
Risk of reactive cutaneous capillary endothelial proliferation induced by camrelizumab in patients with non-small cell lung cancer: a retrospective study
Highlight box
Key findings
• Baseline percentage of eosinophil >1.75% and camrelizumab without an anti-angiogenic agent were risk factors for reactive cutaneous capillary endothelial proliferation (RCCEP) development in patients with advanced-stage non-small cell lung cancer (NSCLC).
What is known and what is new?
• RCCEP is a common immune-related adverse event related to camrelizumab.
• The occurrence of RCCEP might be positively associated with the clinical benefits of immunotherapy in NSCLC patients treated with camrelizumab.
What is the implication, and what should change now?
• Early identification of patients at risk of RCCEP for personalized management may benefit the treatment of NSCLC.
Introduction
Non-small cell lung cancer (NSCLC) is the second most common tumor and the leading cause of cancer death worldwide (1). Immune checkpoint inhibitors (ICIs) have become a new treatment paradigm in NSCLC, either as monotherapy or in combination with chemotherapy, and are now the first-line treatment options for advanced NSCLC (2,3). Still, immune-related adverse events (irAEs) are safety issues and affect the patient’s quality of life (4-6). Severe irAEs may even result in the discontinuation of ICIs, compromising the anti-tumor treatment of patients (6,7). Thus, the prevention and management of irAEs are needed (6), but the proper judgment of irAEs is necessary since some irAEs appear to be biomarkers of immunotherapy efficacy (8-10).
In addition to the common irAEs, some immunotherapies can induce reactive cutaneous capillary endothelial proliferation (RCCEP), which differs from other dermatologic adverse events (AEs) induced by conventional systemic therapies (11,12). RCCEP can be relieved spontaneously or by symptomatic treatments, including laser therapy, minor resection, hemostatic therapy, topical corticosteroids, systemic antibiotic treatment, and cryotherapy (13). Mild RCCEP may affect only the appearance, but severe RCCEP can lead to delay or discontinuation of immunotherapy and life-threatening complications (11). Therefore, predicting the occurrence of RCCEP to take proper means to prevent it is an important issue. A previous phase 2 trial demonstrated that the occurrence of RCCEP was associated with better tumor response and longer survival in patients with hepatocellular carcinoma (13). Whether this conclusion is valid in NSCLC needs validation to guide the proper patient management when RCCEP occurs.
Camrelizumab is a humanized, high-affinity IgG4 kappa monoclonal antibody against programmed cell death-1 (PD-1). It has been approved for the first-line treatment of advanced non-squamous and squamous NSCLC in China based on results from phase 3 trials (14,15). As a human endothelial cell agonist, camrelizumab mediates an aberrant combination of vascular endothelial growth factor (VEGF) and VEGF receptor-2 (VEGFR-2), leading to vascular endothelial cell activation into hemangioma (16). Camrelizumab can activate CD4+ T cells, increasing the release of interleukin (IL)-4, an inflammatory factor, by Th2 cells. IL-4 stimulates CD163+ M2 macrophage differentiation, which promotes vascular regeneration by releasing VEGF-A (17). In addition, camrelizumab targets PD-1-expressing cells in the skin, possibly leading to VEGF synthesis through chemokine release (18). The enhanced immune response induced by camrelizumab might disrupt the dynamic balance between angiogenic and anti-angiogenic factors, leading to RCCEP. Therefore, the increased expression of VEGF-A and the activation of the VEGF-A/VEGFR-2 signaling pathway can partly explain the pathogenesis of RCCEP, but the exact mechanisms of RCCEP remain unclear.
The advantages of VEGF inhibitors in lung cancer include disease control in several patients. Indeed, VEGF inhibitors inhibit the vascularization of the tumors and the normalization of the tumor vasculature. The disadvantages of VEGF inhibitors include potentially life-threatening toxicities (e.g., hemorrhage, febrile neutropenia, cerebrovascular events, and pulmonary thromboembolism) and progressive resistance to therapy. Tyrosine kinase inhibitors have the advantage of the oral route, while bevacizumab has the disadvantages of intravenous infusion (19-21).
This study aimed to explore the baseline characteristics and peripheral blood biomarkers associated with RCCEP and analyzed the association between patient survival and RCCEP and its biomarkers. The results could help identify patients who will develop RCCEP, allowing personalized management. The results might also provide clues for future mechanism exploration of RCCEP. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1144/rc).
Methods
Study design and patients
This retrospective study collected the data of consecutive patients with NSCLC who received camrelizumab at our hospital between January 2019 and December 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Ruijin Hospital (No. 2022-171-72). The need for informed consent was waived by the Institutional Review Board because of the retrospective nature of the study.
All patients were aged ≥18 years and received at least two cycles of camrelizumab-containing treatment (i.e., either as monotherapy or in combination with an anti-angiogenic agent or chemotherapy). There was no limit to the number of treatment lines. A minimum follow-up time of 2 months was required for inclusion in the study. The follow-up end date was 31 June 2022. The exclusion criteria were (I) death within 24 h after admission, (II) history of other malignant tumors within 5 years before the diagnosis of NSCLC, (III) combined with long-term glucocorticoid therapy, (IV) no baseline data available, or (V) no complete records of occurred AEs.
Data collection and outcomes
The baseline characteristics were extracted from the medical record, including age, sex, smoking history, histology, Eastern Cooperative Oncology Group performance status (ECOG PS), programmed cell death-ligand 1 (PD-L1) expression status, treatment line, and camrelizumab-containing treatment regimens. Data of blood cell counts and serum cytokine measured within 1 week before camrelizumab treatment were collected.
The outcomes included the occurrence of RCCEP, AEs, and irAEs, which were graded retrospectively according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. RCCEP was routinely checked and evaluated by oncologists and dermatologists according to the CSCO Guidelines for the Management of Toxicity Associated with Immune Checkpoint Inhibitors for grading criteria for RCCEP (13,22): grade 1, nodule(s) with a maximum diameter of ≤10 mm, with or without rupture and bleeding; grade 2, nodule(s) with a maximum diameter of >10 mm, with or without rupture and bleeding; grade 3, generalized nodules throughout the body, which may be complicated by skin infection; grade 4, multiple and generalized nodules, life-threatening condition; and grade 5, death. The follow-up data were collected to analyze progression-free survival (PFS), defined as the time from the first administration of camrelizumab to disease progression or death from any cause.
Statistical analysis
The continuous variables were presented as means ± standard deviation and were analyzed using Student’s t-test. The categorical variables were presented as n (%) and analyzed using the Chi-squared test. The receiver operating characteristic (ROC) curve method was used to determine the optimal cutoff value of baseline peripheral blood biomarkers. Univariable and multivariable logistic regression analyses were performed to analyze the association between baseline factors and RCCEP; the results were presented as odds ratios (ORs) and 95% confidence intervals (CIs). The variables with P≤0.10 in the univariable analyses were included in the multivariable logistic regression analyses. PFS was analyzed using the Kaplan-Meier method, and the differences between the curves were analyzed using the log-rank test. Two-sided P values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA).
Patient and public involvement
No patient was involved in the development of the research questions and outcome measures, study design or recruitment, and in the conduct of this study.
Results
Characteristics of the patients
Eighty patients with advanced NSCLC who had received camrelizumab were included in this study. Of them, 66 patients (82.5%) were males, and 56 (70.0%) had a smoking history. Patients with squamous and non-squamous NSCLC accounted for 17.5% and 82.5%, respectively. Seventy-four patients (92.5%) had an ECOG PS of 0–1. PD-L1 expression was evaluated in 51 patients (63.8%), of whom 42 (52.5%) had PD-L1 expression of at least 1% of tumor cells. Thirty-seven patients (46.2%) received camrelizumab as the first-line treatment. Forty-seven patients (58.8%) received camrelizumab monotherapy or combined with chemotherapy, and 33 (41.2%) received camrelizumab combined with an anti-angiogenic agent.
This study aimed to explore the characteristics of the patients who developed RCCEP. Among the 80 patients, 24 (30.0%) developed RCCEP, and 56 did not. Among the patients with RCCEP, 20 had grade 1–2 RCCEP, and four had grade 3–4 RCCEP. There were 20 (83.3%) patients with RCCEP after receiving camrelizumab monotherapy or combined with chemotherapy, and only 4 (16.7%) received treatment combined with an anti-angiogenic agent. The median time to the onset of RCCEP was 10.5 weeks. The distributions of age, sex, histology, ECOG PS, PD-L1 expression, and treatment line were similar regardless of RCCEP occurring or not (Table 1). The median follow-up time was 11 (range, 2–26) months.
Table 1
| Characteristics | Total (n=80) | Non-RCCEP (n=56) | RCCEP (n=24) | P value |
|---|---|---|---|---|
| Age (years), n (%) | 0.660 | |||
| ≥65 | 43 (53.8) | 31 (55.4) | 12 (50.0) | |
| <65 | 37 (46.2) | 25 (44.6) | 12 (50.0) | |
| Sex, n (%) | 0.607 | |||
| Male | 66 (82.5) | 47 (83.9) | 19 (79.2) | |
| Female | 14 (17.5) | 9 (16.1) | 5 (20.8) | |
| Smoking status, n (%) | 0.043 | |||
| Smoker | 56 (70.0) | 43 (76.8) | 13 (54.2) | |
| Non-smoker | 24 (30.0) | 13 (23.2) | 11 (45.8) | |
| Histology, n (%) | 0.404 | |||
| Squamous | 14 (17.5) | 8 (14.3) | 6 (25.0) | |
| Non-squamous | 66 (82.5) | 48 (85.7) | 18 (75.0) | |
| ECOG PS, n (%) | 0.781 | |||
| 0–1 | 74 (92.5) | 51 (91.1) | 23 (95.8) | |
| ≥2 | 6 (7.5) | 5 (8.9) | 1 (4.2) | |
| PD-L1 expression, n (%) | 0.062 | |||
| <1% | 9 (11.3) | 9 (16.1) | 0 | |
| 1–49% | 24 (30.0) | 17 (30.4) | 7 (29.2) | |
| ≥50% | 18 (22.5) | 11 (19.6) | 7 (29.2) | |
| Unknown | 29 (36.2) | 19 (33.9) | 10 (41.7) | |
| Treatment line, n (%) | 0.156 | |||
| First-line | 37 (46.2) | 23 (41.1) | 14 (58.3) | |
| Second-line and above | 43 (53.8) | 33 (58.9) | 10 (41.7) | |
| Treatment strategy, n (%) | 0.003 | |||
| Monotherapy or combination with chemotherapy | 47 (58.8) | 27 (48.2) | 20 (83.3) | |
| Combination with an anti-angiogenic agent | 33 (41.2) | 29 (51.8) | 4 (16.7) | |
| Peripheral blood biomarkers, mean ± SD | ||||
| Neu% | – | 66.37±8.39 | 59.18±18.22 | 0.075 |
| L% | – | 22.23±7.06 | 23.29±7.52 | 0.558 |
| EOS% | – | 2.87±2.09 | 4.13±2.90 | 0.064 |
| Neu (×109/L) | – | 4.49±2.28 | 3.97±1.90 | 0.294 |
| L (×109/L) | – | 1.39±0.53 | 1.35±0.51 | 0.707 |
| EOS (×109/L) | – | 0.18±0.12 | 0.23±0.14 | 0.123 |
| CRP (mg/L) | – | 25.13±49.68 | 7.00±10.09 | 0.081 |
| CD3 | – | 963.80±478.39 | 921.67±434.11 | 0.701 |
| CD4 | – | 565.00±309.87 | 580.58±313.34 | 0.839 |
| CD8 | – | 363.18±253.86 | 315.63±173.92 | 0.337 |
| NK | – | 307.29±389.07 | 254.58±161.09 | 0.394 |
RCCEP, reactive cutaneous capillary endothelial proliferation; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death-ligand 1; SD, standard deviation; Neu, neutrophil; L, lymphocyte; EOS, eosinophil; CRP, C-reactive protein; CD3, cluster of differentiation 3; CD4, cluster of differentiation 4; CD8, cluster of differentiation 8; NK, natural killer cell.
irAEs remain a concern with immunotherapy. irAEs of any grades occurred in 47 of the 80 patients (58.8%). No unexpected AEs or significantly increased toxicities were observed. In addition, 41.1% of the patients (23/56) without RCCEP developed irAEs (P<0.001). There were no significant differences in the blood cell count or levels of serum cytokines regardless of RCCEP occurring or not, but the percentage of neutrophil (Neu%), percentage of eosinophil (EOS%), and C-reactive protein (CRP) had P values ≤0.10 (Table 1).
Associations between baseline characteristics and RCCEP
This study used ROC curve analyses to determine the optimal cutoff levels of EOS%, Neu%, and CRP, three continuous variables that were found to be related to RCCEP with P values ≤0.10. According to the ROC curve analysis (Figure 1A), the optimal cutoff value of baseline EOS% for RCCEP was 1.75% [area under the curve (AUC) =0.642; 95% CI: 0.514–0.770]. The optimal cutoff values were 62.15% for Neu% (AUC =0.627; 95% CI: 0.491–0.762) and 9.5 mg/L for CRP (AUC =0.591; 95% CI: 0.464–0.718).

The results of the univariable logistic regression analyses are shown in Table 2. The variables with P≤0.10 (smoking status, treatment strategy, Neu%, EOS%, and CRP) were included in the multivariable analysis for RCCEP. The multivariable logistic regression analysis revealed that EOS% >1.75% was independently associated with a higher risk of RCCEP (OR =4.484; 95% CI: 1.139–17.651; P=0.032) and camrelizumab combined with an anti-angiogenic agent was independently associated with a lower risk of RCCEP compared with camrelizumab monotherapy or combined with chemotherapy (OR =0.188; 95% CI: 0.055–0.639; P=0.007) (Figure 1B).
Table 2
| Variables | OR (95% CI) | P value |
|---|---|---|
| Smoking status (smoker vs. non-smoker) | 2.799 (1.015–7.720) | 0.047 |
| Treatment strategy (combination with an anti-angiogenic agent vs. monotherapy or combination with chemotherapy) | 0.186 (0.056–0.615) | 0.006 |
| Baseline peripheral blood biomarker | ||
| Neu% (>62.15 vs. ≤62.15) | 0.338 (0.126–0.911) | 0.032 |
| EOS% (>1.75 vs. ≤1.75) | 4.529 (1.206–17.011) | 0.025 |
| CRP (mg/L) (>9.5 vs. ≤9.5) | 0.278 (0.074–1.052) | 0.059 |
RCCEP, reactive cutaneous capillary endothelial proliferation; OR, odds ratio; CI, confidence interval; Neu, neutrophil; EOS, eosinophil; CRP, C-reactive protein.
Clinical features of RCCEP
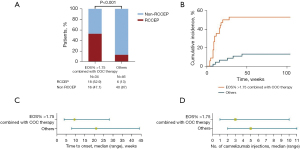
This study aimed to identify patients at a higher risk of RCCEP. For patients with camrelizumab monotherapy or combined with chemotherapy and whose EOS% was >1.75%, the incidence of RCCEP was 52.9% (18/34; 14 patients had grade 1–2 RCCEP, and four had grade 3–4 RCCEP). Of the other patients, 13.0% (6/46) had RCCEP (all grade 1–2). The cumulative incidence of RCCEP was significantly higher in patients with camrelizumab monotherapy or combined with chemotherapy and whose EOS% were >1.75% than the others [hazard ratio (HR) =6.051; 95% CI: 2.587–14.16; P<0.01], with 17.6% and 2.2% at 8 weeks and 52.9% and 8.7% at 28 weeks, respectively (Figure 2A,2B). The median time to the onset of RCCEP after starting camrelizumab was 9.5 weeks in patients with EOS% >1.75% and camrelizumab alone or combined with chemotherapy and 21 weeks in the other patients (P=0.011) (Figure 2C). The median number of camrelizumab injections before the occurrence of RCCEP was 3 (range, 1–10) in patients with EOS% >1.75% and camrelizumab alone or combined with chemotherapy and 5 (range, 2–11) in the other patients (Figure 2D).
Since RCCEP involves abnormal blood vessel proliferation in the skin, anti-angiogenic agents might affect the occurrence of RCEEP. The patients who received camrelizumab combined with an anti-angiogenic agent had a significantly lower incidence of RCCEP than those with camrelizumab monotherapy or combined with chemotherapy (12.1% vs. 42.6%, P=0.003). Among the anti-angiogenic agents used by the patients, bevacizumab was the most frequent, with three of 21 patients (14.3%) developing RCCEP. Ten patients received anlotinib, and none developed RCCEP. Two patients received recombinant human endostatin, an endogenous anti-angiogenic factor, and one of them developed RCCEP (Table 3).
Table 3
| Variables | Total | Non-RCCEP, n (%) | RCCEP, n (%) |
|---|---|---|---|
| Combination with an anti-angiogenic agent | 33 | 29 (87.9) | 4 (12.1) |
| Bevacizumab | 21 | 18 (85.7) | 3 (14.3) |
| Anlotinib | 10 | 10 (100.0) | 0 |
| Recombinant human endostatin | 2 | 1 (50.0) | 1 (50.0) |
| Monotherapy or combination with chemotherapy | 47 | 27 (57.4) | 20 (42.6) |
RCCEP, reactive cutaneous capillary endothelial proliferation.
Associated factors of PFS
Based on the available data, we further evaluated the impact of RCCEP and baseline EOS% >1.75% with camrelizumab monotherapy or combined with chemotherapy on the outcomes of ICI therapy in our cohort. The median PFS was 8 (range, 2–26) months. The Kaplan-Meier curve analysis demonstrated a trend toward a longer median PFS in patients with RCCEP than those without (17 vs. 9 months, P=0.069; Figure 3A). Baseline EOS% >1.75% and the use of camrelizumab monotherapy or combined with chemotherapy were associated with a significantly longer PFS (17 vs. 9 months, P=0.011; Figure 3B).

Discussion
This retrospective study reported that 30.0% of NSCLC patients receiving camrelizumab developed RCCEP, but most patients exhibited mild symptoms of RCCEP. High baseline EOS% (>1.75%) and the use of camrelizumab monotherapy or combined with chemotherapy were independent risk factors of RCCEP. Patients with camrelizumab monotherapy or combined with chemotherapy and high baseline EOS% had a numerically longer median PFS, reflecting the possible prognostic values of these two factors for RCCEP and PFS in patients with NSCLC treated with camrelizumab-containing regimens.
In this study, the EOS% in the patients experiencing RCCEP had a trend to be higher than not occurring and was included in the multivariable logistic regression analysis, which revealed that EOS% >1.75% was an independent risk factor of RCCEP. Over the past decade, eosinophils have been found to have direct proangiogenic effects (23,24). They promote endothelial cell proliferation, induce the production of VEGF in endothelial cells, and make these cells more sensitive to VEGF through the upregulation of their specific surface receptors (25). In addition to VEGF, eosinophils promote the production of other proangiogenic factors, such as IL-8 (26) and the nerve growth factor (27). Therefore, the association of EOS% with RCCEP deserves further exploration.
The multivariable logistic regression analysis also revealed that camrelizumab combined with an anti-angiogenic agent was independently associated with a lower rate of RCCEP compared with camrelizumab monotherapy or combined with chemotherapy. Moreover, the results suggest that camrelizumab combined with an anti-angiogenic agent might prolong the onset time of RCCEP. Theoretically, camrelizumab disrupts the dynamic balance between angiogenic and anti-angiogenic factors, leading to RCCEP (16-18), while anti-angiogenic agents might prevent RCCEP by targeting the VEGF/VEGFR signaling pathway. Li et al. reported the regression of RCCEP after using apatinib in four of eight patients with NSCLC treated with camrelizumab (12). A phase 2 trial showed an RCCEP rate of 29.5% in patients with hepatocellular carcinoma treated with camrelizumab plus apatinib (28), while another phase 2 study of patients with hepatocellular carcinoma showed a rate of RCCEP of 66.8% with camrelizumab monotherapy (29). All these results suggest camrelizumab’s role in preventing RCCEP. Still, research is necessary to determine the real prognostic significance of RCCEP and how the selection of one treatment over another would impact RCCEP occurrence and patient prognosis. Any individual predisposition to erythrosis and/or photosensitivity might favor an exaggerated angiogenic response to a specific monoclonal antibody. Hence, all patients should be carefully assessed before starting an ICI.
Previous studies showed that cutaneous irAEs could predict the efficacy of PD-1 monoclonal antibodies in solid tumors, especially melanoma (30-32). A phase 2 trial demonstrated that the occurrence of RCCEP was associated with significantly longer PFS (3.2 vs. 1.9 months) and overall survival (17.0 vs. 5.8 months) in patients with hepatocellular carcinoma treated with camrelizumab (13). In the present study, a trend toward a longer median PFS (17 vs. 9 months) was also observed in patients who experienced RCCEP occurrence compared with the patients without RCCEP. These results suggest that RCCEP might be a prognostic factor for patients with NSCLC treated with camrelizumab-containing regimens, which deserves further investigation. Especially, whether the association of a lower rate of RCCEP with camrelizumab with anti-angiogenic agents is an effect of the anti-angiogenic agent or related to a potential lower efficacy of the treatment remains to be determined. Indeed, the use of camrelizumab monotherapy or combined with chemotherapy was associated with a significantly longer PFS, which deserves more attention.
This study has several limitations. Due to the single-center design, the sample size was small, with only 24 patients with RCCEP and disease progression, resulting in low power and preventing the analysis of the impact of RCCEP on PFS. Nevertheless, since RCCEP occurs in patients with various solid tumors treated with ICIs, studies with a larger sample size will be performed in the future. The molecular evidence of the association between RCCEP and anti-angiogenic agents also needs to be investigated to assess the role of anti-angiogenic agents in the low rate of RCCEP after camrelizumab treatment. The study was retrospective, and all data were from the patient charts. Histopathological confirmation of RCCEP could not be performed in the setting of the present study, and it was supposed that all due procedures were performed when diagnosing the patients. In addition, there is a possibility that some minor lesions went unreported by the patients. Finally, this study did not use camrelizumab without a VEGF inhibitor and could not determine whether a subgroup of patients might achieve more benefits without a VEGF inhibitor.
Conclusions
This study suggested a high baseline EOS% and camrelizumab monotherapy or combined with chemotherapy were independently associated with the risk of RCCEP in patients with advanced-stage NSCLC, while a possible positive association with the clinical benefits of immunotherapy was observed. This study could help identify patients at risk of RCCEP, allowing personalized management, and might also provide clues for future mechanism exploration of RCCEP.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1144/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1144/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1144/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1144/coif). X.B. reports funding from the Bethune Charitable Foundation Pharmaceutical Research Capacity Building Project (No. Z04JKM2021005) and the Clinical Pharmacy Innovation Institute, Shanghai Jiao Tong University School of Medicine (No. CXYJY2019MS004). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Ruijin Hospital (No. 2022-171-72), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Singh N, Temin S, Baker S Jr, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline. J Clin Oncol 2022;40:3323-43. [Crossref] [PubMed]
- Collins LK, Chapman MS, Carter JB, et al. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer 2017;41:125-8. [Crossref] [PubMed]
- Schulz TU, Zierold S, Sachse MM, et al. Persistent immune-related adverse events after cessation of checkpoint inhibitor therapy: Prevalence and impact on patients' health-related quality of life. Eur J Cancer 2022;176:88-99. [Crossref] [PubMed]
- Thompson JA, Schneider BJ, Brahmer J, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Management of Immunotherapy-Related Toxicities. Version 1.2022. Fort Washington: National Comprehensive Cancer Network; 2022.
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol 2016;2:1346-53. [Crossref] [PubMed]
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. [Crossref] [PubMed]
- Conroy M, Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun 2022;13:392. [Crossref] [PubMed]
- Zhou X, Yao Z, Yang H, et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 2020;18:87. [Crossref] [PubMed]
- Chen X, Ma L, Wang X, et al. Reactive capillary hemangiomas: a novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210. Cancer Biol Med 2019;16:173-81. [Crossref] [PubMed]
- Li W, Wei Z, Yang X, et al. Salvage therapy of reactive capillary hemangiomas: Apatinib alleviates the unique adverse events induced by camrelizumab in non-small cell lung cancer. J Cancer Res Ther 2019;15:1624-8. [Crossref] [PubMed]
- Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol 2020;13:47. [Crossref] [PubMed]
- Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-14. [Crossref] [PubMed]
- Ren S, Chen J, Xu X, et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J Thorac Oncol 2022;17:544-57. [Crossref] [PubMed]
- Teng Y, Guo R, Sun J, et al. Reactive capillary hemangiomas induced by camrelizumab (SHR-1210), an anti-PD-1 agent. Acta Oncol 2019;58:388-9. [Crossref] [PubMed]
- Zhou C, Wang Y, Zhao J, et al. Efficacy and Biomarker Analysis of Camrelizumab in Combination with Apatinib in Patients with Advanced Nonsquamous NSCLC Previously Treated with Chemotherapy. Clin Cancer Res 2021;27:1296-304. [Crossref] [PubMed]
- Lickliter JD, Gan HK, Voskoboynik M, et al. A First-in-Human Dose Finding Study of Camrelizumab in Patients with Advanced or Metastatic Cancer in Australia. Drug Des Devel Ther 2020;14:1177-89. [Crossref] [PubMed]
- Bertino EM, Otterson GA. Benefits and limitations of antiangiogenic agents in patients with non-small cell lung cancer. Lung Cancer 2010;70:233-46. [Crossref] [PubMed]
- Daum S, Hagen H, Naismith E, et al. The Role of Anti-angiogenesis in the Treatment Landscape of Non-small Cell Lung Cancer - New Combinational Approaches and Strategies of Neovessel Inhibition. Front Cell Dev Biol 2020;8:610903. [Crossref] [PubMed]
- Zirlik K, Duyster J. Anti-Angiogenics: Current Situation and Future Perspectives. Oncol Res Treat 2018;41:166-71. [Crossref] [PubMed]
- Wang J, Zhang B, Peng L, et al. Chinese expert consensus recommendations for the administration of immune checkpoint inhibitors to special cancer patient populations. Ther Adv Med Oncol 2023;15:17588359231187205. [Crossref] [PubMed]
- Nissim Ben Efraim AH, Levi-Schaffer F. Roles of eosinophils in the modulation of angiogenesis. Chem Immunol Allergy 2014;99:138-54. [Crossref] [PubMed]
- Varricchi G, Galdiero MR, Loffredo S, et al. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2018;7:e1393134. [Crossref] [PubMed]
- Puxeddu I, Alian A, Piliponsky AM, et al. Human peripheral blood eosinophils induce angiogenesis. Int J Biochem Cell Biol 2005;37:628-36. [Crossref] [PubMed]
- Yousefi S, Hemmann S, Weber M, et al. IL-8 is expressed by human peripheral blood eosinophils. Evidence for increased secretion in asthma. J Immunol 1995;154:5481-90.
- Solomon A, Aloe L, Pe'er J, et al. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J Allergy Clin Immunol 1998;102:454-60. [Crossref] [PubMed]
- Xu J, Shen J, Gu S, et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res 2021;27:1003-11. [Crossref] [PubMed]
- Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21:571-80. [Crossref] [PubMed]
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol 2015;151:1206-12. [Crossref] [PubMed]
- Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016;152:45-51. [Crossref] [PubMed]
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res 2016;22:886-94. [Crossref] [PubMed]


