
Branching patterns and variations of the bronchus and blood vessels in the superior segment of the right lower lobe: a three-dimensional computed tomographic bronchography and angiography study
Highlight box
Key findings
• Anatomical variations of the right S6 segment are much more prevalent than previously reported.
What is known and what is new?
• The S6 segmentectomy is classified as simple due to the single intersegmental plane between the superior and basal segments.
• In the current study, we used three-dimensional computed tomographic bronchography and angiography (3D-CTBA) reconstruction to determine the patterns and variations of the bronchus and blood vessels of the superior segment of the right lower lobe (RS6). In our experience, surgical planning is extremely challenging without first performing a 3D reconstruction if the nodules are in close vicinity of V6b or V6c. We also observed several previously unreported variations (2 B6 variations, 4 A6 variations, and 5 V6 variations).
What is the implication, and what should change now?
• The 3D-CTBA reconstruction should be performed pre-operatively to identify anatomic variations and thus avoid vascular damage and ensure complete resection of the tumor.
Introduction
Segmentectomy has the benefit of conserving lung function with similar therapeutic efficacy to lobectomy for very well selected stage IA lung cancers (1-3), but is technically more challenging due to individual variation of the bronchus and blood vessels (4).
Segmentectomy could be categorized into simple versus complex based on the intersegmental plane (5). Superior segmentectomy is classified as simple due to the single intersegmental plane between the superior and basal segments. However, oncological outcomes in patients undergoing superior segmentectomy tend to be worse compared to those receiving other segmentectomy (6-8). Nakazawa et al. (9) reported that the issue of clipping the intersegmental plane was the cause of the right lower lobe superior segmentectomy’s subpar results. The use of staplers is appropriate in the majority of cases, but may result in residual S6 parenchyma in the cases in which the posterior portion of S6 stretches towards the caudal direction and creates a curved intersegmental plane. Also, segmental volume and surgical margins vary depending on the anatomical landmark used for intersegmental plane identification. Surgical margins tend to be smaller when the bronchi are used instead of intersegmental veins to define the intersegmental plane (9). Considering these anatomical traits, the processing of intraoperative anatomical structures and the individualization of the intersegmental interface ultimately impacts the safety and oncology efficacy of surgery.
In the current study, we used three-dimensional computed tomographic bronchography and angiography (3D-CTBA) reconstruction to determine the patterns and variations of the bronchus and blood vessels of the superior segment of the right lower lobe (RS6). The results were not directly compared to, but were discussed within the context of existing data as reported by Boyden (10) and Yamashita (11). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1607/rc).
Methods
Clinical data and 3D-CTBA reconstruction
All the patients undergoing thoracoscopic segmentectomy for pulmonary nodules at the Thoracic Surgery Department of the First Affiliated Hospital of Nanjing Medical University between November 1, 2018 and March 28, 2021 were retrospectively enrolled in this study. Two-dimensional (2D) computed tomography (CT) images and three-dimensional (3D) reconstructed images were acquired to investigate the anatomical pattern of RS6 bronchial arteries after 316 cases met the inclusion and exclusion criteria. Inclusion criteria: patients who underwent enhanced CT scans and 3D reconstruction. Exclusion criteria: (I) CT images were blurred and 3D-CTBA could not clearly show segmental branches of bronchi and vessels; (II) patients with prior history of lung surgery; (III) incomplete medical records. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Review Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2019-SR-123). Individual consent for this retrospective analysis was waived.
CT scan was performed using iopromide 370 (Uvixian; Bayer Healthcare, Berlin, Germany) as the contrast agent with a Siemens first-generation dual-source CT (Somatom Definition; Siemens, Erlangen, Germany) in all patients. A 20–22 G venous indwelling needle was routinely inserted into the right anterior elbow vein. The patient was supine with head first. Firstly, the density time curve axis was obtained by the method of cluster injection test, and the start time of CT scan and the dosage of iodine contrast agent were set according to the curve axis. A time-density curve was first generated using a bolus test. Dosage of the contrast agent was calculated as follows: (peak time of ascending aorta − peak time of pulmonary artery + scan time) ´ 5. Delay time equaled the peak time of the ascending aorta. CT data were transferred to the DeepInsight software (Deepinsight, Oslo, Norway), a custom-built system developed by this group in collaboration with Neusoft Medical and Northeastern University (12). Data processing prior to 3D reconstruction of the bronchus and blood vessels included tracheal threshold calculation, tracheal extraction, blood vessel extraction, pulmonary nodule extraction, and adjustment of image window width and window position.
The surgical approach was categorized into three types, namely single subsegmentectomy, intrasegmental combined subsegmentectomy, and intersegmental combined subsegmentectomy, based on the nodular classification of subsegmental location. In the process of surgical simulation, a standard virtual margin of 2 cm was typically established for the nodule. However, if the nodule contained a higher proportion of solid components, the virtual margin would be proportionally increased. It is important to note that the virtual margin must not surpass the predetermined resection range; otherwise, an additional resection of adjacent subsegments becomes necessary (13).
Nomenclature
The terminology for the construction of the lung segments is based on Boyden’s nomenclature for segmental bronchi and blood vessels (14), and included: B6, segmental bronchus; B6a, superior subsegmental bronchus; B6b, lateral subsegmental bronchus; B6c, medial subsegmental bronchus; A6, segmental artery; A6a, superior subsegmental artery; A6b, lateral subsegmental artery; A6c, medial subsegmental artery; V6, segmental vein; V6a1, intersubsegmental vein between S6a and S6c; V6a2, intersubsegmental vein between S6a and S6b; V6b1, intersegmental and intersubsegmental vein between S6b and S6c (V6b1); V6b2, intersegmental and intersubsegmental vein between S6b and S9a; V6b3, intersegmental and intersubsegmental vein between S6b and S8a, and V6c, intersegmental and intersubsegmental vein between S6c and S10a or S7b.
Variation definition
The definition of variation is controversial in the existing literature (15). In this study, variations were defined as anatomical patterns that deviate from the normal anatomical location, co-stem origin, or normal return flow. The superior segment bronchus, vascular stems, and subsegmental bronchus of the right lower lobe, as well as the number of vascular bifurcations and the originating location, were used in this study to determine the anatomical pattern.
Statistical analysis
Statistical analysis was performed in SPSS (version 24). For descriptive analysis, the frequency (%) of the categorical variable was assessed. 3D-CTBA was drawn by Deepinsight method. The significance level was set at a two-sided P value below 0.05.
Results
The analysis included 316 patients (53.37±12.21 years of age; 209 women). The average maximum diameter of the nodules was 10.88±6.03 mm. The types of surgery included single pulmonary subsegmental resection (n=51; 16.1%), pulmonary segment combined with adjacent subsegmental resection (n=24; 7.6%), and single segment resections (n=161; 51.0%). Combined subsegmentectomy was conducted in 80 patients (25.3%). Postoperative complications occurred in 9 patients, and included pulmonary air leakage (n=8; treated successfully with infusion of 50 mL 50% glucose solution containing 1 g erythromycin into the pleural cavity) and hemoptysis (n=1; treated successfully with oral carbazochrome). Pulmonary infection, atelectasis, and pleural effusion were not observed.
The main planes included the leaf cleft plane, the rib plane, the spine plane, and the intersegmental plane. Small airways and vascular connections could be detected inside the leaf cleft as well as varying degrees of S2 and S6 fusion on the surface. The rib surface and the leaf cleft surface occasionally exhibited a false crack, typically between the superior segment and the basal segment. The position of the intersegmental plane between segments varied significantly.
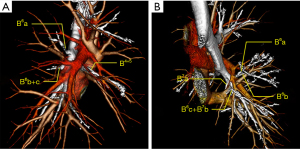
B6 had either a single (n=305, 96.5%) or 2 stems (n=11, 3.5%) (Table 1). For single-stem B6, the patterns of bifurcations of the subsegmental bronchus included B6a, B6b+c (18/316, 5.7%), B6b, B6a+c (178/316, 56.3%), and B6c, B6a+b (52/316, 16.5%), as well as miscellaneous minor branches that crossed complementarily across subsegments (12/316, 3.8%). Trifurcation of the subsegmental bronchus (B6a, B6b, and B6c) was seen in 14.2% (45/316) cases. The 2-stem B6 included B6b, B6a+c (6/316, 1.9%); B6c, B6a+b (1/316, 0.3%) (B6c and B7b co-stem in 1 patient); B6a, B6b+c (B6a alone above the right middle lobe bronchus, B6b+c below the right middle lobe bronchus; 4/316, 1.3%). There were 2 types of B6 variation (n=5; 1.6%). In 4 out of the 5 cases, B6a originated from the intermediate bronchus alone, opposite to and slightly above the orifice of the middle lobe bronchus, and the orifice of B6b+c was opposite and slightly below the orifice of the middle lobe bronchusorificeorificeorifice (Figure 1A). The remaining 1 case was B6c and B7b’s co-trunk variation (Figure 1B).
Table 1
| Branching patterns | Boyden (N=100), n (%) | Yamashita (N=180), n (%) | Our data (N=316), n (%) | P value* |
|---|---|---|---|---|
| B6 arises as a single stem | 94 (94.0) | 180 (100.0) | 305 (96.5) | 0.267; 0.011 |
| Bifurcates into | ||||
| B6b and B6a+c | 79 (79.0) | 112 (62.2) | 178 (56.3) | <0.001; 0.2 |
| B6c and B6a+b | 4 (4.0) | 11 (6.1) | 52 (16.5) | 0.003; 0.001 |
| B6a and B6b+c | 1 (1.0) | 9 (5.0) | 18 (5.7) | 0.05; 0.084 |
| Miscellaneous patterns | 5 (5.0) | 17 (9.4) | 12 (3.8) | 0.597; 0.01 |
| Trifurcates into B6a, B6c and B6b | 5 (5.0) | 22 (12.2) | 45 (14.2) | 0.013; 0.527 |
| Quadrivial type | 0 | 9 (5.0) | 0 | >0.99; <0.001 |
| B6 arises as two separate stems | 6 (6.0) | 0 | 11 (3.5) | 0.267; 0.011 |
| B6b and B6a+c | 4 (4.0) | 0 | 6 (1.9) | 0.232; 0.063 |
| B6c and B6a+b | 0 | 0 | 1 (0.3) | 0.573; 0.45 |
| B6a and B6b+c | 0 | 0 | 4 (1.3) | 0.258; 0.13 |
| Miscellaneous patterns | 2 (2.0) | 0 | 0 | 0.012; >0.99 |
*, P values indicate our data vs. Boyden and our data vs. Yamashita respectively.
The following branch patterns were observed for A6 (Table 2): (I) a single stem: (i) subsegmental artery bifurcates into: A6a, A6b+c (34/316, 10.8%); A6b, A6a+c (65/316, 20.6%); A6c, A6a+b (20/316, 6.3%); and miscellaneous (small branch cross-complementary between subsegments; 7/316, 2.2%); (ii) subsegmental artery trifurcates into: A6a, A6b, and A6c (62/316, 19.6%); (II) 2 stems: A6b, A6a+c (74/316, 23.4%); A6c, A6a+b (29/316, 9.2%); A6a, A6b+c (8/316, 2.5%); and miscellaneous with a tiny branch that was cross-complementary across subsegments (6/316, 1.9%); (III) 3 stems: A6a, A6b, and A6c (11/316,3.5%); and a miscellaneous (identification is challenging due to the short cross-complementary branching between subsegments; 2/316, 0.6%).
Table 2
| Branching patterns | Boyden (N=50), n (%) | Yamashita (N=130), n (%) | Our data (N=316), n (%) | P value* |
|---|---|---|---|---|
| A6 arises as a single stem | 40 (80.0) | 103 (79.2) | 188 (59.5) | 0.005; <0.001 |
| Bifurcates into | ||||
| A6a+c and A6b | 25 (50.0) | 17 (13.1) | 65 (20.6) | <0.001; 0.063 |
| A6a+b and A6c | 4 (8.0) | 10 (7.7) | 20 (6.3) | 0.657; 0.601 |
| A6b+c and A6a | 3 (6.0) | 51 (39.2) | 34 (10.8) | 0.3; <0.001 |
| Miscellaneous patterns | 8 (16.0) | 15 (11.5) | 7 (2.2) | <0.001; <0.001 |
| Trifurcates into A6a, A6c and A6b | – | 10 (7.7) | 62 (19.6) | 0.001; 0.002 |
| A6 arises as two separate stems | 8 (16.0) | 26 (20.0) | 117 (37.0) | 0.004; <0.001 |
| A6a+c and A6b | – | 19 (14.6) | 74 (23.4) | <0.001; 0.038 |
| A6a+b and A6c | – | 7 (5.4) | 29 (9.2) | 0.026; 0.181 |
| A6b+c and A6a | – | 0 | 8 (2.5) | 0.255; 0.067 |
| Miscellaneous patterns | 4 (8.0) | 0 | 6 (1.9) | 0.009; 0.114 |
| A6 arises as three separate stems | 2 (4.0) | 1 (0.8) | 11 (3.5) | 0.854; 0.108 |
*, P values indicate our data vs. Boyden and our data vs. Yamashita respectively.
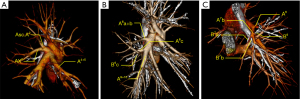
A6 variations included (Table 3) the following: (I) coexistence of A6 and A2 (n=25, 7.9%; Figure 2A); (II) A6b originating from A9+10/A10 alone (n=20, 6.3%); (III) A6c originating from A9+10 (n=10, 3.2%; Figure 2B); (IV) co-draining of A6 and A7 (n=2, 0.6%; Figure 2C).
Table 3
| Anatomical variations | Number | Frequency, % |
|---|---|---|
| A6 arising jointly with A2 | 25 | 7.9 |
| A6b arising A9+10 or A10 | 20 | 6.3 |
| A6c arising A9+10 | 10 | 3.2 |
| A6 arising jointly with A7 | 2 | 0.6 |
| Total | 57 | 18.0 |
The segments and sub-segments of V6 and its branches did not seem to be related to either B6 or A6 and its branches. The backflow patterns of V6 included (Table 4) the following: (I) a single stem: (i) subsegmental vein bifurcates into: V6a, V6b+c (68/316, 21.5%); V6b, V6a+c (44/316, 13.9%); and V6c, V6a+b (92/316, 29.1%); (ii) the subsegmental vein trifurcates into: V6a, V6b, and V6c (94/316, 29.7%); (II) 2 stems: V6a, V6b+c (5/316, 1.6%); V6c, V6a+b [n=12 for V6c backflow to the basal segment vein (3.8%); n=1 for V6c backflow to V10].
Table 4
| Branching patterns | Yamashita (N=130), n (%) | Our data (N=316), n (%) | P value |
|---|---|---|---|
| V6 converges as a single stem | 110 (84.6) | 298 (94.3) | 0.001 |
| Formed by two branches | 91 (70.0) | 204 (64.6) | 0.270 |
| V6a+c and V6b | 24 (18.5) | 44 (13.9) | 0.226 |
| V6a+b and V6c | 19 (14.6) | 92 (29.1) | 0.001 |
| V6b+c and V6a | 20 (15.4) | 68 (21.5) | 0.139 |
| Miscellaneous patterns | 28 (21.5) | 0 | <0.001 |
| Formed by V6a, V6c and V6b | 19 (14.6) | 94 (29.7) | 0.001 |
| V6 converges as two separate stems | 20 (15.4) | 18 (5.7) | 0.001 |
| V6c from V10 | 9 (6.9) | 1 (0.3) | <0.001 |
| V6c from CBV | 8 (6.2) | 12 (3.8) | 0.275 |
| V6b+c and V6a | 0 | 5 (1.6) | 0.149 |
| Miscellaneous patterns | 3 (2.3) | 0 | 0.007 |
CBV, common basal vein.
V6 variations included (Table 5) the following: (I) co-draining of V6 and V2 (n=20, 6.3%; Figure 3A); (II) co-draining of V6 and V4 (n=5, 1.6%); (III) V6 and V8+9 co-draining (n=3, 0.9%); (IV) V6 draining into the superior pulmonary vein (n=4, 1.3%; Figure 3B); and (V) direct V6 draining into the left atrium (n=5, 1.6%; Figure 3C).
Table 5
| Anatomical variations | Number | Frequency, % |
|---|---|---|
| V6 + V2 | 20 | 6.3 |
| V6 + V4 | 5 | 1.6 |
| V6 + V8+9 | 3 | 0.9 |
| V6 drains into SPV | 4 | 1.3 |
| V6 drains into left atrium | 5 | 1.6 |
| Total | 37 | 11.7 |
SPV, superior pulmonary vein.

Discussion
Segmentectomy was first reported in 1972 by Bonfils-Roberts and Clagett (16) as a treatment option for lung cancer patients who are elderly and have poor cardiopulmonary function. Subsequent research by Jensik et al. (17) reported comparable oncological efficacy of segmentectomy versus lobectomy for early-stage lung cancer. Since then, segmentectomy has been increasingly used in patients with early-stage lung cancer. The benefits of segmentectomy in the treatment of peripheral non-small cell lung cancer with diameter 2 cm and with pathologically confirmed no nodal disease by 2 important trials: the Japanese Clinical Oncology Organization JCOG0802/WJOG4607L trial and CALGB study (2,3). However, there have been few studies on the morphological patterns and variant types of segmental bronchi, arteries, and veins, with autopsy studies of limited sample size (18,19) and case reports (20). The use of 3D CT bronchial vascular reconstruction technology has now replaced traditional anatomical studies to examine the variation of the bronchi, pulmonary arteries, and veins (21).
The results from the current study showed that the dominant pattern of the bronchus B6 was single-stem bifurcated 2 branches type (260/316, 82.3%). Under this pattern, the superior subsegment and medial subsegment bronchus co-trunk (B6a+c) was seen in 56.3% of the cases. B6 variation was infrequent. In 4 patients (1.3%), B6a originated from the intermediate bronchus alone, with the orifice above the middle lobe bronchus orifice, and B6b+c opened below the middle lobe bronchus orifice. According to this finding, B6a and B6b+c should be treated individually during right superior segmentectomy to prevent unintentional damage to the right middle lung bronchus, stenosis of the right middle lung bronchus, and omission of B6b+c and thus insufficient resection margin. Another rare variation (n=1) is the co-trunk of B6c and B7b, and the genesis of B6a+b from the intermediate bronchus. Although rare, caution must be exercised to prevent damaging the B7b co-trunk with B6c.
We identified a total of 11 A6 patterns. Consistent with a previous study (22), the most common patterns in the current study included single-stem bifurcation (39.9%) and single-stem trifurcation (19.6%). Notably, 2- and 3-stem patterns (A6a, A6b, or A6c from the interlobar trunk or as an A9/A9+10 alone) were more common than previously reported by Yamashita (20.0% and 0.8%, respectively) (11). Co-stem with A2 was the most frequent variation form of A6 (n=25, 7.9%). Patients who had hypoplasia of the posterior oblique fissure frequently displayed this anomaly. When performing right upper lobectomy or posterior segmentectomy of the right upper lobe, caution should be taken to prevent inaccurate resection of A6. Similarly, attention should be taken when performing right lower pulmonary segmentectomy or right lower lobectomy to avoid inaccurate resection of A2. These patterns highlight the importance of preoperative planning based on CT or 3D-CTBA to achieve safe separation of the oblique fissure between the upper and lower lobes in segmentectomy.
V6 was classified into 7 patterns in the current study, and consistent with the Yamashita report, single-stem bifurcation was the most common pattern (64.6%). V6c, V6a+b accounted for 29.1% of the cases; V6a, V6b+c accounted for 21.5% of the cases; and V6b, V6a+c accounted for 13.9% of the cases. It is important to note that the intersegmental plane was defined by V6c between S6 and S10. When V6c originated from the common basal vein or V10 alone, the superior segment tended to occupy more space of the lower lobe. Such individual variations must be taken into consideration when clipping the intersegmental plane contact between the superior segment and the basal segment to ensure adequate resection margins. The most frequent V6 variation was V6/V2 co-trunk (n=20; 6.3%). When performing S6 or right lower lobectomy and S2 or right upper lobectomy, caution must be exercised to avoid severing the trunk.
In a previous 3D image study of 20 patients undergoing thoracoscopic segmentectomy by Nakao et al. (23), the 3D images were rated as “good” in terms of anatomical validity in 19 (95%) patients and as “good” in terms of anatomical consistency in 18 (90%) patients. Clearly, preoperative assessment based on CT and 3D-CTBA images is helpful to comprehend the relevant anatomical patterns and variations in patients undergoing segmentectomy and subsegmentectomy (24,25). The results in the current study differed significantly from that by either Boyden or Yamashita. Such a discrepancy could be partly attributed to the use of specialized imaging methods and 3D reconstruction in the current study, which in turn allowed us to obtain results that were as close to actual lung ventilation as possible.
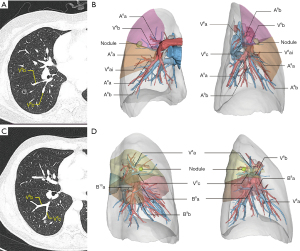
Patients undergoing superior segmentectomy for early-stage lung cancer tend to have worse long-term oncological outcomes than segmentectomy at other locations. Nakazawa et al. attributed this phenomenon to clipping the intersegmental plane. We concur with Nakazawa’s assertion that the right lower lobe superior segmentectomy’s subpar results were likely caused by insufficient surgical margins (9). In our experience, surgical planning is extremely challenging without first performing a 3D reconstruction if the nodules are in close vicinity of V6b or V6c. V6b lies between S6b, S8a, and S9 (26). Accordingly, resection of RS6 alone tends to result in insufficient resection margins for nodules close to V6b (Figure 4A,4B). For such cases, we previously proposed sublobar resection based on subsegment plan (27). Based on the results of the current study, we now believe that the best treatment for such cases is intersegmental combined subsegmentectomy, for example, S6/S6b+S8a or S6/S6b+S8a+S9a resection. Similarly, for lesions in the vicinity of V6c (Figure 4C,4D), we recommend S6/S6c+S10/subsuperior segment/or S7b resection.
The current study has several limitations. First, the data were based on patients at a single center with homogeneous ethnicity, mainly aimed at the Chinese population. Such results may be related to selection bias caused by the small sample size we selected. Whether the findings are generalizable to other populations is unknown. Second, minor branches (for example, <2 mm in diameter) may not be visible in 3D-CTBA, but could be functionally important. Third, to what extent the information obtained from the current study could improve surgical planning requires further studies.
Conclusions
Variation of segmental blood vessels, particularly the arteries and veins, in RS6 is much more common than previously thought. We also observed several previously unreported variations (2 B6 variations, 4 A6 variations, and 5 V6 variations). In particular, when the nodule is close to the V6b or V6c, surgical planning based on 3D reconstruction could be useful.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1607/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1607/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1607/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1607/coif). A.B. reports that he is the member of advisory board and recipient of speaker honoraria with Astra Zeneca, BMS, MSD, Ethicon and Roche, and Director of the Board of the ESTS and STS. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Review Committee of the First Affiliated Hospital of Nanjing Medical University (No.2019-SR-123). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Suzuki K, Watanabe SI, Wakabayashi M, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- White A, Swanson SJ. Video-assisted thoracic surgery (VATS) segmentectomy: state of the art. Minerva Chir 2016;71:61-6.
- Handa Y, Tsutani Y, Mimae T, et al. Surgical Outcomes of Complex Versus Simple Segmentectomy for Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1032-9. [Crossref] [PubMed]
- Jones GD, Caso R, Choe G, et al. Intentional Segmentectomy for Clinical T1 N0 Non-small Cell Lung Cancer: Survival Differs by Segment. Ann Thorac Surg 2021;111:1028-35. [Crossref] [PubMed]
- Handa Y, Tsutani Y, Tsubokawa N, et al. Clinical Prognosis of Superior Versus Basal Segment Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104:1896-901. [Crossref] [PubMed]
- Watanabe S, Suzuki K, Asamura H. Superior and basal segment lung cancers in the lower lobe have different lymph node metastatic pathways and prognosis. Ann Thorac Surg 2008;85:1026-31. [Crossref] [PubMed]
- Nakazawa S, Yajima T, Shirabe K. Superior S(6) Segment, a Wolf in Sheep's Clothing? Ann Thorac Surg 2021;112:686-7. [Crossref] [PubMed]
- Boyden EA. Segmental anatomy of the lung. New York: McGraw-Hill; 1954.
- Yamashita H. Roentgenologic anatomy of the lung. New York: Igaku-Shoin Medical Publishers; 1978.
- Zhang W, Chen L, Wang J, et al. A Study on the Authenticity of Preoperative Pulmonary Bronchial Angiography by DeepInsight Software. Zhongguo Fei Ai Za Zhi 2021;24:88-93. [Crossref] [PubMed]
- Chen L, Zhu Q, Wu W, et al. Atlas of thoracoscopic anatomical pulmonary subsegmentectomy. Amsterdam: Elsevier; 2023.
- Boyden EA. The intrahilar and related segmental anatomy of the lung. Surgery 1945;18:706-31.
- Fan K, Feng JT, Wang HY, et al. Anatomy of upper lung lobes of patients with small pulmonary nodules based on three-dimensional reconstruction of PC. Chinese Journal of Thoracic and Cardiovascular Surgery 2020;36:557-61.
- Bonfils-Roberts EA, Clagett OT. Contemporary indications for pulmonary segmental resections. J Thorac Cardiovasc Surg 1972;63:433-8.
- Jensik RJ, Faber LP, Kittle CF. Segmental resection for bronchogenic carcinoma. Ann Thorac Surg 1979;28:475-83. [Crossref] [PubMed]
- FERRY RM Jr. BOYDEN EA. Variations in the bronchovascular patterns of the right lower lobe of fifty lungs. J Thorac Surg 1951;22:188-201.
- Jiang JY. Surgical anatomy of bronchopulmonary segment. Shanghai: Shanghai Scientific & Technical Publishers; 1960;22-5.
- Akiba T, Marushima H, Kamiya N, et al. Thoracoscopic lobectomy for treating cancer in a patient with an unusual vein anomaly. Ann Thorac Cardiovasc Surg 2011;17:501-3. [Crossref] [PubMed]
- Yoldas B, Gursoy S. A pulmonary vascular variation to be considered in resective lung surgical procedures. Ann Thorac Surg 2014;97:715. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017;65:343-9. [Crossref] [PubMed]
- Nakao M, Omura K, Hashimoto K, et al. Novel three-dimensional image simulation for lung segmentectomy developed with surgeons' perspective. Gen Thorac Cardiovasc Surg 2021;69:1360-5. [Crossref] [PubMed]
- Akiba T, Marushima H, Harada J, et al. Anomalous pulmonary vein detected using three-dimensional computed tomography in a patient with lung cancer undergoing thoracoscopic lobectomy. Gen Thorac Cardiovasc Surg 2008;56:413-6. [Crossref] [PubMed]
- Yamada S, Suga A, Inoue Y, et al. Importance of preoperative assessment of pulmonary venous anomaly for safe video-assisted lobectomy. Interact Cardiovasc Thorac Surg 2010;10:851-4. [Crossref] [PubMed]
- Chen L, Zhu Q, Wu W, et al. Atlas of thoracoscopic anatomical pulmonary subsegmentectomy (Chinese). Nanjing: Southeast University Press; 2021.
- Wu W, He Z, Xu J, et al. Anatomical Pulmonary Sublobar Resection Based on Subsegment. Ann Thorac Surg 2021;111:e447-50. [Crossref] [PubMed]


