
Neoadjuvant sintilimab plus chemotherapy for locally advanced resectable esophageal squamous cell carcinoma: a prospective, single-arm, phase II clinical trial (CY-NICE)
Highlight box
Key findings
• Sintilimab plus platinum-based chemotherapy as neoadjuvant therapy is safe, feasible and effective in locally advanced resectable esophageal squamous cell carcinoma (ESCC).
What is known and what is new?
• NICT can be effective and safe in the short term for locally advanced esophageal cancer (EC), especially for ESCC.
• Sintilimab in combination with chemotherapy as a neoadjuvant treatment for locally advanced ESCC holds the potential to enhance the effectiveness of anti-tumor therapy and offers long-term benefits.
What is the implication, and what should change now?
• Sintilimab plus platinum-based chemotherapy as neoadjuvant therapy is safe, feasible and effective in locally advanced resectable ESCC, suggesting a support rationale for its further evaluation in randomized clinical trials.
Introduction
Esophageal cancer (EC) constituted over 600,000 new cases worldwide in 2020, thereby establishing itself as one of the most prevalent malignancies globally (1). The majority of EC incidences are concentrated in China, where it ranks 6th in terms of incidence and 5th in mortality among all types of cancers (2). Histologically, EC can be categorized into two main subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). Notably, ESCC accounts for the primary subtype of EC in China, affecting approximately 90% of patients (3).
Currently, the established approach for managing locally advanced ESCC involves employing neoadjuvant chemoradiotherapy (nCRT) or neoadjuvant chemotherapy (nCT) followed by esophagectomy, as supported by various clinical trials (4,5). Generally, nCRT has shown superior effectiveness in achieving a pathological complete response (pCR) compared to nCT. However, it is noteworthy that patients undergoing nCRT have exhibited a high recurrence rate with an elevated risk of distant metastasis, further compounded by the increased surgical complexity (6,7). As a result, there is an urgent demand for novel therapeutic strategies to address these challenges.
In recent years, immune checkpoint inhibitors (ICIs) therapy has shown remarkable efficacy in treating various types of cancers, positioning them as promising therapeutic agents in the management of EC. The impressive outcomes of ICIs in neoadjuvant therapy for other solid tumors (8,9), where adverse reactions have also been controllable, have spurred growing interest in applying ICIs to neoadjuvant therapy for EC. This emerging approach holds great promise in the quest for more effective and well-tolerated treatments for EC.
Multiple clinical trials have explored the efficacy and safety of immunotherapy against locally advanced resectable EC in the neoadjuvant setting. The latest meta-analysis illustrated that neoadjuvant immunochemotherapy (nICT) can be effective and safe in the short term for locally advanced EC, especially for ESCC, and can be used as a reference for future trials. At present, nICT is still in the clinical trial stage, and no indications for neoadjuvant immunotherapy are currently authorized.
Sintilimab is a fully recombinant human IgG4 anti-programmed death-1 (PD-1) monoclonal antibody that has shown promise in cancer therapy. In the ORIENT-15 study (10), the combination of sintilimab with chemotherapy demonstrated a significant improvement in overall survival (OS) with a remarkable median OS of 16.7 months in patients with advanced or metastatic ESCC. And it also showed that the OS advantage with sintilimab in combination with chemotherapy was not related to expression of programmed death-ligand 1 (PD-L1). Based on these encouraging results, utilizing sintilimab in combination with chemotherapy as a neoadjuvant treatment for locally advanced ESCC holds the potential to enhance the effectiveness of anti-tumor therapy and offers long-term benefits. In this phase II study, we aimed to evaluate the efficacy and safety of the combination of sintilimab with cisplatin plus paclitaxel in a cohort of patients diagnosed with locally advanced ESCC by exploring the potential of sintilimab-based neoadjuvant therapy. We present this article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1388/rc).
Methods
Study design and patients
This study was a prospective, single-arm, phase II exploratory clinical trial, registered on the website of the China Clinical Trial Registration Center (registration number: ChiCTR2200056558). The objective of this study was to evaluate the rate of pCR after surgery and assess the safety of neoadjuvant treatment with sintilimab in combination with platinum-containing chemotherapy for patients with locally advanced resectable ESCC at Lanzhou University Second Hospital. The study included adult patients aged between 18 and 75 years, who were histopathologically confirmed to have ESCC. Eligible patients were in stage II/III disease according to the 8th edition of the American Joint Committee on Cancer (AJCC) Tumor Node Metastasis (TNM) staging system, with cT1-3N1-2M0 or cT3-4aN0M0 disease. Exclusion criteria encompassed patients with a history of other malignant tumors within the past 5 years that were not cured, individuals with ongoing autoimmune conditions or a history of autoimmune disease, and those who had received any previous anti-tumor treatments. The complete list of inclusion and exclusion criteria can be found in the supplementary materials (Appendix 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the Lanzhou University Second Hospital (No. 2021A 053) and informed consent was taken from all the patients.
Neoadjuvant therapy
In this study, all eligible patients meeting the inclusion criteria received a minimum of two cycles of neoadjuvant treatment with sintilimab plus chemotherapy, with each cycle administered every 3 weeks, as per the research protocol. The specific regimen for the treatment is outlined as follows: sintilimab: 200 mg intravenous (IV) infusion on day 1 of each cycle, cisplatin: 75 mg/m2 IV infusion on day 2 of each cycle, or carboplatin: area under the curve (AUC) 5 IV infusion on day 2 of each cycle. Paclitaxel liposome: 175 mg/m2 IV infusion on day 2 of each cycle, or nab-paclitaxel: 200 mg/m2 IV infusion on day 2 of each cycle. The treatment protocol aimed to assess the effectiveness and safety of the combination therapy in patients with locally advanced resectable ESCC, with the goal of improving the rate of pCR and overall treatment outcomes.
Surgery
Within 4–6 weeks after completing the last cycle of nICT, all enrolled patients underwent a series of evaluations to assess the treatment’s effectiveness and the feasibility of tumor resection. The assessments included gastroscopy and contrast-enhanced thoracoabdominal computed tomography (CT) scans. Patients who had received at least two cycles of nICT also underwent a thorough evaluation through physical examination, routine laboratory tests, echocardiography, and pulmonary function examination. The purpose of these evaluations was to determine whether they are suitable candidates for surgery. For patients without any contraindications for surgery, the recommended approach was the McKeown minimally invasive esophagectomy (MIE), which may or may not involve the use of robot-assisted techniques. The evaluation process aimed to guide appropriate treatment decisions and ensure the safety and suitability of the patients for surgical intervention, ultimately enhancing the chances of successful outcomes in the management of locally advanced resectable ESCC.
Staging and assessment
To stage and evaluate the disease, contrast-enhanced thoracoabdominal CT scan, endoscopic ultrasound (EUS), endoscopic duodenal gastroscopy, cranial magnetic resonance imaging (MRI), and whole-body bone scanning were conducted within 2 weeks before treatment initiation and again during 4–6 weeks after the completion of the last nICT. And for patients with suspected cervical lymph node (LN) involved, external ultrasonography of the neck with fine needle aspiration or positron emission tomography-CT (PET-CT) was required. Histopathological examination of the surgical specimens followed established norms, and the clinical and postoperative pathological staging were based on the AJCC 8th edition TNM staging system. Radiological response was assessed to confirm the efficacy of the neoadjuvant therapy by two independent central expert radiologists based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (11). Pathological examination of the surgically resected specimens was performed independently by two senior pathologists using the tumor regression grade (TRG), which assessed the percentage of residual viable tumor after primary tumor resection (12,13). All surgeries were performed by a professional and experienced thoracic surgeon at Lanzhou University Second Hospital, who performed more than 100 surgeries annually. All of the radiologists, pathologists and the surgeon were aware of the ongoing study priorly.
Adverse events were documented following the Common Terminology Criteria for Adverse Events v5.0 (CTCAE v5.0) (14). To determine any improvement in patients’ quality of life (QOL) after nICT treatment, the researchers utilized the European Organization for Research and Treatment of Cancer Quality of Life-Core 30 Questionnaire (EORTC QLQ-C30) Version 3.0 (15) and EORTC QLQ-esophageal-specific module-18 (OES18) (16) questionnaires. The comprehensive assessment of treatment response, adverse events, and QOL outcomes provided valuable insights into the safety and effectiveness of the nICT approach for locally advanced resectable ESCC patients.
Outcomes
The primary endpoint of this clinical trial was the rate of pCR, defined as the absence of residual viable tumor in the resected specimen, including LNs (ypT0N0M0). The secondary endpoints included the rate of major pathological response (MPR), the objective response rate (ORR), treatment-related adverse events (TRAEs) and immune-related adverse events (irAEs), as well as the evaluation of QOL during the nICT treatment period. MPR was defined as less than 10% of residual tumor cells within the primary tumor bed after neoadjuvant treatment and resection. ORR represented the percentage of patients achieving either a complete response (CR) or a partial response (PR). Other secondary indicators comprised the tumor downstaging rate, surgical rate, R0 resection rate (indicating the absence of residual tumor at the resection margins), and the incidence of perioperative complications. Additionally, OS and relapse-free survival (RFS) were considered exploratory endpoints in this study. By assessing these various endpoints, the researchers aimed to comprehensively evaluate the efficacy, safety, and overall impact of the nICT approach in patients with locally advanced resectable ESCC. The study outcomes would contribute valuable insights to the potential benefits and challenges associated with this treatment strategy.
Statistical analysis
The reported pCR rate of primary esophagus after nCT was approximately 14.8% (17), considering the primary endpoint, we assumed the regimen of the neoadjuvant sintilimab plus chemotherapy would achieve a pCR rate of 30.5%. Type I error was controlled within α=0.05 and power of test was 80%. Using the one-proportion Z-test of PASS software, the sample size was calculated to be 39 cases, and with a dropout rate of 10%, 43 cases were needed to be enrolled in this trial.
All demographic information and baseline characteristics of the patients were compiled into a table and subjected to analysis. The effectiveness and safety of the treatment were assessed, with categorical variables described using frequency and percentage (%). To compare the outcomes among the three groups (pCR, MPR, and TRG3/4 grade), Fisher’s exact test for categorical variables was employed. Changes in QOL scores before and after nICT were analyzed using the Wilcoxon signed-rank sum test. All statistical tests were two-tailed, and a significance level of 0.05 was used for determining statistical significance. The analysis was conducted as at May 10, 2023. For statistical analyses, SPSS software (version 27.0) was utilized. This comprehensive approach allowed for a robust evaluation of the treatment outcomes and potential differences between the response groups, providing valuable insights into the efficacy and safety of the nICT for locally advanced resectable ESCC patients.
Results
Baseline characteristics
Between March 2021 and January 2023, a total of 56 patients were initially screened for eligibility in this study. Ultimately, 43 eligible patients were included in the analysis and received a minimum of two cycles of nICT treatment. Of these, 32 patients (74.4%) successfully completed the subsequent surgery (Figure 1 illustrates the study flowchart). The majority of patients in this study cohort were male (n=41, 95.3%), and the most common location of esophageal tumors was in the middle and lower third segments (n=40, 93.0%). The median age of the patients was 63 years, with an age range of 55 to 67.5 years. All patients were clinically classified as stage II–III, with cT3 (n=37, 86.0%) being the predominant T category and cN1 (n=21, 48.8%) in the N category. A summary of baseline characteristics can be found in Table 1. This patient cohort’s demographic and clinical features provide valuable context for understanding the treatment outcomes and the potential impact of nICT in locally advanced resectable ESCC patients.
Table 1
| Baseline characteristics | All patients (n=43) |
|---|---|
| Age (years) | |
| Mean ± SD | 61.6±7.8 |
| Median (range) | 63 (55–67.5) |
| Gender | |
| Male | 41 (95.3) |
| Female | 2 (4.7) |
| ECOG | |
| 0 | 33 (76.7) |
| 1 | 10 (23.3) |
| Smoking | |
| Yes | 31 (72.1) |
| No | 12 (27.9) |
| Alcohol drinking | |
| Yes | 16 (37.2) |
| No | 27 (62.8) |
| Tumor location | |
| Upper third | 3 (7.0) |
| Middle third | 27 (62.8) |
| Lower third | 13 (30.2) |
| cTNM stage (AJCC-TNM 8th) | |
| II | 11 (25.6) |
| III | 32 (74.4) |
| cT stage | |
| T2 | 4 (9.3) |
| T3 | 37 (86.0) |
| T4 | 2 (4.7) |
| cN stage | |
| N0 | 9 (20.9) |
| N1 | 21 (48.8) |
| N2 | 13 (30.2) |
| Neoadjuvant treatment cycles | |
| 2 | 34 (79.1) |
| >2 | 9 (20.9) |
Data are expressed as n (%), median (range), or mean ± SD. Tumor stage was evaluated following AJCC 8th edition TNM staging system. SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; TNM, Tumor Node Metastasis; AJCC, American Joint Committee on Cancer.
Radiological and pathological response
In the total of 43 patients, 4 (9.3%) achieved a CR and 21 patients (48.8%) attained a PR based on the RECIST 1.1 criteria, resulting in an ORR of 58.1%. Additionally, 17 patients (39.5%) had stable disease (SD), and only one patient (2.3%) had progressive disease (PD), resulting in a disease control rate (DCR) of 97.7% (42/43). The radiological response evaluation was summarized in Table 2. Out of the 43 enrolled patients, 32 eventually underwent surgery, while ten patients declined surgery due to significant tumor shrinkage and symptomatic improvement, and one patient experienced disease progression (Figure 1).
Table 2
| Tumor response | N (%) |
|---|---|
| Radiological response (n=43) | |
| CR | 4 (9.3) |
| PR | 21 (48.8) |
| SD | 17 (39.5) |
| PD | 1 (2.3) |
| ORR | 25 (58.1) |
| DCR | 42 (97.7) |
| R0 resection (n=32) | 32 (100.0) |
| Pathological response (n=32) | |
| pCR (TRG1) | 9 (28.1) |
| MPR (TRG2) | 12 (37.5) |
| TRG3/4 | 11 (34.4) |
| ypTNM stage | |
| ypT0N0M0 (pCR) | 9 (28.1) |
| I | 18 (56.3) |
| II | 2 (6.3) |
| III | 12 (37.5) |
| Downstaging of TNM stage | |
| Yes | 20 (62.5) |
| No | 12 (37.5) |
| Downstaging of N stage | |
| Yes | 20 (62.5) |
| No | 12 (37.5) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate; pCR, pathological complete response; MPR, major pathological response; TRG, tumor regression grade; TNM, Tumor Node Metastasis.
Among the patients who underwent surgery, all 32 achieved R0 resection (100.0%). Based on post-surgery pathological evaluation, the rate of pCR (TRG1) was 28.1% (9/32), while 12 patients (37.5%) showed a MPR (TRG2). Compared with the clinical stage before treatment, overall clinical stage downstaging was observed in 20 patients (62.5%), and 20 patients (62.5%) achieved downstaging of clinical N stage (Table 2). These findings suggested that nICT demonstrated favorable treatment responses, including high rates of CR and PR, and a notable reduction in tumor stage. The achievement of R0 resection in all surgical patients further supported the potential effectiveness of this approach in locally advanced resectable ESCC.
A weak association was observed between pathological response and radiological response to nICT, with a P value of 0.014 (Table 3). However, no significant correlation was found between pathological response and the number of neoadjuvant treatment cycles (P>0.05; Table 3).
Table 3
| Variables | Pathological response | P value | ||
|---|---|---|---|---|
| pCR (n=9) | MPR (n=12) | TRG3/4 (n=11) | ||
| Radiological response | 0.014 | |||
| CR | 2 | 0 | 0 | |
| PR | 5 | 8 | 2 | |
| SD | 2 | 4 | 9 | |
| Neoadjuvant treatment cycles | 0.109 | |||
| 2 | 9 | 8 | 7 | |
| >2 | 0 | 4 | 4 | |
Statistically significant (P<0.05). pCR, pathological complete response; MPR, major pathological response; TRG, tumor regression grade; CR, complete response; PR, partial response; SD, stable disease.
TRAEs
The safety of the neoadjuvant treatment was assessed in the 43 patients who underwent the therapy. Based on follow-up and clinical data, TRAEs were reported in 41 patients (95.3%) with varying grades, with 10 patients (23.3%) experiencing grade 3 adverse events. No grade 4 or higher events occurred, and there were no instances of treatment discontinuation or death related to TRAEs. The most common grade 3 TRAEs primarily affected the blood system including neutropenia (9.3%), leukopenia (7.0%), anemia (7.0%), and thrombocytopenia (7.0%). These adverse events were manageable through drug withdrawal or symptomatic treatment. During the treatment, nine cases of irAEs were observed. Among these, eight patients did not require medical intervention, and their conditions returned to normal within 2 weeks. However, 1 patient (2.3%) experienced delayed surgical treatment due to checkpoint inhibitor pneumonitis (CIP). The details of the adverse events and irAEs related to the treatment are summarized in Table 4.
Table 4
| Treatment-related adverse events | Patients (n=43), n (%) | ||
|---|---|---|---|
| Any grade | Grade 1–2 | Grade 3 | |
| Total number | 41 (95.3) | 40 (93.0) | 10 (23.3) |
| Treatment-related AEs | |||
| Treatment-related hematological toxicity | |||
| Leukopenia | 18 (41.9) | 15 (34.9) | 3 (7.0) |
| Neutropenia | 15 (34.9) | 11 (25.6) | 4 (9.3) |
| Thrombocytopenia | 14 (32.6) | 11 (25.6) | 3 (7.0) |
| Anemia | 20 (46.5) | 17 (39.5) | 3 (7.0) |
| Febrile neutropenia | 1 (2.3) | 1 (2.3) | 0 |
| Treatment-related nonhematological toxicity | |||
| Nausea | 26 (60.5) | 26 (60.5) | 0 |
| Alopecia | 37 (86.0) | 37 (86.0) | 0 |
| Rash | 6 (14.0) | 6 (14.0) | 0 |
| Pruritus | 6 (14.0) | 6 (14.0) | 0 |
| Vomiting | 17 (39.5) | 16 (37.2) | 1 (2.3) |
| Dizziness | 8 (18.6) | 8 (18.6) | 0 |
| Diarrhea | 12 (27.9) | 12 (27.9) | 0 |
| Constipation | 2 (4.7) | 2 (4.7) | 0 |
| Asthenia or fatigue | 30 (69.8) | 30 (69.8) | 0 |
| Arthralgia | 9 (20.9) | 9 (20.9) | 0 |
| Muscle soreness | 8 (18.6) | 8 (18.6) | 1 (2.3) |
| Immune-related AEs | 9 (20.9) | 8 (18.6) | 0 |
| Increased AST/ALT | 4 (9.3) | 4 (9.3) | 0 |
| Hyperthyroidism/hypothyroidism | 5 (11.6) | 5 (11.6) | 0 |
| CIP | 1 (2.3) | 0 | 1 (2.3) |
Data are expressed as n (%). AEs, adverse events; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CIP, checkpoint inhibitor pneumonitis.
QOL
In this study, the baseline and post-neoadjuvant therapy QOL were assessed and compared using the EORTC QLQ-C30 and QLQ-OES18 questionnaires. The original scores were converted into a 0–100-point scale using a linear formula (18,19). Regarding the QLQ-C30 functional scale, there were no significant changes between pre-nICT and post-nICT assessments. However, in the symptom domain, there was a significant increase in fatigue (P=0.047) and diarrhea (P=0.033) scores after nICT while pain (P=0.011) showed a significant decrease. In the QLQ-OES18 questionnaire, several symptoms were significantly alleviated after neoadjuvant treatment compared to baseline. These improvements were observed in dysphagia (P=0.007), eating difficulties (P=0.005), pain (P=0.024), swallowing saliva (P=0.011), choking when swallowing (P=0.007), and cough (P=0.009). However, no significant changes were observed in other symptoms. The variations in QOL scores before and after treatment for the enrolled patients are presented in Table 5.
Table 5
| Variables | Pre-nICT | Post-nICT | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Min–Max | Median | Range | Mean (SD) | Min–Max | Median | Range | |||
| QLQ-C30 | ||||||||||
| Functional scales | ||||||||||
| Physical functioning | 85.4 (13.3) | 46.7–100 | 86.7 | 80–96.7 | 82.2 (13.5) | 46.7–100 | 86.7 | 73.3–90 | 0.266 | |
| Role functioning | 79.1 (20.6) | 16.7–100 | 83.3 | 66.7–100 | 81.0 (24.6) | 0–100 | 83.3 | 66.7–100 | 0.317 | |
| Emotional functioning | 82.9 (15.6) | 33.3–100 | 83.3 | 75–100 | 81.2 (13.9) | 33.3–100 | 83.3 | 75–91.7 | 0.519 | |
| Cognitive functioning | 76.0 (17.9) | 33.3–100 | 83.3 | 66.7–83.3 | 74.4 (19.0) | 33.3–100 | 83.3 | 66.7–83.3 | 0.535 | |
| Social functioning | 71.0 (28.4) | 0–100 | 66.67 | 50–100 | 72.1 (24.9) | 16.7–100 | 83.33 | 66.7–83.3 | 0.900 | |
| Symptom scales | ||||||||||
| Fatigue | 24.3 (16.1) | 0–66.7 | 22.2 | 11.1–33.3 | 31.5 (23.5) | 0–100 | 33.3 | 16.7–44.4 | 0.047 | |
| Pain | 14.7 (15.9) | 0–66.7 | 16.7 | 0–16.7 | 8.9 (12.8) | 0–50 | 0 | 0–16.7 | 0.011 | |
| Nausea and vomiting | 13.5 (19.9) | 0–66.7 | 0 | 0–16.7 | 16.0 (16.6) | 0–66.7 | 16.7 | 0–22.2 | 0.276 | |
| Symptom items | ||||||||||
| Dyspnea | 7.8 (14.2) | 0–33.3 | 0 | 0–0 | 7.0 (13.7) | 0–33.3 | 0 | 0–0 | 0.796 | |
| Insomnia | 14.7 (16.7) | 0–33.3 | 0 | 0–33.3 | 15.1 (18.3) | 0–66.7 | 0 | 0–33.3 | 0.808 | |
| Appetite loss | 20.9 (23.0) | 0–66.7 | 33.3 | 0–33.3 | 16.3 (25.6) | 0–100 | 0 | 0–33.3 | 0.230 | |
| Constipation | 18.6 (25.5) | 0–100 | 0 | 0–33.3 | 12.4 (16.3) | 0–33.3 | 0 | 0–33.3 | 0.163 | |
| Diarrhea | 3.9 (10.8) | 0–33.3 | 0 | 0–0 | 7.8 (14.2) | 0–33.3 | 0 | 0–0 | 0.033 | |
| Financial difficulties | 48.1 (32.8) | 0–100 | 33.3 | 33.3–66.7 | 50.8 (27.8) | 0–100 | 50 | 33.3–66.7 | 0.241 | |
| Global health status | 59.7 (21.7) | 16.7–100 | 50 | 50–75 | 64.5 (24.9) | 8.3–100 | 66.7 | 45.8–83.3 | 0.086 | |
| QLQ-OES18 | ||||||||||
| Symptom scales | ||||||||||
| Dysphagia | 35.4 (26.9) | 0–100 | 33.3 | 16.7–44.4 | 24.5 (22.8) | 0–88.9 | 22.2 | 11.1–38.9 | 0.007 | |
| Eating | 32.8 (20.4) | 0–75 | 25 | 25–45.8 | 25.2 (15.3) | 0–75 | 25 | 16.7–33.3 | 0.005 | |
| Reflux | 16.3 (15.6) | 0–66.7 | 16.7 | 0–25 | 15.5 (15.2) | 0–55.6 | 16.7 | 0–33.3 | 0.44 | |
| Pain | 22.5 (16.9) | 0–55.6 | 22.2 | 11.1–33.3 | 17.6 (14.2) | 0–100 | 11.1 | 11.1–27.8 | 0.024 | |
| Symptom items | ||||||||||
| Swallowing saliva | 16.3 (26.6) | 0–100 | 0 | 0–33.3 | 8.5 (16.4) | 0–100 | 0 | 0–0 | 0.011 | |
| Choking when swallowing | 38.0 (33.8) | 0–100 | 33.3 | 0–66.7 | 24.8 (25.3) | 0–100 | 33.3 | 0–33.3 | 0.007 | |
| Dry mouth | 26.4 (29.6) | 0–100 | 33.3 | 0–33.3 | 31.0 (30.3) | 0–100 | 33.3 | 0–33.3 | 0.283 | |
| Taste | 25.6 (28.9) | 0–100 | 33.3 | 0–33.3 | 25.6 (28.0) | 0–100 | 33.3 | 0–33.3 | 0.956 | |
| Cough | 13.2 (24.3) | 0–66.7 | 0 | 0–16.7 | 6.2 (13.1) | 0–66.7 | 0 | 0–0 | 0.009 | |
| Speech | 5.4 (14.4) | 0–66.7 | 0 | 0–0 | 3.1 (9.8) | 0–33.3 | 0 | 0–0 | 0.102 | |
Statistically significant (P<0.05). QLQ-C30, Quality of Life-Core 30 Questionnaire; QLQ-OES18, Quality of Life-esophageal-specific module 18; nICT, neoadjuvant immunochemotherapy; SD, standard deviation; Min, minimum; Max, maximum.
Surgical outcomes
Out of the 43 patients enrolled in the study, 32 patients underwent surgery, and the average operative time was 390.3±113.9 minutes. The surgical interval between neoadjuvant therapy and surgeries was 37.6±15.4 days. One patient experienced a delay in surgery due to irAEs (CIP). During the surgery, an average of 27.2±11.2 LNs were dissected. Perioperative complications were observed in 8 patients (25.0% of surgical cases), with pneumonia being the most common complication, occurring in 15.6% of the surgical cases (5 out of 32). Fortunately, there were no instances of perioperative mortality. A summary of the surgical findings is provided in Table 6.
Table 6
| Variables | Values |
|---|---|
| Surgical approach | |
| Robot-assisted McKeown esophagectomy | 25 (78.1) |
| Thoracoscopic-assisted McKeown esophagectomy | 7 (21.9) |
| Operative time (min) | 390.3±113.9 |
| Surgical interval (days) | 37.6±15.4 |
| Blood loss (mL) | 86.9±75.7 |
| Number of dissected LNs | 27.2±11.2 |
| Postoperative hospital stay (days) | 14.6±7.5 |
| ICU stay (days) | 0 |
| 30-day readmission | 0 |
| 30-day mortality | 0 |
| Surgical complications | 8 (25.0) |
| Pulmonary complications | |
| Pneumonia | 5 (15.6) |
| Pleural effusion requiring drainage | 1 (3.1) |
| Respiratory insufficiency | 1 (3.1) |
| Gastrointestinal complications | |
| Anastomotic leakage | 2 (6.3) |
| Anastomotic stricture | 1 (3.1) |
| Operation-related complications | |
| Atrial fibrillation | 2 (6.3) |
| Unhealing wound after surgery | 2 (6.3) |
| Hemothorax | 1 (3.1) |
| Postoperative chylothorax | 1 (3.1) |
Data are expressed as n (%) or mean ± SD. LNs, lymph nodes; ICU, intensive care unit; SD, standard deviation.
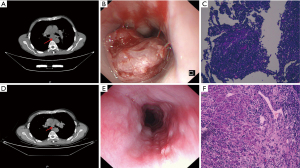
Presentation of typical case data
For patients with pathological response of pCR, MPR, and TRG3 after neoadjuvant therapy, the comparative images of chest CT, gastroscopy, and pathology before and after treatment are shown in Figures 2-4. The comparative images of chest CT and gastroscopy before and after treatment for patients with PD after neoadjuvant therapy are shown in Figure 5.



Discussion
In this prospective, phase II study, we explored the safety and efficacy of sintilimab plus nCT for locally advanced ESCC patients, and results demonstrated that this neoadjuvant treatment contributed to a significant and clinically promising improvement in the rates of pCR (28.1%), MPR (37.5%), and ORR (58.1%). Moreover, it also showed a manageable safety profile, causing a small number of grade ≥3 TRAEs, a low rate of occurrence for postoperative complications and no 30-day mortality. Furthermore, this study presented a great achievement in QOL among patients receiving nICT.
In terms of the therapeutic efficacy of neoadjuvant PD-1 inhibitor plus chemotherapy, our study (CY-NICE) showed a rate of pCR (28.1%) which is far higher than the results (0–9%) from the previous randomized controlled trial nCT (20,21), and even higher than the pCR rate (19.8%) of the latest DCF nCT scheme discussed in JCOG1109 (22). Although the pCR rate is inferior compared to that of CROSS study (5) (49% for squamous cell carcinoma) and 5010 study (23) (43.2%), it was sufficient to show that this neoadjuvant treatment for ESCC is effective. A multicenter single-arm clinical trial by Duan et al. (24), explored the efficacy and adverse events of sintilimab combined with nCT. The final results presented that six patients (6/17, 35.3%) ultimately obtained a pCR, which was similar to our findings and demonstrated the potential of sintilimab in a neoadjuvant therapy setting. Meanwhile, the rate of pCR in some other nICT studies was not identical to that in this study, such as NICE study (42.5%) (18), TD-NICE study (50%) (19), NIC-ESCC2019 study (31.4%) (25) and KEYSTONE-001 study (41.4%) (26), which indicated that the choice of ICIs and nCT regimen may have different effects on the final resolution for patients. Thus, randomized studies are necessary to confirm the optimal neoadjuvant treatment regimen among locally advanced ESCC.
Among patients undergoing neoadjuvant therapy, tumor downstaging and post-treatment LN status are considered important for prognosis (27). It has been reported (28) that the number of LNs removed in the neoadjuvant immunotherapy group is significantly lower than that in the surgery alone group in non-small cell lung cancer (NSCLC) patients. However, in this study, the regimen did not affect the number of LN resected during surgery, the median number of LN dissection was 26 comparable to that of the surgery alone group in Wang’s research (29), which was beneficial for achieving a more precise LN staging and for enhancing the prognosis of the patient (30). In addition, 62.5% of patients achieved tumor downstaging and 83.3% achieved LN downstaging, significantly higher than the rates of nCT and nCRT (31), which indicated that PD-1 inhibitors can not only induce anti-tumor immune response in primary tumors, but also enhance systemic activation of the immune system to eliminate micrometastasis in other tissues.
The design of this neoadjuvant research also concerned the safety, which was evaluated by recording the incidence of grade 3–4 TRAEs (32). In this study, the incidence of grade 3 AEs was 23.3%, which appeared to be less than that of nCRT (23.3% vs. 48.8%) (23), and there were no grade 4–5 AEs, indicating that this neoadjuvant regimen did not cause an additional risk of toxicity. However, irAEs deserved special attention during neoadjuvant immunotherapy. In this study, one patient had grade 3 CIP and resulted in operation delay. Hence, early warnings and prompt management of irAEs are essential. During the perioperative period, data were also collected on these patients to analyze if neoadjuvant therapy had any impact on surgery. The results indicated that the difficulty of subsequent surgical procedure didn’t increase basically, and there is no significant impact on operative methods, operative time, intraoperative bleeding, LN dissection, etc. Postoperative complications were relatively controllable and caused no new unexpected safety signals. In contrast, several studies have shown that surgical procedure that follows neoadjuvant therapy is generally more difficult than surgery alone due to dense fibrosis (33,34).
In the field of cancer treatment in China, QOL assessment is widely used in clinical treatment decision-making, treatment effect evaluation, and has become one of the endpoints of cancer treatment research. In our study, some typical symptoms of EC, such as dysphagia (P=0.007), eating difficulties (P=0.005), choking when swallowing (P=0.007), and pain (P=0.024) in the QLQ-OES18 score, showed significant improvement after neoadjuvant therapy, presumably due to treatment effectiveness. On the basis of our data, the tendency to refuse surgery was identified to be associated with the QOL. Patients who had significant improvement in QOL were more likely to refuse surgery, considering that 58.1% of patients in this study were able to achieve ORR after at least two cycles of nICT and the corresponding clinical symptoms were alleviated after tumor reduction.
In addition, patients with clinical CR (cCR) had chosen to refuse surgery, indicating that, for patients who achieved cCR in neoadjuvant therapy, organ preservation strategies may have become a research hotspot (35). The preSANO study (36) preliminarily explored the correlation between clinical response and the final pathological response of excised specimens, and our study also revealed a certain correlation between pathological response and radiological response (P=0.014), which indicated that imaging assessment may be a potential predictor of pathological response. Based on the preSANO study, the preSINO study (37) aimed to evaluate the accuracy of response evaluation of ESCC after nCRT, and a prospective trial will be conducted comparing active surveillance with standard esophagectomy in patients with a cCR after nCRT (SINO trial). We look forward to these studies for answering the question of “observation and waiting or active surgery” to some extent, and providing more valuable basis for the selection of treatment plans for ESCC patients after nICT. In the future, we will continue to follow up those cCR patients who refuse surgery, and conduct a simple comparative analysis with patients who undergo radical surgery.
There are several limitations to our study. First, the efficacy and safety of this regimen have been demonstrated initially, but it was still a single-arm study that requires a control group to confirm these findings compared to standard therapy. Secondly, the enrolled patients were only recruited from a single institution in the Chinese population and the case capacity was not large, which may cause selection bias. A further limitation is that our study did not reach the exploratory endpoints RFS and OS because of the relatively short follow-up period. However, our study is still being followed up, and the long-term results will be reported after completion.
Conclusions
CY-NICE study proved that sintilimab combined with paclitaxel and platinum as a neoadjuvant therapy for locally advanced resectable ESCC achieved promising antitumor activity, with considerable rates of pCR, MPR and R0 resection, as well as manageable safety profile. Further phase III randomized controlled trial is warranted.
Acknowledgments
We would like to extend our sincere appreciation to each and every patient as well as their families for consenting to take part in this research, as well as all the radiologists, pathologists, nurses, and anyone who assisted us in this trial.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1388/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1388/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1388/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1388/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the Lanzhou University Second Hospital (No. 2021A 053) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zheng RS, Zhang SW, Sun KX, et al. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi 2023;45:212-20. [Crossref] [PubMed]
- Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med 2017;14:33-41. [Crossref] [PubMed]
- Zheng Y, Li Y, Liu X, et al. Reevaluation of Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma: A Meta-Analysis of Randomized Controlled Trials Over the Past 20 Years. Medicine (Baltimore) 2015;94:e1102. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Barbetta A, Sihag S, Nobel T, et al. Patterns and risk of recurrence in patients with esophageal cancer with a pathologic complete response after chemoradiotherapy followed by surgery. J Thorac Cardiovasc Surg 2019;157:1249-1259.e5. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Lu Z, Wang J, Shu Y, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ 2022;377:e068714. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521-30. [Crossref] [PubMed]
- National Cancer Institute. Common Terminology Criteriafor Adverse Events (CTCAE). Version 5.0. U.S. Department of Health and Human Services. Accessed November 27, 2017.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Blazeby JM, Conroy T, Hammerlid E, et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 2003;39:1384-94. [Crossref] [PubMed]
- Chen Y, Wu X, Hao D, et al. Neoadjuvant nimotuzumab plus chemoradiotherapy compared to neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma. Oncotarget 2019;10:4069-78. [Crossref] [PubMed]
- Li Z, Liu J, Zhang M, et al. A phase II study of neoadjuvant immunotherapy combined with chemotherapy (camrelizumab plus albumin paclitaxel and carboplatin) in resectable thoracic esophageal squamous cell cancer (NICE study): Interim results. J Clin Oncol 2021;39:4060.
- Yan X, Duan H, Ni Y, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg 2022;103:106680. [Crossref] [PubMed]
- Zhao X, Ren Y, Hu Y, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: A meta-analysis based on clinical trials. PLoS One 2018;13:e0202185. [Crossref] [PubMed]
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. [Crossref] [PubMed]
- Kato K, Ito Y, Daiko H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol 2022;40:238.
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Duan H, Wang T, Luo Z, et al. A multicenter single-arm trial of sintilimab in combination with chemotherapy for neoadjuvant treatment of resectable esophageal cancer (SIN-ICE study). Ann Transl Med 2021;9:1700. [Crossref] [PubMed]
- Liu J, Li J, Lin W, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2 study. Int J Cancer 2022;151:128-37. [Crossref] [PubMed]
- Shang X, Zhao G, Liang F, et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage III) esophageal squamous cell carcinoma: a study protocol for a prospective, single-arm, single-center, open-label, phase-II trial (Keystone-001). Ann Transl Med 2022;10:229. [Crossref] [PubMed]
- Kamarajah SK, Navidi M, Wahed S, et al. Significance of Neoadjuvant Downstaging in Carcinoma of Esophagus and Gastroesophageal Junction. Ann Surg Oncol 2020;27:3182-92. [Crossref] [PubMed]
- Zhang F, Qiu B, Ji Y, et al. Comparison of surgical difficulty in patients with resectable non-small cell lung cancer under different neoadjuvant treatment modes: a retrospective cohort study. J Thorac Dis 2021;13:5604-16. [Crossref] [PubMed]
- Wang K, Liang Y, Huang J, et al. A propensity score-matched analysis of neoadjuvant chemoimmunotherapy versus surgery alone for locally advanced esophageal squamous cell carcinoma. J Surg Oncol 2023;128:207-17. [Crossref] [PubMed]
- Wu LL, Zhong JD, Zhu JL, et al. Postoperative survival effect of the number of examined lymph nodes on esophageal squamous cell carcinoma with pathological stage T1-3N0M0. BMC Cancer 2022;22:118. [Crossref] [PubMed]
- Jiang D, Wang H, Song Q, et al. Comparison of the prognostic difference between ypTNM and equivalent pTNM stages in esophageal squamous cell carcinoma based on the 8th edition of AJCC classification. J Cancer 2020;11:1808-15.
- Wang Z, Shao C, Wang Y, et al. Efficacy and safety of neoadjuvant immunotherapy in surgically resectable esophageal cancer: A systematic review and meta-analysis. Int J Surg 2022;104:106767. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Chaft JE, Hellmann MD, Velez MJ, et al. Initial Experience With Lung Cancer Resection After Treatment With T-Cell Checkpoint Inhibitors. Ann Thorac Surg 2017;104:e217-8. [Crossref] [PubMed]
- Fitzgerald RC. Organ-preserving approaches in oesophageal cancer. Lancet Oncol 2018;19:858-9. [Crossref] [PubMed]
- Noordman BJ, Spaander MCW, Valkema R, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol 2018;19:965-74. [Crossref] [PubMed]
- Zhang X, Eyck BM, Yang Y, et al. Accuracy of detecting residual disease after neoadjuvant chemoradiotherapy for esophageal squamous cell carcinoma (preSINO trial): a prospective multicenter diagnostic cohort study. BMC Cancer 2020;20:194. [Crossref] [PubMed]



