
Retrospective cohort study on the correlation analysis among peri-procedural factors, complications, and local tumor progression of lung tumors treated with CT-guided microwave ablation
Highlight box
Key findings
• The Hounsfield unit (HU) difference may predict the local ablation condition. Managing pneumothorax may reduce tumor progression and infection.
What is known and what is new?
• Despite adherence to guidelines, recurrence of lesions remains possible in lung tumor microwave ablation even when termination is enabled by 5–10 mm ground glass changes. Limited evidence exists regarding the correlation between timely management of perioperative complications (including pneumothorax, pleural effusion, hemorrhage, cavity formation, and infection) and local tumor progression.
• A greater difference between HU before and after the procedure or a decrease in computed tomography (CT) values immediately after ablation may predict a higher rate of local complete ablation. Prompt management of intraoperative pneumothorax may lower local tumor progression rates and decrease incidence of post-procedural infection.
What is the implication, and what should change now?
• The treatment’s effectiveness can be enhanced by monitoring CT HU differences, adjusting the needle position, and modifying ablation termination time during the operation. Timely management of pneumothorax has the potential to reduce tumor progression and infection incidence.
Introduction
Computed tomography-guided microwave ablation (CT-MWA) has been shown to be a safe and effective technique for local tumors (1), with the best complete ablation rates of 80–90% obtained from tumors <3 cm (2). With the advantages including a shorter procedure time and less ‘heat sink’ effect, MWA has become one of the most popular minimally invasive techniques (3). Local tumor progression was reported to be related to a larger number of lesions and a larger tumor size (4). While many proceduralists who perform lung ablation expect to see a zone of ground glass opacity (GGO) surrounding the tumor after ablation. The relationship between CT features of the ablation zone/tumor and local tumor progression remains unknown.
Currently, GGO surrounding the ablation zone is used to assess for technical effectiveness. The guidelines suggest that complete ablation can be achieved when the ground glass change surrounding the lesion measures 5–10 mm, allowing for the termination of the ablation (5). The presence or recurrence of lesions may still occur despite strict adherence to these requirements during MWA of lung tumors. The immediate pathological changes in a tumor after MWA may indicate the treatment response or long-term prognosis. Many radiomic studies have reported that quantitative features from medical images have the potential to indicate the treatment response and prognosis (6-8). The convective effect and low heat deposition of microwave thermal radiation in the lung can lead to heat gasification after ablation, potentially impacting the local density of lung tumors and resulting in changes to CT values. Hence, CT figures hold promise as predictive factors for local tumor progression although the relationship between Hounsfield unit (HU) value.
MWA carries a significant risk of complications. The incidence rates of pneumothorax vary widely, ranging between 8.5% and 63% (9). Most patients with pneumothorax are managed conservatively without intervention (9), with 0.8–15% receiving closed thorax drainage (10). The incidence of pleural effusion is approximately 1–60%, and about 1–7% of patients require puncture or catheter drainage (11). The frequency of hemorrhage during MWA varies between 3% and 8% (12), mostly manifested by hemoptysis and hemothorax. The formation of cavities is another significant complication of lung ablation. There is limited evidence indicating whether timely management of these perioperative complications are related to local tumor progression.
In this study, a retrospective analysis was conducted to investigate the correlation among peri-procedural factors including imaging characteristics of ablation zone, complications (such as hemorrhage, pneumothorax, pleural effusion, pulmonary infection, etc.), and the local outcomes [complete response (CR), incomplete response (ICR), and progressive disease (PD)]. The study is expected to contribute significantly to reducing complications in CT-guided lung tumor ablation techniques, improving local prognosis, and providing new insights for radiomics research in CT-MWA. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1799/rc).
Methods
CT-MWA procedures
- Pre-procedural preparation: a chest CT and enhanced CT were completed before procedure to determine the gross tumor region (GTR), measure the size of the lesion (maximum diameter of the cross-section under the pulmonary window), and evaluate the anatomical relationship between the lesion and adjacent organs (blood vessels, trachea, heart, etc.). The best puncture site and puncture route were determined according to the CT results; ablation needle size, number, and parameters were planned.
- Puncture: after local anesthesia, the needle was advanced to the predetermined position in the lesion under the guidance of CT. The ablation needle was passed through the center of the largest cross-section of the lesion, with the needle tip extending beyond the tumor by 0.5–1 cm.
- Ablation: ablation was performed at previously set parameters, such as power and time. When the GGO area in the post-ablation target zone (PTZ) extended the boundary of the pre-ablation lesion by more than 5 mm, the ablation was terminated. Each lesion used one probe.
- Management of complications: intra-procedural and post-procedural vital signs were monitored, and complications such as pneumothorax, pleural effusion, pulmonary inflammation, and bleeding were treated.
Study population
A total of 164 patients who underwent CT-MWA for primary or secondary lung cancer at the Minimally Invasive Therapy Center of Fudan University Shanghai Cancer Center from September 2019 to May 2020 were enrolled. The criteria for inclusion were as follows: (I) ≤3 unilateral pulmonary lesions (≤5 bilateral pulmonary lesions), maximum diameter of ≤3 cm in multiple metastases, and maximum diameter ≤5 cm in unilateral metastases; (II) no extensive extrapulmonary metastases (except for bone metastases after systemic therapy or radiotherapy); and (III) no severe coagulopathy or lung failure. Patients who have had prior surgery or previous MWA were excluded. For all patients, CT-MWA was the first choice of all treatments after assessment from a tumor board that allocates the patients into the best practice, and all patients who have had other treatments were excluded.
Data collection, follow-up, and radiologic assessment
Information mainly including demographic data, procedure details, and CT results were collected from electronic records which were well protected by researchers. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Minimally Invasive Therapy Center, Fudan University Shanghai Cancer Center (No. KS(Y)21316). The requirement for informed consent was waived due to the retrospective nature of this study. Patients were followed every 3 months with CT to determine patterns of recurrence and efficacy of ablation. Treatment response was captured and defined as follows: (I) CR (Figure 1A): (i) lesion disappearance; (ii) complete cavity formation; (iii) fibrosis or scarring; and (iv) solid nodules were reduced or did not change or increase, and CT showed no contrast-enhancement. (II) ICR (Figure 1B): (i) partial reduction or enlargement of the lesion (<20%), contrast-enhanced CT signs or positron emission tomography (PET)-CT indicating current metabolic activity of the tumor; (ii) part of the lesion showed cavity or fibrosis, and there were contrast-enhanced CT signs or PET-CT indicating current metabolic activity of the tumor. (III) PD (Figure 1C): (i) enlargement lesion size ≥20%, CT contrast-enhanced signs or PET-CT suggesting metabolic activity; and (ii) presence of new GGO or nodules around the ablated lesion, contrast-enhanced CT signs or PET-CT suggestive of tumor metabolic activity.

Main consumables and equipment
Disposable water-cooled MWA needle and MTC-3C MWA therapy instrument (Viking Jiuzhou Medical Device City Research and Development Center, Nanjing, China); Optima CT670 64-Row 128-Slice Spiral CT (GE Company, Boston, MA, USA).
Statistical analysis
The statistical processing was conducted using the software SPSS 20.0 (IBM Corp., Armonk, NY, USA). Measurement data were presented as , and data were compared using the two independent samples t-test. Frequency (%) was used to present enumeration data, and enumeration data were compared using the chi-square test or Fisher exact probability method. The Pearson correlation coefficient was utilized to conduct correlation analysis on two sets of measurement data that conformed to a normal distribution. The Spearman’s rank correlation coefficient was used to analyze the correlation between two sets of measurement data that did not follow a normal distribution. The Kruskal-Wallis test was employed to conduct statistical analysis among several sets of measurement data that were not normally distributed. Rank data or count data were analyzed using the rank-Spearman correlation coefficient. Two count data (categorical variables) were subjected to the chi-square test to determine the number of column associations. Statistical significance was considered when P≤0.05.
Results
Demographic results (Table 1)
Table 1
| Baseline characteristics | Values |
|---|---|
| Gender | |
| Male | 98 |
| Female | 68 |
| Age (years) | 56.1 [21–76] |
| Pathological type | |
| Colorectal cancer | 52 |
| Non-colorectal cancer | 112 |
| Tumor location | |
| Right lung | 94 |
| Left lung | 70 |
| Tumor size (cm) | |
| ≤1 | 82 |
| >1, ≤3 | 70 |
| 3–5 | 12 |
| Ablation power (W) | 50 [35–60] |
| Ablation time (min) | 7.6 [3–14] |
Values are presented as n or median [range].
The study included 98 male and 68 female participants with an average age of 56.1 years (range, 21 to 76 years). Among them, there were 52 patients diagnosed with colorectal cancer, 20 patients with hepatocellular carcinoma, 15 patients with lung cancer, 9 patients with nasopharyngeal carcinoma, and 68 patients with other types of cancers; 94 tumor lesions were in the right lung and 70 were in the left lung. A total of 82 tumor lesions measured ≤1 cm in diameter, 70 measured ≤3 cm, and 12 measured 3–5 cm. The average ablation power was 35–60 W, and the average ablation time was approximately 7.6 minutes (range, 3 to 14 minutes). The mean follow-up time of lesions was 31 months.
CT-MWA outcomes of complications and the local effective rate
Among the 164 patients, pneumothorax occurred in 58 patients (35.4%), post-procedural pulmonary infection in 15 cases (9.1%), and pleural effusion in 20 cases (12.2%). There was no hemorrhage, serious, or fatal adverse events. Local CR was achieved in 90, local ICR in 56, and local PD in 18 lesions. The local effective rate, which is the composite of CR and ICR, reached 89% over a mean follow-up time of 31 months.
A positive correlation of local tumor progression with average immediate posto-procedural CT value, and a negative correlation with difference between pre- and post-procedural CT value
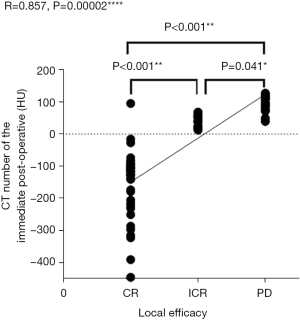
As illustrated in Figure 2, A thickness of 1.25 mm was chosen for thin-slice CT. The CT screenshot displayed a long axis of the ablated lesion, with the needle at two points (a red dot measuring 0.05 cm2) 0.05 cm bilateral to the tumor center. As illustrated in Figures 3,4, the local tumor progression exhibited a positive correlation with the average value of immediate post-procedural CT at the measurement points (R=0.857, P=0.00002) (Figure 3) and a negative correlation with the difference between pre- and post-procedural CT values (R=−0.855, P=0.006) (Figure 4). The average value of immediate post-procedural CT at the measurement point was predominantly negative in the CR group, positive in the PD group, and either positive or negative in the ICR group. The CR group had the greatest difference in CT value between pre- and post-procedural measurements; the PD group had the smallest difference; the ICR group exhibited a moderate difference. By conducting pairwise comparisons between groups, the average value of immediate post-procedural CT and the difference between pre-MWA and post-MWA showed statistically significant differences between the CR group and ICR group, the CR group and PD group, and the ICR group and PD group (the average value of immediate post-procedural CT: P<0.001, P<0.001, P=0.041, respectively) (Figure 3); CT value difference: P<0.001, P<0.001, P=0.018, respectively (Figure 4). Thus, the total ablation rate in patients with negative average values of immediate post-procedural CT after ablation was significantly higher than that in those with positive values. A larger difference between pre- and post-procedural CT values indicated a higher rate of complete local ablation. Figure 5 displays a 61-year-old male patient with sigmoid carcinoma with lung metastasis. Figure 6 shows a 60-year-old male patient with lung metastasis of sigmoid carcinoma. Both patients showed a decrease in CT values after ablation, and subsequent CT reexamination revealed that the ablation lesion had transformed into fibrous tissue. Contrastingly, if the average value of immediate post-procedural CT was positive, and the pre- and post-procedural CT values were similar, the lesion was more likely to remain or recur.




Post-procedural refractory pulmonary infection was positively correlated with pneumothorax
We observed that 58 (35%) patients had intra-procedural pneumothorax needing chest tube drainage. Of 58 patents needing chest tube, 36 (62%) patients received immediate chest tube drainage, and 22 (38%) patients was given supplemental oxygen after procedure. As a result, none of patients with chest tube developed infection, but 13 (59%) cases of patients without chest tube developed post-procedural pulmonary infection. According to the correlation analysis, there was a positive correlation between intra-procedural pneumothorax and post-procedural lung infection (R=0.340 and P=0.0001) (Table 2). Linear regression analysis indicated a significant difference in the post-procedural lung infection between the two groups with and without immediate treatment of intra-procedural pneumothorax (F=22.824, P=0.0003; Table 3).
Table 2
| Factors | Gender | Age | Pathological type | Tumor location | Tumor size | Ablation power | Ablation time | Pre-procedural CT value | The immediate post-procedural CT value | CT difference | Local tumor progression | Pneumothorax | Post-procedural infection | Pleural effusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||||||
| R | 1 | 0.121 | −0.012 | −0.024 | 0.025 | −0.041 | −0.039 | 0.108 | −0.112 | 0.112 | −0.071 | −0.053 | −0.076 | −0.087 |
| P | – | 0.277 | 0.917 | 0.828 | 0.822 | 0.715 | 0.726 | 0.333 | 0.317 | 0.315 | 0.523 | 0.636 | 0.499 | 0.439 |
| Age | ||||||||||||||
| R | 0.121 | 1 | −0.061 | 0.078 | −0.165 | 0.063 | −0.05 | 0.116 | −0.045 | 0.059 | −0.019 | 0.025 | 0.149 | 0.078 |
| P | 0.277 | – | 0.586 | 0.485 | 0.138 | 0.573 | 0.653 | 0.299 | 0.691 | 0.6 | 0.863 | 0.825 | 0.183 | 0.486 |
| Pathological type | ||||||||||||||
| R | −0.012 | −0.061 | 1 | −0.058 | −0.052 | −0.085 | 0.008 | −0.169 | −0.094 | −0.001 | −0.063 | −0.099 | −0.089 | −0.066 |
| P | 0.917 | 0.586 | – | 0.604 | 0.64 | 0.446 | 0.944 | 0.128 | 0.403 | 0.992 | 0.574 | 0.377 | 0.426 | 0.553 |
| Tumor location | ||||||||||||||
| R | −0.024 | 0.078 | −0.058 | 1 | −0.113 | −0.149 | −0.083 | −0.057 | 0.087 | −0.191 | −0.071 | 0.123 | 0.081 | −0.055 |
| P | 0.828 | 0.485 | 0.604 | – | 0.311 | 0.181 | 0.461 | 0.612 | 0.437 | 0.085 | 0.525 | 0.272 | 0.47 | 0.623 |
| Tumor size | ||||||||||||||
| R | 0.025 | −0.165 | −0.052 | −0.113 | 1 | 0.442 | 0.511 | −0.008 | 0.035 | −0.016 | 0.106 | 0.03 | −0.132 | 0.07 |
| P | 0.822 | 0.138 | 0.64 | 0.311 | – | 0.001** | <0.001** | 0.944 | 0.756 | 0.888 | 0.341 | 0.787 | 0.236 | 0.529 |
| Ablation power | ||||||||||||||
| R | −0.041 | 0.063 | −0.085 | −0.149 | 0.442 | 1 | 0.218 | 0.066 | −0.1 | 0.176 | 0.059 | 0.098 | −0.067 | 0.103 |
| P | 0.715 | 0.573 | 0.446 | 0.181 | 0.001** | – | 0.049* | 0.558 | 0.373 | 0.114 | 0.596 | 0.38 | 0.548 | 0.359 |
| Ablation time | ||||||||||||||
| R | −0.039 | −0.05 | 0.008 | −0.083 | 0.511 | 0.218 | 1 | −0.2 | −0.074 | 0.086 | −0.091 | 0.022 | −0.015 | −0.106 |
| P | 0.726 | 0.653 | 0.944 | 0.461 | <0.001** | 0.049* | – | 0.072 | 0.508 | 0.443 | 0.414 | 0.841 | 0.897 | 0.345 |
| Pre-procedural pulmonary infection | ||||||||||||||
| R | −0.133 | 0.003 | −0.062 | 0.136 | −0.104 | −0.047 | −0.037 | −0.11 | 0.185 | −0.21 | 0.228 | 0.214 | 0.698 | 0.183 |
| P | 0.233 | 0.976 | 0.579 | 0.222 | 0.352 | 0.675 | 0.739 | 0.324 | 0.095 | 0.058 | 0.039* | 0.054 | <0.001* | 0.1 |
| Pre-procedural CT value | ||||||||||||||
| R | 0.108 | 0.116 | −0.169 | −0.057 | −0.008 | 0.066 | −0.2 | 1 | 0.267 | 0.306 | −0.216 | −0.097 | 0.043 | 0.017 |
| P | 0.333 | 0.299 | 0.128 | 0.612 | 0.944 | 0.558 | 0.072 | – | 0.015* | 0.005** | 0.052 | 0.386 | 0.701 | 0.883 |
| The immediate post-procedural CT value | ||||||||||||||
| R | −0.112 | −0.045 | −0.094 | 0.087 | 0.035 | −0.1 | −0.074 | 0.267 | 1 | −0.737 | 0.857 | −0.017 | 0.215 | 0.13 |
| P | 0.317 | 0.691 | 0.403 | 0.437 | 0.756 | 0.373 | 0.508 | 0.015* | – | <0.001** | 0.00002**** | 0.878 | 0.052 | 0.245 |
| CT difference | ||||||||||||||
| R | 0.112 | 0.059 | −0.001 | −0.191 | −0.016 | 0.176 | 0.086 | 0.306 | −0.737 | 1 | −0.855 | −0.067 | −0.178 | −0.214 |
| P | 0.315 | 0.6 | 0.992 | 0.085 | 0.888 | 0.114 | 0.443 | 0.005** | <0.001** | – | 0.006** | 0.548 | 0.109 | 0.053 |
| Local tumor progression | ||||||||||||||
| R | −0.071 | −0.019 | −0.063 | −0.071 | 0.106 | 0.059 | −0.091 | −0.216 | 0.857 | −0.855 | 1 | 0.135 | 0.191 | 0.161 |
| P | 0.523 | 0.863 | 0.574 | 0.525 | 0.341 | 0.596 | 0.414 | 0.052 | 0.00002**** | 0.006** | – | 0.226 | 0.086 | 0.147 |
| Pneumothorax | ||||||||||||||
| R | −0.053 | 0.025 | −0.099 | 0.123 | 0.03 | 0.098 | 0.022 | −0.097 | −0.017 | −0.067 | 0.135 | 1 | 0.340 | 0.192 |
| P | 0.636 | 0.825 | 0.377 | 0.272 | 0.787 | 0.38 | 0.841 | 0.386 | 0.878 | 0.548 | 0.226 | – | 0.0001** | 0.084 |
| Post-procedural infection | ||||||||||||||
| R | −0.076 | 0.149 | −0.089 | 0.081 | −0.132 | −0.067 | −0.015 | 0.043 | 0.215 | −0.178 | 0.191 | 0.340 | 1 | 0.262 |
| P | 0.499 | 0.183 | 0.426 | 0.47 | 0.236 | 0.548 | 0.897 | 0.701 | 0.052 | 0.109 | 0.086 | 0.0001** | – | 0.018* |
| Pleural effusion | ||||||||||||||
| R | −0.087 | 0.078 | −0.066 | −0.055 | 0.07 | 0.103 | −0.106 | 0.017 | 0.13 | −0.214 | 0.161 | 0.192 | 0.262 | 1 |
| P | 0.439 | 0.486 | 0.553 | 0.623 | 0.529 | 0.359 | 0.345 | 0.883 | 0.245 | 0.053 | 0.147 | 0.084 | 0.018* | – |
*, P<0.05; **, P<0.01; ****, P<0.0001. CT, computed tomography.
Table 3
| Post-procedural infection | Intra-procedural pneumothorax | R | P† | F | P‡ | |
|---|---|---|---|---|---|---|
| − | + | |||||
| − | 104 | 45 | 0.340 | 0.0001*** | 22.824 | 0.0003*** |
| + | 2 | 13 | ||||
***, P<0.001. †, P value of the correlation coefficient; ‡, P value of the chi-square test.
A negative correlation between intra-procedural management of pneumothorax and local tumor progression
The correlation between intra-procedural management of pneumothorax and local tumor progression was significant (R=−0.550 and P=0.0003) (Figure 7). There was a significant difference in the local tumor progression between the two groups with and without intra-procedural management of pneumothorax (F=25.090, P=0.016; Table 4). Prompt management of intraoperative pneumothorax may lower local tumor progression rates.

Table 4
| Groups | Intraoperative management of pneumothorax | R | P† | F | P‡ | |
|---|---|---|---|---|---|---|
| − | + | |||||
| CR | 2 | 18 | −0.550 | 0.0003*** | 25.090 | 0.016** |
| ICR | 5 | 13 | ||||
| PD | 15 | 5 | ||||
**, P<0.01; ***, P<0.001. †, P value of the correlation coefficient; ‡, P value of the chi-square test. CR, complete response; ICR, incomplete response; PD, progressive disease.
Discussion
In this study, the results showed that a greater HU difference between pre- and post-procedural CT values or a decrease in CT values immediately after ablation predicted a higher rate of local complete ablation. In addition, correlation analysis results indicated a positive correlation between post-procedural refractory infection and pneumothorax, and a negative correlation between intra-procedural management of pneumothorax and local tumor progression.
Local MWA technique has grown in popularity recently as a precise and minimally invasive therapy for lung tumors. However, the incidence of pneumothorax after ablation remains high at approximately 8.5–63% (13). It has been shown that most cases of pneumothorax are self-limiting and can be treated conservatively with oxygen inhalation, whereas only 13–20% of cases require chest tube drainage (14). In our study, we discovered that intra-procedural management of pneumothorax was negatively correlated to the local tumor progression rate of lung lesion ablation. The relationships may involve the following mechanisms: (I) intra-procedural pneumothorax results in sudden and conspicuous compression of the lung, leading to the displacement of the lung and the tumor, which impairs accuracy of the puncture and efficacy of the ablation. Although several studies have shown that artificial pneumothorax before puncture was helpful to relieve pain and avoid damage of vital organs adjacent to the tumor (15-18), concerns remain that pneumothorax increases local pulmonary motion, making tumors more likely to displace during puncture and ablation, resulting in impairment of ablation efficacy. Considering the low incidence of vital organs damage, ablation efficacy was felt to overweigh the relatively little discomfort during surgery for patients with malignant tumors. (II) The occurrence of intra-procedural pneumothorax reduces the convective effect of lung ablation and increases the heat-sink effect, reducing efficacy of the ablation. In addition, intra-procedural pneumothorax caused discomfort for patients. Therefore, pneumothorax should be managed during operation.
In this study, we found that a greater decrease in CT value was related to a higher rate of CR, whereas a smaller decrease may imply a higher risk of local recurrence. This phenomenon might be attributed to histological changes in the ablation zone. After ablation, local tissue underwent coagulation necrosis, destruction of cell structures, and local alveolar vaporization, all decreasing CT values. A more complete ablation suggested a more obvious tissue shrinkage and local gasification, and thus a greater decrease in CT value. Liu et al., Chetan et al., and Alexander et al. reported that the mean density and the mean CT values both decreased in the ablation zone (19-21), which is consistent with our results. Several studies have suggested that radiomics analysis using image analysis tools had the potential to recognize recurrence early, but its reliance on particular computer software limited availability in the clinic (22-24). There have also been reports about the utilization of post-ablation CT densitometry to predict recurrence, but added radiation dose conferred by CT densitometry remained a concern (25). Therefore, the post-ablation changes in CT value can be a more available and safer way to predict recurrence and instruct optimal timing of ablation termination. Our results suggest that if there is no difference in immediate post-ablation HU, there is a high rate of ICR or PD suggesting that additional treatment should be considered in that session.
A limitation of this study was the difference in CT scanners, which likely created variability in absolute CT values. In addition, the study included tumor lesions diagnosed with different pathological types, which may have different treatment responses to ablation. Although our results showed a satisfactory local effective rate of 89%, the limitations of this study were its retrospective design, the possibility of selection bias, the length of follow-up and the irregular follow-up due to the inconvenience of travel from remote areas. In addition, our follow-up results lack histological proof of CR.
Conclusions
CT-MWA procedure exerts significant local effects in the treatment of primary or metastatic lung tumors. Intra-procedural pneumothorax may affect the efficacy of this procedure, and immediate CT value may be a predictive index of local tumor progression.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1799/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1799/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1799/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1799/coif). B.B.P. serves as Consultant of GE Healthcare, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Minimally Invasive Therapy Center, Fudan University Shanghai Cancer Center [No. KS(Y)21316]. The need for patient consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shang Y, Li G, Zhang B, et al. Image-guided percutaneous ablation for lung malignancies. Front Oncol 2022;12:1020296. [Crossref] [PubMed]
- Ye X, Fan W, Wang Z, et al. Expert consensus on thermal ablation therapy of pulmonary subsolid nodules (2021 Edition). J Cancer Res Ther 2021;17:1141-56. [Crossref] [PubMed]
- Blackmon SH, Sterner RM, Eiken PW, et al. Technical and safety performance of CT-guided percutaneous microwave ablation for lung tumors: an ablate and resect study. J Thorac Dis 2021;13:6827-37. [Crossref] [PubMed]
- Shan YQ, Wang HY, He XN, et al. Feasibility analysis of CT-guided thermal ablation of multiple pulmonary nodules combined with intraoperative biopsy. Front Radiol 2022;2:1036026. [Crossref] [PubMed]
- Ye X, Fan W, Wang H, et al. Expert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J Cancer Res Ther 2018;14:730-44. [Crossref] [PubMed]
- Hu Y, Xue G, Liang X, et al. The safety and feasibility of three-dimensional visualization planning system for CT-guided microwave ablation of stage I NSCLC (diameter ≤2.5 cm): A pilot study. J Cancer Res Ther 2023;19:64-70. [Crossref] [PubMed]
- Shen X, Chen T, Yang B, et al. Magnetic resonance imaging-guided microwave ablation for lung tumor: a case report. Quant Imaging Med Surg 2021;11:2780-4. [Crossref] [PubMed]
- Du K, Liu Y, Wu K, et al. Percutaneous microwave ablation for lung tumors: a retrospective case-control study of conventional CT and C-arm CT guidance. Quant Imaging Med Surg 2023;13:5737-47. [Crossref] [PubMed]
- Meng L, Wu B, Zhang X, et al. Microwave ablation with local pleural anesthesia for subpleural pulmonary nodules: our experience. Front Oncol 2022;12:957138. [Crossref] [PubMed]
- Harvey J, Windsor MN, Steinke K. Delayed complications following microwave ablation of lung tumours. J Med Imaging Radiat Oncol 2019;63:770-8. [Crossref] [PubMed]
- Reisenauer JS, Eiken PW, Callstrom MR, et al. A prospective trial of CT-guided percutaneous microwave ablation for lung tumors. J Thorac Dis 2022;14:939-51. [Crossref] [PubMed]
- Nour-Eldin NE, Naguib NN, Mack M, et al. Pulmonary hemorrhage complicating radiofrequency ablation, from mild hemoptysis to life-threatening pattern. Eur Radiol 2011;21:197-204. [Crossref] [PubMed]
- Yang Q, Qi H, Zhang R, et al. Risk Factors for Local Progression after Percutaneous Radiofrequency Ablation of Lung Tumors: Evaluation Based on a Review of 147 Tumors. J Vasc Interv Radiol 2017;28:481-9. [Crossref] [PubMed]
- Yan P, Tong AN, Nie XL, et al. Assessment of safety margin after microwave ablation of stage I NSCLC with three-dimensional reconstruction technique using CT imaging. BMC Med Imaging 2021;21:96. [Crossref] [PubMed]
- Li C, Wang J, Shao JB, et al. Microwave ablation combined with chemotherapy improved progression free survival of IV stage lung adenocarcinoma patients compared with chemotherapy alone. Thorac Cancer 2019;10:1628-35. [Crossref] [PubMed]
- Jia H, Tian J, Liu B, et al. Efficacy and safety of artificial pneumothorax with position adjustment for CT-guided percutaneous transthoracic microwave ablation of small subpleural lung tumors. Thorac Cancer 2019;10:1710-6. [Crossref] [PubMed]
- Wei Z, Yang X, Ye X, et al. Microwave ablation plus chemotherapy versus chemotherapy in advanced non-small cell lung cancer: a multicenter, randomized, controlled, phase III clinical trial. Eur Radiol 2020;30:2692-702. [Crossref] [PubMed]
- Chen J, Qi L, Chen J, et al. Microwave ablation therapy assisted by artificial pneumothorax and artificial hydrothorax for lung cancer adjacent to the vital organs. Front Oncol 2022;12:981789. [Crossref] [PubMed]
- Liu B, Li C, Sun X, et al. Assessment and Prognostic Value of Immediate Changes in Post-Ablation Intratumor Density Heterogeneity of Pulmonary Tumors via Radiomics-Based Computed Tomography Features. Front Oncol 2021;11:615174. [Crossref] [PubMed]
- Chetan MR, Gleeson FV. Radiomics in predicting treatment response in non-small-cell lung cancer: current status, challenges and future perspectives. Eur Radiol 2021;31:1049-58. [Crossref] [PubMed]
- Alexander ES, Xiong L, Baird GL, et al. CT Densitometry and Morphology of Radiofrequency-Ablated Stage IA Non-Small Cell Lung Cancer: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) Trial. J Vasc Interv Radiol 2020;31:286-93. [Crossref] [PubMed]
- Markich R, Palussière J, Catena V, et al. Radiomics complements clinical, radiological, and technical features to assess local control of colorectal cancer lung metastases treated with radiofrequency ablation. Eur Radiol 2021;31:8302-14. [Crossref] [PubMed]
- Crombé A, Palussière J, Catena V, et al. Radiofrequency ablation of lung metastases of colorectal cancer: could early radiomics analysis of the ablation zone help detect local tumor progression? Br J Radiol 2023;96:20201371. [Crossref] [PubMed]
- Zhang G, Yang H, Zhu X, et al. A CT-Based Radiomics Nomogram to Predict Complete Ablation of Pulmonary Malignancy: A Multicenter Study. Front Oncol 2022;12:841678. [Crossref] [PubMed]
- Huang H, Zheng D, Chen H, et al. A CT-based radiomics approach to predict immediate response of radiofrequency ablation in colorectal cancer lung metastases. Front Oncol 2023;13:1107026. [Crossref] [PubMed]


