascending, % | 72.8±2.4 | 68.6±2.5 | 53.4±3.6 |
| + Root, % | 80.0±4.0 | 72.7±4.5 | 55.6±6.4 |
| + Arch, % | 70.0±4.5 | 61.3±5.2 | At risk <10 |
| + Root & arch, % | 75.0±8.8 | 68.7±10.1 | At risk <10 |
| P value | 0.56 | ||
| Survival according to urgency status at presentation | |||
| Urgent, % | 84.0±2.1 | 80.2±2.4 | 66.8±3.9 |
| Emergency 1, % | 66.9±4.6 | 58.3±5.0 | 50.2±5.8 |
| Emergency 2, % | 62.1±3.8 | 56.9±4.0 | 35.9±5.6 |
| Salvage 1, % | 50±10.2 | 50±10.2 | At risk <10 |
| Salvage 2, % | 40.0±21.9 | At risk <10 | At risk <10 |
| P value | <0.01 | ||
Data are represented as mean ± SE. Survival after type A aortic dissection repair in the overall sample, and across subgroups according to the aortic segment replaced and according to urgency status at presentation. SE, standard error.
Predictors of in-hospital and follow-up mortality
Age, intubation at hospitalization, raised lactate levels, and “emergency/salvage” status at admission were independent predictors of mortality. While in-hospital mortality was forecasted by “poor mobility” status, follow-up mortality was higher in patients with congenital bicuspid aortic valves. Tables 6,7 indicate the univariate and multivariable predictors for operative and follow-up mortality respectively.
Table 6
| Predictors | Mortality | |
|---|---|---|
| Operative | Follow-up | |
| Age | <0.01 | <0.01 |
| Body mass index | 0.43 | 0.84 |
| Female | 0.37 | 0.24 |
| Creatinine | <0.01 | <0.01 |
| Hemoglobin | 0.07 | 0.15 |
| Platelet count | 0.13 | 0.46 |
| Arterial lactate | <0.01 | <0.01 |
| Cardiac biomarkers increase | <0.01 | 0.48 |
| Diabetes | 0.61 | 0.60 |
| Prior CVA | 0.35 | 0.14 |
| Pulmonary disease | 0.01 | <0.01 |
| Extracardiac arteriopathy | 0.64 | 0.16 |
| Poor mobility | 0.15 | 0.27 |
| Moderate-to-severe frailty | 0.13 | 0.32 |
| Recent myocardial infarction | 0.02 | 0.02 |
| Preoperative cardiac massage | <0.01 | <0.01 |
| Intubated/sedated at arrival | <0.01 | 0.11 |
| Status: emergency or salvage | <0.01 | <0.01 |
| Bicuspid aortic valve | 0.46 | 0.14 |
| Aortic regurgitation | 0.65 | 0.53 |
| Malperfusion | <0.01 | <0.01 |
| Cerebral perfusion | 0.37 | 0.07 |
| Root or arch replaced | 0.98 | 0.99 |
Univariable predictors of operative mortality and follow-up mortality in patients who underwent repair of acute type A aortic dissection. Univariate logistic regression to determine the association between dependent (i.e., in-hospital and follow-up mortality) and independent variables. Predictors that presented an association with P<0.2 were entered into the multivariable models. CVA, cerebro-vascular accident.
Table 7
| Predictor | Estimate | 95% confidence interval | P value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Operative mortality | ||||
| Age | 1.04 | 1.02 | 1.06 | <0.01 |
| Creatinine | 1.00 | 0.99 | 1.01 | 0.17 |
| Hemoglobin | 0.99 | 0.98 | 1.01 | 0.51 |
| Platelet count | 1.00 | 0.00 | 1.00 | 0.57 |
| Arterial lactate | 1.37 | 1.20 | 1.58 | <0.01 |
| Cardiac biomarkers increment | 1.14 | 0.64 | 1.98 | 0.65 |
| Pulmonary disease | 2.06 | 0.74 | 5.52 | 0.15 |
| Poor mobility | 3.03 | 1.20 | 7.52 | 0.02 |
| Moderate-to-severe frailty | 1.58 | 0.11 | 39.77 | 0.73 |
| Recent myocardial infarction | 1.81 | 0.54 | 5.67 | 0.31 |
| Preoperative cardiac massage | 1.30 | 0.45 | 3.69 | 0.62 |
| Intubated/sedated at arrival | 2.74 | 1.65 | 4.59 | <0.01 |
| Emergency or salvage | 2.60 | 1.32 | 5.14 | <0.01 |
| Malperfusion | 1.02 | 0.53 | 2.00 | 0.93 |
| Follow-up mortality | ||||
| Age | 1.03 | 1.01 | 1.04 | <0.01 |
| Creatinine | 1.00 | 0.99 | 1.00 | 0.07 |
| Hemoglobin | 0.99 | 0.99 | 1.00 | 0.53 |
| Arterial lactate | 1.21 | 1.14 | 1.29 | <0.01 |
| Cerebro-vascular accident | 1.47 | 0.80 | 2.70 | 0.21 |
| Pulmonary disease | 1.70 | 0.94 | 3.05 | 0.07 |
| Extra-cardiac arteriopathy | 0.92 | 0.45 | 1.90 | 0.83 |
| Recent myocardial infarction | 1.05 | 0.53 | 2.09 | 0.88 |
| Preoperative cardiac massage | 1.29 | 0.68 | 2.44 | 0.43 |
| Intubated/sedated at arrival | 1.68 | 1.21 | 2.32 | <0.01 |
| Emergency or salvage | 2.04 | 1.37 | 3.04 | <0.01 |
| Bicuspid aortic valve | 2.70 | 1.23 | 5.92 | 0.01 |
| Malperfusion | 0.82 | 0.54 | 1.25 | 0.37 |
Multivariable predictors of operative mortality and follow-up mortality in patients who underwent repair of acute type A aortic dissection. Only candidate variables with a P value ≤0.2 specifically for hospital or follow-up mortality were inserted respectively into the multivariable logistic regression and Cox regression models. The estimates express odds ratios for in-hospital mortality and hazard ratios for follow-up mortality.
Discussion
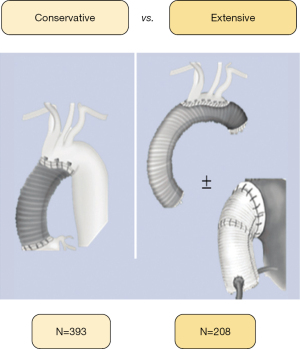
In this extensive evaluation, we assessed the survival rates following surgical repair for type A acute aortic dissection. The study is founded on a 14-year experience [2008–2021] from a multicentre dataset of three reputable centres. The majority of patients opted for the ‘conservative’ approach, with root sparing-AAR administered using a Dacron graft interposition. Only a small fraction of patients underwent AAR conjoined with arch repair with or without root replacement. Figure 4 shows a raw OM rate of 24.3%, with 1-, 5-, and 10-year survival rates of 73.3%, 68.2%, and 53.5%, respectively. Operative and follow-up mortality risk factors include age, arterial lactate standards, intubation state, and severity categories, such as emergency or salvage status at hospital admission (P<0.01).
The results of this 14-year study have advanced our understanding of the benefits of surgical management of ATAAD. Successful surgery for ATAAD involves four general principles. Firstly, the aortic replacement must restore a competent thoracic aorta. Secondly, the entire portion of the aorta involved in the dissection should be replaced to minimize the risk of further reoperation. Thirdly, the surgeon should ensure the lowest OM for the index procedure. Finally, achieving these results may require more complicated surgery, leading to longer operating times and an increased risk of prolonged organ and cardiac ischemia.
We observed a significant disparity in OM rates based on the urgency categories determined by an individual’s clinical condition. The “urgent” patients exhibited a rate of 15.8%, while the “salvage 2” patients displayed a rate of 60%. The OM reported here is comparable to that which has been described in various national and international registries (3-5,17-20). Despite increased awareness of advances in accurate image processing, which leads to faster diagnosis and better perioperative care, the reported OM rate remains alarmingly high. The STS data from 2014 to 2016 showed an intra-hospital mortality rate of 26.9% in patients who underwent TARP, versus 16.3% of those managed with hemiarch repair (19). Comparable figures were recorded in the International Registry of Acute Aortic Dissection (IRAD) (5,17) and the German Registry for Acute Aortic Dissection Type A (GERAADA) (4,20) databases. In the IRAD registry, the overall mortality rate during hospital admission for ATAAD was 5.8% at 48 hours, with a significant contrast between non-surgical and surgical cohorts. In the non-surgical group, the death rate was 0.5% per hour (23.7% at 48 hours), which decreased significantly to 4.4% in the surgical group at 48 hours (17). The initial mortality rate reported in the UK National Adult Cardiac Surgery Audit (UK National Adult Cardiac Surgical Audit) for ATAAD procedures was 17.8%, which decreased over time from 22% in 2009 to 15% in 2018 (18) and was comparable to that observed in the Nordic Consortium for Acute Type A Aortic Dissection (NORCAAD) of 16% (3).
These findings highlight two key points to note. First, high-volume patient registries have demonstrated that severe neurological damage, renal failure, and postoperative complications are significant barriers to successful surgical recovery (3-5,18). Secondly, the average age across these registries (60–64 years) and the risk factors for inpatient mortality in our study coincide with those reported in similar registries, including clinical conditions such as preoperative resuscitation, mechanical ventilation, and coexisting comorbidities (e.g., advanced age) (3,18,20-24).
Varying outcomes in average OM have been found among individual centres, which have reported consistent figures of metric discrepancies ranging from rates as high as 20% and 24% (25) to as low as 5.5% (26). These discrepancies also take into account the scope of the necessary surgical intervention. Recently, a referral centre with extensive experience in ATAAD (27) procedures reported a mean OM of 5.6%. There were no significant differences observed between patients who received ‘conservative’ surgical repair involving root sparing or a limited hemiarch procedure and those who underwent the most frequent surgical option, which involved replacing the aortic root and/or a TARP. This disparity is especially evident in high-volume aortic centres of excellence (26-30). In these centres, patients with ATAAD who received complex repairs experienced approximately half the OM reported in the GERAADA and NORCAAD registries (3,4). Specific surgical teams performing a higher volume of ATAAD procedures and with more experience in aortic surgery have highlighted this strategy for improving early outcomes and achieving successful surgical treatments for specific cohorts of patients (31-33).
Whole intimal tear resections associated with root-sparing aortic arch replacement (AAR) using interposition Dacron grafts with or without hemiarch repair remain the most commonly adopted surgical option for patients with ATAAD (5,17-20,23). Therefore, our findings emphasize the necessity of evaluating different surgical procedures, ranging from ascending aortic replacement to hemiarch repair or total arch replacement, based on thorough assessment of the aortic lesion. The surgical repair, excising the affected portion of the aorta, was determined by the location of the dissection entry tear. It is noteworthy that patients presenting with an entrance tear in the aortic arch aligned with the lesser curvature of the aorta receive conservative surgical treatment, where the ascending aortic replacement with an interposed prosthetic graft and hemiarch repair is prioritised. In contrast, patients with an entry tear near the supra-aortic branches require extensive surgery that involves replacement of the aortic arch. Similarly, in our series, the surgeon’s decision to pursue extended surgical treatment was based on their clinical judgment, considering factors such as the patient’s condition upon hospitalization, the severity of the aortic arch lesion, and the surgeon’s technical expertise (1,34-36).
Significant advancements have been made in aortic surgery in recent years, making it advisable to refer patients to dedicated aortic centers with specialized expertise. The implementation of innovative technologies, improved surgical procedures, and greater employment of cerebral protection have facilitated more extensive surgical interventions to treat ATAAD. These may involve replacing the aortic arch, possibly including extensions into the proximal descending aorta, along with hybrid staged approaches (24-27,37). There are numerous advantages to expanding the initial ATAAD operation to encompass a larger section of the dissected aorta. The long-term risk of increased expansion and rupture of the resected aorta is lower; furthermore, a notable benefit for managing subsequent intravascular treatment options was observed. However, an anticipated medium to long-term benefit from a more extensive surgical option necessitates proper evaluation against the potential rise in morbidity and mortality related to greater complexity that must be managed in the primary procedure.
The study shows that patients who underwent extensive surgical options including TARP procedures had a higher likelihood of experiencing adverse events. While previous studies have established the safety of TARP in elective procedures, the risk/benefit ratio has not yet been proposed. Surgical risks and potential benefits remain a topic of debate in the context of ATAAD. Studies have reported conflicting outcomes for open surgery and endovascular management (OM and PND). Consequently, the current data present contradictory findings that may misinform shared decision-making for ATAAD patients (24,25,27,38). One study revealed that there was an increased mortality rate associated with the extent of the procedure, which included the replacement of the aortic arch. The reported percentage of mortality ranged from 9.8% for a surgery limited to AAR, up to 21.6% for hemiarch repair and 28% for TARP (24). Another study similarly reported an OM of 13.4% in patients who received total arch replacement, and 9.7% in those who received hemiarch repair. It is worth noting that a higher incidence of PND was observed in TARP patients (22.7% vs. 6.3%) (39). However, two other studies contradict these findings. One report (25) noted that recipients of conservative surgical options had comparable rates of OM and PND to those managed with a more aggressive surgical option that included the use of TARP (24.1% vs. 22.6% and 9.1% vs. 7.5%). Similarly, another study (6) comparing the conservative approach of limited repair of the hemiarch with the more extensive treatment of FET showed no disparity in OM or PND.
In an ideal scenario, it is advisable to carry out thorough root and arch procedures on patients with low-risk, particularly those at a higher risk of reoperation. In the centers participating in the study, a modified Bentall procedure with a mechanical or bioprosthetic valve, was considered the gold standard intervention for TAAAD when the ARR needed a replacement for dilatation (larger than 4.5 cm), contains the intimal tear, or if the patient suffers by aortic valvulopathy or connective tissue disorder. The rare cases of valve-sparing aortic root replacement (VSARR) with reimplantation technique (David procedure) or remodeling technique (Yacoub procedure) were performed for younger patients by a surgeon with large experience with VSARR.
A recent meta-analysis (33) compared long-term outcomes of VSARR versus composite aortic valve graft replacement (CAVGR) shows well that VSARR has not any difference in terms of in-hospital mortality and long-term survival. Still, it presents a higher risk of reoperation and in the older population a higher risk of all-cause death.
This approach provides greater long-term survival benefits and reduces the likelihood of reintervention in patients who have received treatment for ATAAD. Sá et al. (33) noted a statistically significant variation in overall survival rates, suggesting that patients receiving an aggressive TARP approach had a better outcome (P=0.022), while those undergoing the aggressive surgical intervention were less likely to require reoperation (P=0.02) beyond 7 years. Our findings suggest that TARP should be performed taking into account the patient’s clinical condition at the time of admission and following a precise evaluation of the lesion’s size.
We acknowledge that our patient group has a lower proportion of individuals receiving the TARP procedure, at 17.2%, compared to other studies. This has resulted in a certain level of caution that has become a prevailing mindset among most surgeons, favouring simpler procedures. The conservative approach to surgery is rational, as using an interposition Dacron graft with or without hemiarch repair to replace the ascending aorta while preserving the root seems to be the favoured method over more daunting and intricate procedures. Benedetto and colleagues 18 recommended a comparable operative plan for patients referred for TARP surgery. This approach seems to be influenced by the existing UK policy that entails public reporting of individual surgeons’ operative outcomes, which could encourage more cautious decisions like avoiding high-risk procedures that could result in poorer hospital outcomes. This is particularly relevant in the case of aortic arch replacement in the context of ATAAD (40-43).
In our analysis, we found that 21.7% of patients underwent total root replacement either with or without valve-sparing, and there was no difference in OM. Whilst the technical difficulty of ARR surgery in the setting of ATAAD may have a limiting effect, other studies with a vast number of patients have highlighted similar instances of OM, linked with lower rates of reoperation required for treatment of aortic root dysfunction (1,2,4,17,19). There have been reports stating TARP to be a priority surgical option for patients suffering from Ao-R destruction, simultaneous Ao-R aneurysm, bicuspid aortic disease, or a history of CTD (28,30,38,41,42). Recently, Lau and colleagues (23) found that a more extensive repair that included Ao-R and/or the entire aortic arch resulted in a higher predictive rate for repeat surgery compared to the less aggressive root sparing-AAR option with or without hemiarch surgery involvement (P=0.01) (27). Surgeons need to consider the anatomical extent of the aortic laceration when deciding on the appropriate surgical option. Our findings indicate that favorable outcomes were more influenced by the grade of the aortic lesion and the individual’s deteriorating condition at admission rather than the percentage of surgical repair performed. Surgeons may inherently prefer less aggressive surgical options for subjects with multiple comorbidities and/or elderly individuals, compared to more extensive procedures aimed at low-risk and/or younger subjects. It is also necessary to establish clear and widely-shared criteria for evaluating the extent of aortic disease. Different assessment methods exist for determining the entry point and size of a laceration, while some may assess the extension of the dissected aorta. Nonetheless, these criteria play a crucial role in directing the most suitable surgical option to attain optimal results (27,30,31,38).
In our analysis, the use of cerebral perfusion as a strategy alone did not pose a risk in the multivariate analysis. When the strategy was taken into account, there was no discernible difference in postoperative mortality (P=0.37) or follow-up (P=0.07) between patients who underwent extensive surgery involving hemiarch and arch replacement. The study’s findings align with those of the STS (19,23) and GERAADA (4,20) registries that examined unselected patients and conducted prospective single-center analysis. Both reports concluded that no correlation exists between the different cerebral perfusion approaches and outcomes. Further analysis suggests that various inherent biases may have influenced these outcomes.
Neuroprotective strategies were primarily based on the surgeon’s discretion, with consideration for the patient’s clinical condition and extensions during the resection of the dissected aorta. Cerebral perfusion strategies have shown notable advantages for patients undergoing complicated procedures with circulatory arrest for more than 30 minutes (20-22,30-32,38,41-46). Composite outcomes of significant adverse events were more frequent in the groups with worse clinical states (66.7% and 50.9% vs. 32.9% and 36.4%; P<0.01), indicating preoperative comorbidity rather than surgical technique (P=0.11). Despite extensive surgery and the anticipated prolonged operation time, careful preoperative patient selection, as observed in urgent and emergency 1 procedures, could reduce the added risk and improve the rates of early and late survival. The survival rates at 5 years (68.6% and 72.7% vs. 61.3% and 68.7%) and 10 years (53.4% and 55.6% vs. risk <10) were similar for both groups (P=0.56).
Although the comprehensive approach may serve as a substitute for high-risk populations with an inaccessible tear in the ascending aorta, CTD, or an existing large aneurysm that may indicate the requirement for further surgery, the patient’s clinical condition upon admission ultimately determines the surgical option. While root replacement, TAR, and FET are potentially beneficial for these patients, their routine use may not be necessary or appropriate for most hemodynamically unstable patients exhibiting signs of malperfusions.
Our analysis has shown a significant association between mortality and compromised clinical condition upon hospital admission. The findings indicate that the rise in surgery-related fatalities may stem from exacerbated symptoms, unstable blood flow, and/or insufficient oxygen supply compounded by the need for emergency resuscitation.
Limitations
The study is limited by its retrospective design, which resulted in two groups of different sizes being compared for arch surgery versus surgery without arch involvement. Additionally, the multicentre nature of the study was impacted by variations in surgeon and centre preferences for cannulation and neuroprotective strategies, which could not be stratified. Furthermore, variances existed in the patient intake at each centre, which could have contributed to intangible factors including socioeconomic demographics and centre-specific experience.
Conclusions
Mortality rates for ATAAD remain high, with a strong association to the degree of presentation acuity. Outcomes for both operative and long-term treatment were primarily dependent on malperfusion, patient age, conscious state on arrival, and urgency, particularly within the emergency and salvage cohorts. The surgeon and centre experience should determine whether to include arch replacement as part of the repair, personalised to the patient and their presentation. This decision should weigh the additional risks associated with more extensive surgery against the need for further reintervention. Our study found that patients who underwent arch replacements during dissection had a higher stroke rate, despite being younger.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1137/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1137/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1137/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1137/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study complied with the Declaration of Helsinki (as revised in 2013). Patient consent was obtained after the assigned ethics approval from institutional review board of Montpellier University Hospital (IRB approval No. 202201173) in accordance with the research guidance. The other institutions are informed and agreed with the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Biancari F, Juvonen T, Fiore A, et al. Current Outcome after Surgery for Type A Aortic Dissection. Ann Surg 2023;278:e885-92. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Geirsson A, Shioda K, Olsson C, et al. Differential outcomes of open and clamp-on distal anastomosis techniques in acute type A aortic dissection. J Thorac Cardiovasc Surg 2019;157:1750-8. [Crossref] [PubMed]
- Czerny M, Schoenhoff F, Etz C, et al. The Impact of Pre-Operative Malperfusion on Outcome in Acute Type A Aortic Dissection: Results From the GERAADA Registry. J Am Coll Cardiol 2015;65:2628-35. [Crossref] [PubMed]
- Evangelista A, Isselbacher EM, Bossone E, et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018;137:1846-60. [Crossref] [PubMed]
- Uimonen M, Olsson C, Jeppsson A, et al. Outcome After Surgery for Acute Type A Aortic Dissection With or Without Primary Tear Resection. Ann Thorac Surg 2022;114:492-501. [Crossref] [PubMed]
- Moore NR, Parry AJ, Trottman-Dickenson B, et al. Fate of the native aorta after repair of acute type A dissection: a magnetic resonance imaging study. Heart 1996;75:62-6. [Crossref] [PubMed]
- Uchida K, Minami T, Cho T, et al. Results of ascending aortic and arch replacement for type A aortic dissection. J Thorac Cardiovasc Surg 2021;162:1025-31. [Crossref] [PubMed]
- Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg 2019;55:133-62. [Crossref] [PubMed]
- Arabkhani B, Verhoef J, Tomšič A, et al. The Aortic Root in Acute Type A Dissection: Repair or Replace? Ann Thorac Surg 2023;115:1396-402. [Crossref] [PubMed]
- Kallenbach K, Büsch C, Rylski B, et al. Treatment of the Aortic Root in Acute Aortic Dissection Type A: Insights from the German Registry for Acute Aortic Dissection Type A (GERAADA) Registry. Eur J Cardiothorac Surg 2022;ezac261. [Crossref] [PubMed]
- Biancari F, Mariscalco G, Yusuff H, et al. European registry of type A aortic dissection (ERTAAD) - rationale, design and definition criteria. J Cardiothorac Surg 2021;16:171. [Crossref] [PubMed]
- Harris KM, Nienaber CA, Peterson MD, et al. Early Mortality in Type A Acute Aortic Dissection: Insights From the International Registry of Acute Aortic Dissection. JAMA Cardiol 2022;7:1009-15. [Crossref] [PubMed]
- Benedetto U, Dimagli A, Kaura A, et al. Determinants of outcomes following surgery for type A acute aortic dissection: the UK National Adult Cardiac Surgical Audit. Eur Heart J 2021;43:44-52. [Crossref] [PubMed]
- O'Hara D, McLarty A, Sun E, et al. Type-A Aortic Dissection and Cerebral Perfusion: The Society of Thoracic Surgeons Database Analysis. Ann Thorac Surg 2020;110:1461-7. [Crossref] [PubMed]
- Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. [Crossref] [PubMed]
- Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation 2002;105:200-6. [Crossref] [PubMed]
- Czerny M, Siepe M, Beyersdorf F, et al. Prediction of mortality rate in acute type A dissection: the German Registry for Acute Type A Aortic Dissection score. Eur J Cardiothorac Surg 2020;58:700-6. [Crossref] [PubMed]
- Lee TC, Kon Z, Cheema FH, et al. Contemporary management and outcomes of acute type A aortic dissection: An analysis of the STS adult cardiac surgery database. J Card Surg 2018;33:7-18. [Crossref] [PubMed]
- Rylski B, Beyersdorf F, Kari FA, et al. Acute type A aortic dissection extending beyond ascending aorta: Limited or extensive distal repair. J Thorac Cardiovasc Surg 2014;148:949-54; discussion 954. [Crossref] [PubMed]
- Di Eusanio M, Berretta P, Cefarelli M, et al. Total Arch Replacement Versus More Conservative Management in Type A Acute Aortic Dissection. Ann Thorac Surg 2015;100:88-94. [Crossref] [PubMed]
- Zhang H, Lang X, Lu F, et al. Acute type A dissection without intimal tear in arch: proximal or extensive repair? J Thorac Cardiovasc Surg 2014;147:1251-5. [Crossref] [PubMed]
- Lau C, Robinson NB, Farrington WJ, et al. A tailored strategy for repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2022;164:1698-1707.e3. [Crossref] [PubMed]
- Yang B, Norton EL, Hobbs R, et al. Short- and long-term outcomes of aortic root repair and replacement in patients undergoing acute type A aortic dissection repair: Twenty-year experience. J Thorac Cardiovasc Surg 2019;157:2125-36. [Crossref] [PubMed]
- Omura A, Miyahara S, Yamanaka K, et al. Early and late outcomes of repaired acute DeBakey type I aortic dissection after graft replacement. J Thorac Cardiovasc Surg 2016;151:341-8. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch?. Circulation 2011;123:971-8. [Crossref] [PubMed]
- Rylski B, Milewski RK, Bavaria JE, et al. Long-term results of aggressive hemiarch replacement in 534 patients with type A aortic dissection. J Thorac Cardiovasc Surg 2014;148:2981-5. [Crossref] [PubMed]
- Sievers HH, Richardt D, Diwoky M, et al. Survival and reoperation after valve-sparing root replacement and root repair in acute type A dissection. J Thorac Cardiovasc Surg 2018;156:2076-2082.e2. [Crossref] [PubMed]
- Andersen ND, Ganapathi AM, Hanna JM, et al. Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol 2014;63:1796-803. [Crossref] [PubMed]
- Di Bartolomeo R, Leone A, Di Marco L, et al. When and how to replace the aortic arch for type A dissection. Ann Cardiothorac Surg 2016;5:383-8. [Crossref] [PubMed]
- Ikeno Y, Yokawa K, Matsueda T, et al. Long-term outcomes of total arch replacement using a 4-branched graft. J Thorac Cardiovasc Surg 2019;157:75-85.e3. [Crossref] [PubMed]
- Kim JB, Chung CH, Moon DH, et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2011;40:881-7. [Crossref] [PubMed]
- Sá MP, Jacquemyn X, Tasoudis PT, et al. Long-term outcomes of total arch replacement versus proximal aortic replacement in acute type A aortic dissection: Meta-analysis of Kaplan-Meier-derived individual patient data. J Card Surg 2022;37:4256-66. [Crossref] [PubMed]
- Juvonen T, Jormalainen M, Mustonen C, et al. Direct Aortic Versus Supra-Aortic Arterial Cannulation During Surgery for Acute Type A Aortic Dissection. World J Surg 2023;47:2899-908. [Crossref] [PubMed]
- Nappi F, Avtaar Singh SS, Gambardella I, et al. Surgical Strategy for the Repair of Acute Type A Aortic Dissection: A Multicenter Study. J Cardiovasc Dev Dis 2023;10:253. [Crossref] [PubMed]
- Nappi F, Petiot S, Salsano A, et al. Sex-Based Difference in Aortic Dissection Outcomes: A Multicenter Study. J Cardiovasc Dev Dis 2023;10:147. [Crossref] [PubMed]
- Radford PD, Derbyshire LF, Shalhoub J, et al. Publication of surgeon specific outcome data: a review of implementation, controversies and the potential impact on surgical training. Int J Surg 2015;13:211-6. [Crossref] [PubMed]
- Leshnower BG, Chen EP. When and how to replace the aortic root in type A aortic dissection. Ann Cardiothorac Surg 2016;5:377-82. [Crossref] [PubMed]
- Geirsson A, Bavaria JE, Swarr D, et al. Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 2007;84:1955-64; discussion 1955-64. [Crossref] [PubMed]
- Berretta P, Patel HJ, Gleason TG, et al. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg 2016;5:346-51. [Crossref] [PubMed]
- Preventza O, Simpson KH, Cooley DA, et al. Unilateral versus bilateral cerebral perfusion for acute type A aortic dissection. Ann Thorac Surg 2015;99:80-7. [Crossref] [PubMed]
- Krüger T, Weigang E, Hoffmann I, et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation 2011;124:434-43. [Crossref] [PubMed]
- Benedetto U, Dimagli A, Cooper G, et al. Neuroprotective strategies in acute aortic dissection: an analysis of the UK National Adult Cardiac Surgical Audit. Eur J Cardiothorac Surg 2021;60:1437-44. [Crossref] [PubMed]
- Nardi P, Olevano C, Bassano C, et al. The effect of postoperative malperfusion after surgical treatment of type A acute aortic dissection on early and mid-term survival. Vessel Plus 2017;1:77-83.
- Bassano C, Pugliese M, Mve Mvondo C, et al. Initial Surgical Strategy for the Treatment of Type A Acute Aortic Dissection: Does Proximal or Distal Extension of the Aortic Resection Influence the Outcomes? Int J Environ Res Public Health 2022;19:8878. [Crossref] [PubMed]
- Mussa FF, Horton JD, Moridzadeh R, et al. Acute Aortic Dissection and Intramural Hematoma: A Systematic Review. JAMA 2016;316:754-63. [Crossref] [PubMed]


