
A retrospective analysis of prognostic factors and treatment choices in small cell lung cancer with liver metastasis
Highlight box
Key findings
• Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2 and mixed small cell lung cancer (SCLC) as independent adverse prognostic factors for SCLC with liver metastatic.
• Chemotherapy is still the main treatment option for SCLC with liver metastasis.
What is known and what is new?
• The prognosis of SCLC with liver metastasis is extremely dismal. However, data on clinical features, prognostic factors, treatment options, and outcomes are scarce in SCLC with liver metastasis.
• Our study suggested that ECOG PS ≥2 and mixed SCLC increased risk of death, while treatment including systemic therapy or systemic therapy combined with local therapy reduced the risk of death.
What is the implication, and what should change now?
• This study provides important information of the current treatment and survival status of SCLC with liver metastasis in China.
Introduction
Small cell lung cancer (SCLC) is a high-grade pulmonary neuroendocrine tumor, accounting for approximately 15% of all lung cancers (1). This malignancy is characterized by its aggressive nature and early dissemination, accounting for its high mortality rate. At the time of diagnosis, nearly two-thirds of SCLC patients have already developed metastases (2). The prognosis for SCLC patients is dismal, with a 5-year survival rate of less than 5% (3).
The liver is the most common location for SCLC metastasis. Approximately 17% of SCLC patients present with liver metastases at diagnosis, a prevalence much higher than patients with non-SCLC (NSCLC) (around 4%) (4). Furthermore, the incidence of liver metastasis is highest in SCLC (5). In extensive-stage SCLC (ES-SCLC), the proportion of liver metastasis exceeds 30%, even soaring to 60% (6,7). Most of these patients (62%) present with liver metastases at the time of diagnosis or within 30 days of diagnosis (8). Unfortunately, SCLC patients with liver metastasis face the poorest prognoses. With the best supportive care, the median overall survival (OS) is a mere 3–4 months, and the 1-year OS rate falls below 20% (5,9). The 3-year OS rate plummets to as low as 1.7% (10). A retrospective study involving 200 SCLC patients demonstrated that those with liver metastasis had a 2.52-fold increased mortality risk compared to those without liver involvement (11). Additionally, numerous studies have shown that liver metastasis constitutes an independent adverse prognostic factor for SCLC (7,9,12-14). Nonetheless, the clinicopathological features influencing the prognosis of SCLC patients with liver metastasis have not been comprehensively elucidated.
Current clinical treatment options for SCLC, whether with or without liver metastasis, do not differ significantly. The management of SCLC with liver metastasis remains a formidable challenge, given that limited specific studies have focused on treatment strategies for this patient population. To bridge this knowledge gap, we undertook a retrospective analysis to assess the clinicopathological features, clinical treatment choices, outcomes, and prognostic factors of SCLC patients with liver metastasis. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1294/rc).
Methods
Patient selection and data collection
We retrospectively analyzed data from patients diagnosed with SCLC and liver metastasis at Jilin Cancer Hospital between January 1, 2013, and January 1, 2022. The inclusion criteria were as follows: (I) confirmation of SCLC through cytological or pathological means; (II) verification of liver metastasis through magnetic resonance imaging (MRI) or computerized tomography (CT) at the time of diagnosis; (III) availability of survival data in the follow-up system. Exclusion criteria included: (I) coexistence of other malignancies; (II) missing survival data.
Demographic and clinicopathological characteristics at baseline, as well as the treatment information for patients, were retrieved from electronic medical records. Survival data were obtained from records in eSuizhen v2.7.1.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Jilin Cancer Hospital (No. 202308-07-01) and individual consent for this retrospective analysis was waived.
Response and outcome evaluation
The Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1 (15) was employed to assess treatment efficacy. OS1 was defined as the time from the date of diagnosis to the date of death, while OS2/OS3 were defined as the time from the initiation of second-line or third-line treatment to death. Progression-free survival (PFS) was defined as the time from the start of treatment to disease progression or death. The last follow-up visit occurred on January 1, 2023.
Statistical analysis
Patient characteristics were summarized using descriptive statistics. Kaplan-Meier methodology and log-rank statistics were utilized to estimate OS and PFS. Independent prognostic factors for OS were assessed through univariate and multivariate regression analyses employing Cox proportional hazards regression models. A two-sided P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, version 26, and bar charts were generated using GraphPad Prism 8. Missing data were excluded from the analysis.
Results
Patient characteristics and treatment
In this study, 349 eligible patients with liver metastasis of SCLC at the time of diagnosis were included. The median age was 64 years (range, 24–87 years), with 151 patients (43.3%) aged ≥65 years. Most patients had pure SCLC (97.7%), and 66.8% of patients were male. A smoking history was present in 243 patients (69.6%). Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1 was observed in 78.8% of patients, while 66 patients (18.9%) had an ECOG PS ≥2. At baseline, the majority of patients (64.8%) had metastases to organs other than the liver, including bone (42.4%), intrapulmonary metastasis (14.9%), pleura (13.5%), and brain (10.3%). Notably, 85 patients (24.4%) had metastases in more than three organs. Lymph node metastasis (N1–N3) was detected in most patients (89.4%). Among the patients, 286 patients (81.9%) received first-line systemic therapy (Table S1), with 263, 14, and 9 patients treated with chemotherapy, chemotherapy combined with immunotherapy, and chemotherapy with angiogenesis inhibitors, respectively, 43.8% of patients received second-line treatment (Table S2), while 23.2% of patients (n=81) entered third-line treatment (Table S3). Radiotherapy was the local treatment for 69 patients. There were 60 patients without receiving first-line systemic treatment including 30 patients were provided with optimal supportive care due to inadequate organ function or poor performance status, based on the recommendation of oncologists, 17 patients refused anti-tumor treatment, and 13 patients opted for Chinese herbal medicine treatment alone. Additionally, there were three patients who received palliative radiotherapy alone due to poor performance status. The baseline characteristics of the patients are summarized in Table 1.
Table 1
| Characteristic | Statistic result |
|---|---|
| Age, years | |
| Median [range] | 64 [24–87] |
| <65 | 198 (56.7%) |
| ≥65 | 151 (43.3%) |
| Gender | |
| Male | 233 (66.8%) |
| Female | 116 (33.2%) |
| Smoking | |
| Never | 106 (30.4%) |
| Current/former | 243 (69.6%) |
| ECOG PS | |
| 0–1 | 275 (78.8%) |
| ≥2 | 66 (18.9%) |
| Missing | 8 (2.3%) |
| Pathology | |
| SCLC | 341 (97.7%) |
| Mixed-SCLC | 8 (2.3%) |
| N-stage | |
| N0 | 15 (4.3%) |
| N1 | 5 (1.4%) |
| N2 | 156 (44.7%) |
| N3 | 151 (43.3%) |
| Missing | 22 (6.3%) |
| Liver metastases | |
| Only liver | 123 (35.2%) |
| Involved other organs | 226 (64.8%) |
| Brain metastases | |
| No | 313 (89.7%) |
| Yes | 36 (10.3%) |
| Bone metastases | |
| No | 201 (57.6%) |
| Yes | 148 (42.4%) |
| Intrapulmonary metastasis | |
| No | 297 (85.1%) |
| Yes | 52 (14.9%) |
| Malignant pleural effusion | |
| No | 302 (86.5%) |
| Yes | 47 (13.5%) |
| Number of involved metastatic organs | |
| <3 | 264 (75.6%) |
| ≥3 | 85 (24.4%) |
| First-line systemic therapy | 286 (81.9%) |
| Chemotherapy | 263 (92.0%) |
| Chemotherapy + immunotherapy | 14 (4.9%) |
| Chemotherapy + angiogenesis inhibitors | 9 (3.1%) |
| Second-line systemic treatment | 153 (43.8%) |
| Chemotherapy | 131 (85.6%) |
| Chemotherapy + immunotherapy | 5 (3.3%) |
| Chemotherapy + angiogenesis inhibitors | 8 (5.2%) |
| Immunotherapy | 1 (0.7%) |
| Angiogenesis inhibitors | 4 (2.6%) |
| Immunotherapy + angiogenesis inhibitors | 2 (1.3%) |
| Chemotherapy + immunotherapy + angiogenesis inhibitors | 2 (1.3%) |
| Third-line or beyond treatment | 81 (23.2%) |
| Chemotherapy | 58 (71.6%) |
| Chemotherapy + immunotherapy | 7 (8.6%) |
| Chemotherapy + angiogenesis inhibitors | 11 (13.6%) |
| Angiogenesis inhibitors | 5 (6.2%) |
| Thorax radiotherapy | |
| No | 330 (94.6%) |
| Yes | 19 (5.4%) |
| Brain radiotherapy | |
| No | 302 (86.5%) |
| Yes | 47 (13.5%) |
| Other radiotherapy | |
| No | 323 (92.6%) |
| Yes | 26 (7.4%) |
| Treatment | |
| No systemic treatment | 60 (17.2%) |
| Systemic treatment | 220 (63.0%) |
| Systemic treatment + local radiotherapy | 69 (19.8%) |
ECOG PS, Eastern Cooperative Oncology Group performance status; SCLC, small cell lung cancer.
Objective response rate (ORR) and disease control rate (DCR)
Among patients receiving chemotherapy as first-line treatment, 217 patients were eligible for evaluation. Of these, 8, 110, 87, and 12 patients exhibited complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD), respectively. The ORR and DCR for first-line chemotherapy were 54.4% and 94.5%, respectively. For patients receiving chemotherapy combined with immunotherapy as first-line treatment (n=14), none achieved CR, while 11 patients showed PR, and 2 patients exhibited SD among the 13 patients eligible for evaluation. The ORR and DCR for first-line chemotherapy plus immunotherapy were 84.6% and 100%, respectively. Among patients treated with chemotherapy combined with angiogenesis inhibitors as first-line therapy, all 9 patients were evaluable, with 7 patients achieving PR and 2 patients showing SD. The ORR and DCR were 77.8% and 100%, respectively. Among the patients treated with second-line chemotherapy, 97 patients had efficacy evaluation data, with no patients achieving CR. Among them, 12, 52, and 33 patients exhibited PR, SD, and PD, respectively. The ORR and DCR for second-line chemotherapy were 12.4% and 66.0%, respectively. For patients who received chemotherapy alone as their third-line treatment (n=58), 41 patients were evaluable, and the ORR and DCR were 4.9% and 51.2%, respectively. Due to the limited number of cases, data on response were not collected for patients receiving second- or third-line treatment, including immunotherapy or angiogenesis inhibitors (Table 2).
Table 2
| Treatment | CR, N (%) | PR, N (%) | SD, N (%) | PD, N (%) | NA, N | ORR, % | DCR, % |
|---|---|---|---|---|---|---|---|
| First-line systemic treatment | |||||||
| Chemotherapy | 8 (3.7) | 110 (50.7) | 87 (40.1) | 12 (5.5) | 46 | 54.4 | 94.5 |
| Chemotherapy + immunotherapy | 0 | 11 (84.6) | 2 (15.4) | 0 | 1 | 84.6 | 100.0 |
| Chemotherapy + angiogenesis inhibitors | 0 | 7 (77.8) | 2 (22.2) | 0 | 0 | 77.8 | 100.0 |
| Second-line systemic treatment | |||||||
| Chemotherapy | 0 | 12 (12.4) | 52 (53.6) | 33 (34.0) | 34 | 12.4 | 66.0 |
| Third-line treatment | |||||||
| Chemotherapy | 0 | 2 (4.9) | 19 (46.3) | 20 (48.8) | 17 | 4.9 | 51.2 |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NA, not assessed; ORR, objective response rate; DCR, disease control rate.
OS
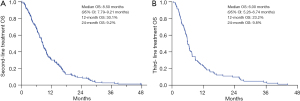
In this retrospective study, the median OS for all enrolled patients was 10.00 months [95% confidence interval (CI): 9.12–10.88]. The 12- and 24-month survival rates were 39.8% and 11.7%, respectively (Figure 1A). Untreated patients had a significantly shorter median OS of only 2.67 months (95% CI: 1.20–4.14). The median OS significantly improved for patients who received systemic treatment (10.33 months, 95% CI: 9.08–11.59, P<0.001) or systemic treatment combined with local radiotherapy (13.40 months, 95% CI: 10.45–16.35, P<0.001) (Figure 1B). Although systemic treatment plus local radiotherapy was associated with superior OS compared to systemic treatment alone, the difference did not reach statistical significance (P=0.082) (Figure 1B). The median OS1 for patients who received first-line treatment was 10.97 months (95% CI: 9.88–12.06). The 12- and 24-month survival rates were 45.7% and 13.8%, respectively (Figure 2A). There was no significant difference in the median OS1 for patients who received chemotherapy alone (11.37 months, 95% CI: 10.27–12.47), chemotherapy plus immunotherapy (7.7 months, 95% CI: 5.43–9.97), and chemotherapy plus angiogenesis inhibitors (11.97 months, 95% CI: 8.93–15.01) as their first-line chemotherapy (P=0.489) (Figure 2B). The median OS2 for patients who received second-line systemic treatment was 8.5 months (95% CI: 7.79–9.21), with 12- and 24-month survival rates of 30.1% and 9.2%, respectively (Figure 3A). For patients receiving third-line treatment, the median OS3 was 6.00 months (95% CI: 5.26–6.74), with 12- and 24-month survival rates of 23.2% and 9.8%, respectively (Figure 3B). A comparison was made among patients (n=133) who exclusively received first-line treatment, those who underwent second-line treatment (n=72), and those who received third-line therapy (n=81). The median OS from diagnosis to death was 7.57, 10.83, and 16.17 months for these groups, respectively (Figure S1). A better OS was observed for patients who received second-line treatment than those receiving first-line therapy alone, and the OS was significantly improved for those who received third-line therapy, suggesting that subsequent second and third-line therapies were associated with improved outcomes for patients with liver metastases.


PFS
The median PFS for patients who underwent first-line treatment was 5.27 months (95% CI: 4.75–5.79). The 6- and 12-month PFS rates were 35.6% and 2.5%, respectively (Figure 4A). Among patients who received chemotherapy, chemotherapy plus immunotherapy, or chemotherapy plus angiogenesis inhibitors, the median PFS was 5.07, 6.00, and 9.70 months, respectively. Significantly longer PFS was observed for patients who received chemotherapy plus angiogenesis inhibitors than those who received chemotherapy alone (P=0.027) (Figure 4B). PFS for chemotherapy and chemotherapy combined with immunotherapy was similar (P=0.152). The median PFS for second- and third-line treatments was 2.80 and 2.17 months, respectively (Figure 4C,4D).

Prognosis factors
During univariate analysis, 14 clinical parameters were included. Age ≥65 years, ECOG PS ≥2, mixed-SCLC, metastasis to other organs, malignant pleural effusion, receipt of systemic treatment, and receipt of systemic treatment plus local radiotherapy were significantly associated with prognosis. Furthermore, multivariate analysis identified ECOG PS ≥2 [hazard ratio (HR): 1.373, 95% CI: 1.036–1.820, P=0.028] and mixed-SCLC (HR: 2.724, 95% CI: 1.337–5.578, P=0.006) as independent predictors of poor prognosis factors in SCLC with liver metastasis. Conversely, systemic treatment (HR: 0.365, 95% CI: 0.267–0.498, P<0.001) or systemic treatment plus local radiotherapy (HR: 0.308, 95% CI: 0.211–0.450, P<0.001) was associated with a reduced risk of mortality (Table 3).
Table 3
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age, years | |||||
| <65 | 1 | 1 | |||
| ≥65 | 1.429 (1.155–1.769) | 0.001* | 1.124 (0.892–1.416) | 0.322 | |
| Gender | |||||
| Male | 1 | ||||
| Female | 1.041 (0.832–1.302) | 0.727 | |||
| Smoking | |||||
| Never | 1 | ||||
| Yes or ever | 0.910 (0.723–1.144) | 0.417 | |||
| ECOG PS score | |||||
| 0–1 | 1 | 1 | |||
| ≥2 | 1.612 (1.229–2.113) | 0.001* | 1.373 (1.036–1.820) | 0.028* | |
| Pathology | |||||
| SCLC | 1 | 1 | |||
| Mixed-SCLC | 2.243 (1.107–4.546) | 0.025* | 2.724 (1.337–5.578) | 0.006* | |
| First-line response | |||||
| PR + CR | 1 | ||||
| SD + PD | 1.246 (0.964–1.611) | 0.093 | |||
| Liver metastases | |||||
| Only liver | 1 | 1 | |||
| Involved other organs | 1.351 (1.083–1.687) | 0.008* | 1.206 (0.953–1.525) | 0.119 | |
| Brain metastases | |||||
| No | 1 | ||||
| Yes | 1.274 (0.900–1.804) | 0.171 | |||
| Bone metastases | |||||
| No | 1 | ||||
| Yes | 1.051 (0.849–1.302) | ||||
| Intrapulmonary metastasis | |||||
| No | 1 | ||||
| Yes | 1.301 (0.967–1.752) | 0.082 | |||
| Malignant pleural effusion | |||||
| No | 1 | 1 | |||
| Yes | 1.412 (1.035–1.926) | 0.029* | 1.261 (0.892–1.781) | 0.189 | |
| Number of involved metastatic organs | |||||
| <3 | 1 | ||||
| ≥3 | 1.241 (0.970–1.586) | 0.085 | |||
| N-stage | |||||
| N0 | 1 | ||||
| N1 | 0.894 (0.325–2.463) | 0.829 | |||
| N2 | 0.926 (0.544–1.576) | 0.777 | |||
| N3 | 1.097 (0.641–1.859) | 0.747 | |||
| Treatment | |||||
| No treatment | 1 | 1 | |||
| Systemic treatment | 0.339 (0.252–0.455) | <0.001* | 0.365 (0.267–0.498) | <0.001* | |
| Systemic treatment + local radiotherapy | 0.269 (0.189–0.383) | <0.001* | 0.308 (0.211–0.450) | <0.001* | |
*, P<0.05. OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; SCLC, small cell lung cancer; PR, partial response; CR, complete response; SD, stable disease; PD, progressive disease.
Discussion
In this retrospective study, we comprehensively investigated the clinical features of SCLC with liver metastasis. We found that most SCLC cases with liver metastasis were pure SCLC and often coexisted with bone metastasis. Approximately one-fourth of patients had metastases in more than three organs, and the proportion of patients with poor physical status was higher. Our study demonstrated that patients with an ECOG PS of ≥2 experienced a 1.4-fold increased risk of death, and patients with mixed SCLC had a 2.7-fold increased risk of death, while treatment including systemic therapy or systemic therapy combined with local therapy reduced the risk of death by approximately 60%. In our study, chemotherapy remained the primary choice for first-, second-, and third-line treatment. The OS of SCLC with liver metastasis was similar to historical data on ES-SCLC (16-18). This study provides important information about the current treatment and survival status of SCLC with liver metastasis in China. To our knowledge, this is the first real-world study to focus on SCLC with liver metastasis at the time of diagnosis.
At present, there is a paucity of research on the optimal treatment of SCLC with liver metastasis, accounting for a similar treatment approach for cases with or without liver metastasis. Before the advent of immunotherapy improving the survival of ES-SCLC, platinum plus etoposide was the treatment paradigm for SCLC with liver metastases. A retrospective study analyzed the treatment and survival of 28 patients with liver metastasis in SCLC, 27 of whom received chemotherapy, with a median OS of only 6 months (4). Another retrospective study included 507 patients with ES-SCLC, of whom 141 had liver metastases. The median OS was 9.0 months for patients with liver metastases and 12 months for patients without liver metastases (P=0.016) (19). Therefore, the efficacy of chemotherapy for SCLC with liver metastasis is very limited. In our study, more than 80% of patients also chose chemotherapy as their first-line treatment, and the OS was approximately 11 months, consistent with historical data on OS in patients undergoing chemotherapy ES-SCLC.
SCLC has strongly been associated with tobacco exposure. While the proportion of non-smokers among Caucasians with SCLC generally does not exceed 10% (20-22), the proportion of non-smokers was higher, usually in the range of 20–37%, in clinical trials and observational studies from China (23-25). There were 30.4% of non-smoker patients in our study, consistent with other studies conducted in China. We also analyzed the outcomes of SCLC cases with liver metastases who were smokers and non-smokers and found no significant difference in OS between the two groups (10.23 vs. 9.73, P=0.416) (Figure S2). For Chinese patients with ES-SCLC liver metastasis, smoking did not significantly affect prognosis, although relevant studies are warranted for other races.
Recently, the addition of programmed cell death (ligand) 1 [PD-(L)1] inhibitors to first-line chemotherapy increased the median OS by 2–4.7 months in ES-SCLC (20,21,23,24), becoming the new standard-of-care therapy. Several phase 3 studies of first-line immunotherapy for ES-SCLC have enrolled approximately 25–41% of patients with liver metastases (20,21,23,24). In the subgroup analysis of the ASTRUM-005 study, serplulimab plus chemotherapy improved OS in patients with liver metastases compared to chemotherapy (HR: 0.58, 95% CI: 0.40–0.84) (24). In the IMpower133 study, the median OS of the atezolizumab and placebo groups were 9.3 and 7.8 months for patients with liver metastases, respectively. Although the median OS was numerically longer, the difference was not significant (HR: 0.81, 95% CI: 0.55–1.20) (20). The OS of immunotherapy combined with chemotherapy was similar to that of chemotherapy alone in the CAPSTONE-1 study (HR: 0.92, 95% CI: 0.65–1.31) and the KEYNOTE-604 study (HR: 0.90, 95% CI: 0.67–1.21) in the liver metastasis subgroup (22,23). In patients without liver metastasis, the results of four phase 3 studies were consistent, showing that immunotherapy combined with chemotherapy significantly improved OS. In a meta-analysis (26), immunotherapy achieved only marginal efficacy in SCLC with liver metastases (HR: 0.94, 95% CI: 0.73–1.23). SCLC with liver metastasis had very limited benefit from immunotherapy compared with patients without liver metastasis (ratio of OS-HRs: 1.22, 95% CI: 1.01–1.46; P=0.036). In our study, only 14 patients with liver metastases received immunotherapy combined with chemotherapy as first-line treatment, and the median OS was 7.7 months, with no advantage over chemotherapy. Given that the sample size of immunotherapy in our study is very limited, a larger sample of specific studies is needed to clarify the value of immunotherapy in SCLC with liver metastasis.
Angiogenesis inhibitors, especially small molecule multi-target tyrosine kinase inhibitors, have been explored in relapsed SCLC. The ALTER 1202 study, a Phase 2 study evaluating the efficacy and safety of anlotinib versus placebo for SCLC as third-line and post-line therapy, confirmed that anlotinib could improve PFS and OS (25). In the liver metastasis subgroup of the ALTER 1202 study, the median PFS of the anlotinib group and placebo groups were 1.51 and 0.71 months, respectively (HR: 0.365, 95% CI: 0.17–0.78; P=0.0064) (10). Therefore, anlotinib improved PFS in treating SCLC with liver metastasis, but OS did not differ significantly. A real-world study assessed the efficacy and safety of anlotinib combined with the EP regimen (etoposide plus cisplatin) as the first-line treatment of ES-SCLC. The study included 58 patients, 16 (27.6%) with liver metastases. The PFS and OS were 4.7 and 5.4 months in patients with liver metastases, respectively, while the PFS and OS of those without liver metastases were 8.0 and 15.0 months (27). Anlotinib combined with chemotherapy did not result in a significant improvement in survival for SCLC with liver metastasis. Only nine patients in our study received chemotherapy combined with anlotinib as first-line treatment, and the median OS was not different from that of other regimens. Although multi-target angiogenesis inhibitors have achieved good efficacy in primary liver cancer, the value still needs further exploration in liver metastases, especially for SCLC.
There is an ongoing phase 2 study evaluating the efficacy and safety of bevacizumab in combination with chemoimmunotherapy in patients with liver metastases in ES-SCLC (BELIEVE study, NCT05588388). In addition, a phase 3 study of anlotinib combined with chemoimmunotherapy as the first-line treatment in ES-SCLC, in which liver metastases are used as one of the stratification factors, is also ongoing. These studies will provide answers to whether angiogenesis inhibitors combined with chemoimmunotherapy can provide a survival benefit in SCLC with liver metastasis.
Our study had several limitations. Firstly, it was a retrospective analysis conducted at a single center, and it only encompassed patients whose survival data were available in our follow-up system. Potential bias might have influenced treatment selection. To assess whether distinct chemotherapy regimens yield varying efficacy in patients with ES-SCLC and liver metastasis, future research will involve the utilization of multicenter data. Second, although immunotherapy combined with chemotherapy has become the new standard of first-line treatment for ES-SCLC, chemotherapy alone is still the main option for the first-line treatment choice for most patients in this study, and only a very limited number of patients chose immunotherapy combined with chemotherapy or angiogenesis inhibitors combined with chemotherapy as the first-line treatment. Therefore, the results of these two regimens in SCLC with liver metastasis should be interpreted with caution. Finally, we only analyzed the prognostic factors of SCLC with liver metastasis from the clinical features, and the analysis of molecular markers related to the prognosis was lacking.
Conclusions
In this real-world analysis, we validated poor physical status and mixed SCLC as independent adverse prognostic factors for SCLC with liver metastasis. Chemotherapy remains the primary therapeutic approach for individuals with SCLC and liver metastasis. The combination of chemotherapy and radiotherapy has demonstrated a potential for extending OS, though further validation is required through extensive sample studies. The potential benefits of integrating immunotherapy or angiogenesis inhibitors with chemotherapy in managing SCLC with liver metastasis warrant investigation in prospective studies. Indeed, understanding the molecular mechanisms and comprehensively exploring microenvironment characteristics may be key to addressing the treatment complexities associated with SCLC and liver metastasis.
Acknowledgments
The authors would like to thank Yidu Cloud (Beijing) Technology Co., Ltd for their assistance in data searching, extraction, and processing. We would like to thank Freescience Editorial Team for their help in polishing our paper.
Funding: This study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1294/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1294/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1294/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1294/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Jilin Cancer Hospital (No. 202308-07-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021;7:3. [Crossref] [PubMed]
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664-72. [Crossref] [PubMed]
- Jeon DS, Kim HC, Kim SH, et al. Five-Year Overall Survival and Prognostic Factors in Patients with Lung Cancer: Results from the Korean Association of Lung Cancer Registry (KALC-R) 2015. Cancer Res Treat 2023;55:103-11. [Crossref] [PubMed]
- Kagohashi K, Satoh H, Ishikawa H, et al. Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol 2003;20:25-8. [Crossref] [PubMed]
- Wang X, Wang Z, Pan J, et al. Patterns of Extrathoracic Metastases in Different Histological Types of Lung Cancer. Front Oncol 2020;10:715. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. [Crossref] [PubMed]
- Arriola E, Trigo JM, Sánchez-Gastaldo A, et al. Prognostic Value of Clinical Staging According to TNM in Patients With SCLC: A Real-World Surveillance Epidemiology and End-Results Database Analysis. JTO Clin Res Rep 2022;3:100266. [Crossref] [PubMed]
- Megyesfalvi Z, Tallosy B, Pipek O, et al. The landscape of small cell lung cancer metastases: Organ specificity and timing. Thorac Cancer 2021;12:914-23. [Crossref] [PubMed]
- Nakazawa K, Kurishima K, Tamura T, et al. Specific organ metastases and survival in small cell lung cancer. Oncol Lett 2012;4:617-20. [Crossref] [PubMed]
- Cheng Y, Wang Q, Li K, et al. Anlotinib for patients with small cell lung cancer and baseline liver metastases: A post hoc analysis of the ALTER 1202 trial. Cancer Med 2022;11:1081-7. [Crossref] [PubMed]
- Wu C, Li F, Jiao SC. Prognostic factors for survival of patients with extensive stage small cell lung cancer--a retrospective single institution analysis. Asian Pac J Cancer Prev 2012;13:4959-62. [Crossref] [PubMed]
- Yuan J, Cheng F, Xiao G, et al. Efficacy and Safety of Anlotinib in the Treatment of Small Cell Lung Cancer: A Real-World Observation Study. Front Oncol 2022;12:917089. [Crossref] [PubMed]
- Reck M, Mok TSK, Mansfield A, et al. Brief Report: Exploratory Analysis of Maintenance Therapy in Patients With Extensive-Stage SCLC Treated First Line With Atezolizumab Plus Carboplatin and Etoposide. J Thorac Oncol 2022;17:1122-9. [Crossref] [PubMed]
- Li Y, Jing W, Jing X, et al. Role of consolidative thoracic radiation in extensive-stage small-cell lung cancer with first-line chemoimmunotherapy: a retrospective study from a single cancer center. Discov Oncol 2023;14:55. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471-7. [Crossref] [PubMed]
- Evans WK, Shepherd FA, Feld R, et al. First-line therapy with VP-16 and cisplatin for small-cell lung cancer. Semin Oncol 1986;13:17-23.
- Niell HB, Herndon JE 2nd, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J Clin Oncol 2005;23:3752-9. [Crossref] [PubMed]
- Ma X, Zhang Z, Chen X, et al. Prognostic factor analysis of patients with small cell lung cancer: Real-world data from 988 patients. Thorac Cancer 2021;12:1841-50. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 2020;38:2369-79. [Crossref] [PubMed]
- Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:739-47. [Crossref] [PubMed]
- Cheng Y, Han L, Wu L, et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022;328:1223-32. [Crossref] [PubMed]
- Cheng Y, Wang Q, Li K, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled Phase 2 study. Br J Cancer 2021;125:366-71. [Crossref] [PubMed]
- Xia H, Zhang W, Zhang Y, et al. Liver metastases and the efficacy of immune checkpoint inhibitors in advanced lung cancer: A systematic review and meta-analysis. Front Oncol 2022;12:978069. [Crossref] [PubMed]
- Zheng HR, Jiang AM, Gao H, et al. The efficacy and safety of anlotinib combined with platinum-etoposide chemotherapy as first-line treatment for extensive-stage small cell lung cancer: A Chinese multicenter real-world study. Front Oncol 2022;12:894835. [Crossref] [PubMed]


